Body Weight Gain Status during the Incubator Weaning Process in Very Low Birth Weight Premature Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
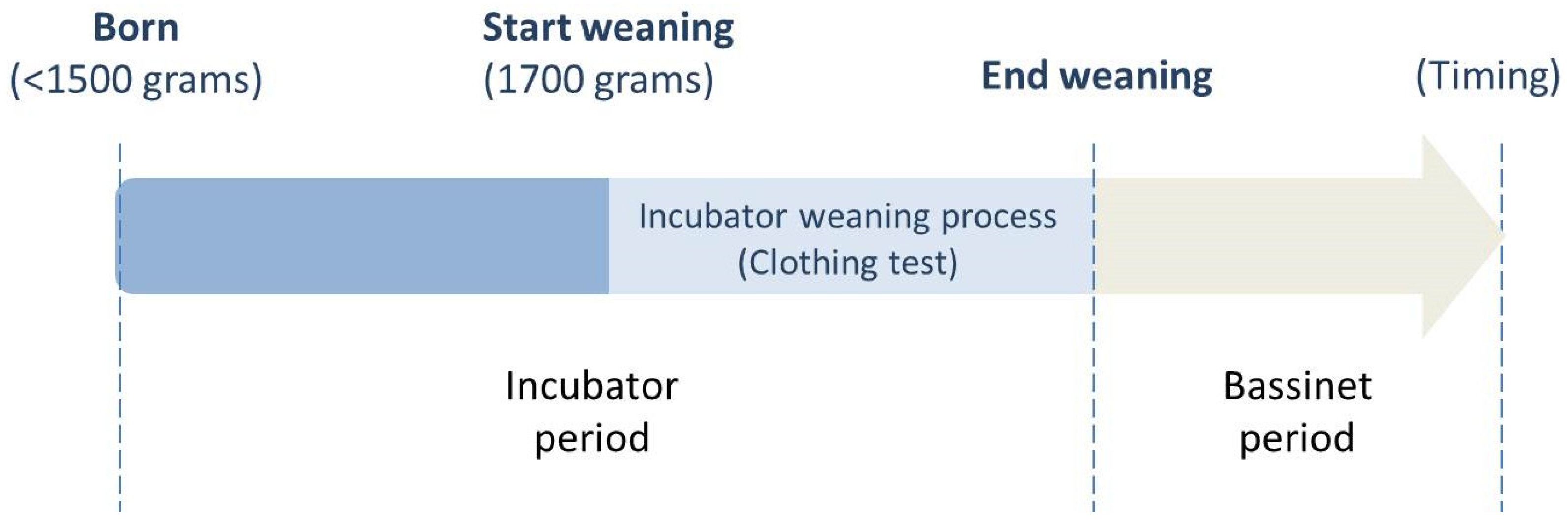
2.2. Care System
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walani, S.R. Global burden of preterm birth. Int. J. Gynaecol. Obstet. 2020, 150, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Heal. 2018, 7, e37–e46. [Google Scholar] [CrossRef]
- World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth. 2012. Available online: https://www.who.int/publications/i/item/9789241503433 (accessed on 27 March 2022).
- Shankaran, S.; Bell, E.; Laptook, A.R.; Saha, S.; Newman, N.S.; Kazzi, S.N.J.; Barks, J.; Stoll, B.J.; Bara, R.; Gabrio, J.; et al. Weaning of Moderately Preterm Infants from the Incubator to the Crib: A Randomized Clinical Trial. J. Pediatr. 2018, 204, 96–102. [Google Scholar] [CrossRef]
- Berger, I.; Marom, R.; Mimouni, F.; Kopelovich, R.; Dollberg, S. Weight at weaning of preterm infants from incubator to bassinet: A randomized clinical trial. Am. J. Perinatol. 2014, 31, 535–540. [Google Scholar] [CrossRef]
- Merritt, T.A.; Pillers, D.; Prows, S.L. Early NICU discharge of very low birth weight infants: A critical review and analysis. Semin. Neonatol. 2003, 8, 95–115. [Google Scholar] [CrossRef]
- Zecca, E.; Corsello, M.; Priolo, F.; Tiberi, E.; Barone, G.; Romagnoli, C. Early weaning from incubator and early discharge of preterm infants: Randomized clinical trial. Pediatrics 2010, 126, e651–e656. [Google Scholar] [CrossRef]
- Sutter, T.W.; Phan, D.; Pierchala, C.E.; Rishel, W. Weaning of premature infants from the incubator to an open crib. J. Perinatol. 1988, 8, 193–198. [Google Scholar]
- Razak, A. At what weight should preterm infants be transferred from incubator to open cot. Arch. Dis. Child 2019, 104, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Medoff-Cooper, B. Transition of the preterm infant to an open crib. J. Obstet. Gynecol. Neonatal Nurs. 1994, 23, 329–335. [Google Scholar] [CrossRef] [PubMed]
- West, C.R.; Williams, M.; Weston, P.J. Feasibility and safety of early transfer of premature infants from incubators to cots: A pilot study. J. Paediatr. Child Health 2005, 41, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, R.; Kirkby, S.; Turenne, W.; Greenspan, J. Incubator weaning in preterm infants and associated practice variation. J. Perinatol. 2009, 29, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Casatelli, J. Developing a guideline for transferring premature infants from an incubator to an open crib. J. Neonatal Nurs. 2020, 26, 162–166. [Google Scholar] [CrossRef]
- New, K.; Flint, A.; Bogossian, F.; East, C.; Davies, M.W. Transferring preterm infants from incubators to open cots at 1600 g: A multicentre randomised controlled trial. Arch. Dis. Child Fetal Neonatal Ed. 2012, 97, F88–F92. [Google Scholar] [CrossRef]
- Heimler, R.; Sumners, J.E.; Grausz, J.P.; Kien, C.L.; Glaspey, J.C. Thermal environment change in growing premature infants: Effect on general somatic growth and subcutaneous fat accumulation. Pediatrics 1981, 68, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Cleminson, J.S.; Zalewski, S.P.; Embleton, N.D. Nutrition in the preterm infant: What’s new. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 220–225. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics Committee on Nutrition. References. In Paediatric Nutrition Handbook, 4th ed.; Kleinman, R.E., Ed.; American Academy of Pediatrics: Elk Grove Village, IL, USA, 1998; pp. 55–58. ISBN 978-1-5811-0005-1. [Google Scholar]
- Ritchie, K.; McClure, G. Prematurity. Lancet 1979, 2, 1227–1229. [Google Scholar] [CrossRef]
- Gregory, K. Update on nutrition for preterm and full-term infants. J. Obstet. Gynecol. Neonatal Nurs. 2005, 34, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.H.; Lien, R.; Hsu, J.F.; Chiang, M.C.; Fu, R.H. Effect of body weight on temperature control and energy expenditure in preterm infants. Pediatr. Neonatol. 2010, 51, 178–181. [Google Scholar] [CrossRef][Green Version]
- Gregory, K.E.; Deforge, C.E.; Natale, K.M.; Phillips, M.; Van Marter, L.J. Necrotizing enterocolitis in the premature infant: Neonatal nursing assessment, disease pathogenesis, and clinical presentation. Adv. Neonatal Care 2011, 11, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Hulzebos, C.V.; Sauer, P.J. Energy requirements. Semin. Fetal Neonatal Med. 2007, 12, 2–10. [Google Scholar] [CrossRef] [PubMed]
| Basic Characteristics | * Preterm Newborn (n = 127) |
|---|---|
| Sex | |
| Male | 62 (48.8) |
| Female | 65 (51.2) |
| † GA (week) | 29.5 ± 2.5 |
| <30+0 weeks | 68 (53.2) |
| ≥30+0 weeks | 59 (46.8) |
| Delivery (cesarean) | 50 (39.4) |
| Multiple birth | 39 (30.7) |
| APGAR score | |
| 1 min | 5.4 ± 1.9 |
| 5 min | 7.2 ± 1.8 |
| §BBW (gram) | 1188.9 ± 255.3 |
| <1000 g | 35 (27.6) |
| ≥1000 g | 92 (72.4) |
| ‡ SGA | 43 (34.1) |
| Clinical Characteristics | ‡‡ Preterm Newborn (n = 127) |
|---|---|
| Disease pattern | |
| Early sepsis | 0 (0) |
| ‡ RDS | 123 (96.9) |
| ¶ PDA | 40 (30.7) |
| Ø NEC | 2 (1.6) |
| Ð BPD | 16 (12.5) |
| ð ROP | 119 (93.7) |
| Start weaning | |
| † PMA (week) | 34.5 ± 1.3 |
| § PND (day) | 35.3 ± 18.1 |
| Body weight (gram) | 1794.7 ± 119.2 |
| End weaning | |
| † PMA (week) | 35.1 ± 1.3 |
| § PND (day) | 37.7 ± 18.2 |
| Body weight (gram) | 1882.8 ± 157.1 |
| Total weaning period (day) | 3.5 ± 3.1 |
| Fenton growth weight at incubator weaning (z-score) | −1.4 ± 0.9 |
| Intake-calorie status | |
| * Daily intake-calories (kcal/kg) | 124.2 ± 8.9 |
| Intake-calorie gain status | |
| ** Change of daily intake-calories (kcal/kg) | 0.9 ± 3.9 |
| *** Variation of change of daily intake-calories (kcal/kg) | 5.4 ± 7.2 |
| Body weight status | |
| # Daily body weight (gram) | 1816.0 ± 155.8 |
| Body weight gain status | |
| ## Change of daily body weight (gram) | 37.6 ± 14.5 |
| ### Variation of change of daily body weight (gram) | 23.3 ± 19.9 |
| Preterm Newborn (n = 127) | Standardized β | p Value |
|---|---|---|
| ‡‡ GA (week) | 0.064 | 0.489 |
| § BBW (gram) | 0.051 | 0.578 |
| Disease pattern | ||
| ‡ RDS | 0.015 | 0.873 |
| ¶ PDA | 0.035 | 0.706 |
| Ø NEC | 0.085 | 0.351 |
| Ð BPD | −0.014 | 0.877 |
| ð ROP | −0.050 | 0.582 |
| † PMA (week) | ||
| Start-weaning | 0.029 | 0.753 |
| End-weaning | 0.299 | 0.001 |
| Intake-calorie status | ||
| * Daily intake-calories (kcal/kg) | 0.049 | 0.618 |
| Intake-calorie gain status | ||
| ** Change of daily intake-calories (kcal/kg) | 0.141 | 0.154 |
| *** Variation of change of daily intake-calories (kcal/kg) | −0.010 | 0.921 |
| Body weight status | ||
| # Daily body weight (gram) | 0.571 | <0.001 |
| Body weight gain status | ||
| ## Change of daily body weight (gram) | −0.087 | 0.342 |
| ### Variation of change of daily body weight (gram) | 0.069 | 0.455 |
| Preterm Newborn (n = 127) | Standardized β | p Value |
|---|---|---|
| * GA (week) | −0.005 | 0.956 |
| § BBW (gram) | 0.014 | 0.876 |
| Disease pattern | ||
| ‡ RDS | −0.001 | 0.988 |
| ¶ PDA | −0.089 | 0.325 |
| Ø NEC | 0.179 | 0.046 |
| Ð BPD | −0.065 | 0.484 |
| Ð ROP | 0.024 | 0.787 |
| † PMA (week) | ||
| Start-weaning | 0.138 | 0.134 |
| End-weaning | 0.305 | 0.001 |
| Intake-calorie status | ||
| * Daily intake-calories (kcal/kg) | −0.070 | 0.478 |
| Intake-calorie gain status | ||
| ** Change of daily intake-calories (kcal/kg) | −0.029 | 0.771 |
| *** Variation of change of daily intake-calories (kcal/kg) | −0.065 | 0.508 |
| Body weight gain status | ||
| ## Change of daily body weight (gram) | 0.093 | 0.306 |
| ### Variation of change of daily body weight (gram) | 0.109 | 0.230 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-W.; Ko, H.-Y.; Huang, C.-C.; Yeh, C.-Y.; Chiu, Y.-C.; Chen, H.-L. Body Weight Gain Status during the Incubator Weaning Process in Very Low Birth Weight Premature Infants. Children 2022, 9, 985. https://doi.org/10.3390/children9070985
Lin C-W, Ko H-Y, Huang C-C, Yeh C-Y, Chiu Y-C, Chen H-L. Body Weight Gain Status during the Incubator Weaning Process in Very Low Birth Weight Premature Infants. Children. 2022; 9(7):985. https://doi.org/10.3390/children9070985
Chicago/Turabian StyleLin, Chung-Wei, Hsiang-Yun Ko, Chih-Chi Huang, Chiu-Yu Yeh, Yen-Chun Chiu, and Hsiu-Lin Chen. 2022. "Body Weight Gain Status during the Incubator Weaning Process in Very Low Birth Weight Premature Infants" Children 9, no. 7: 985. https://doi.org/10.3390/children9070985
APA StyleLin, C.-W., Ko, H.-Y., Huang, C.-C., Yeh, C.-Y., Chiu, Y.-C., & Chen, H.-L. (2022). Body Weight Gain Status during the Incubator Weaning Process in Very Low Birth Weight Premature Infants. Children, 9(7), 985. https://doi.org/10.3390/children9070985







