Effects of Treatment of Sleep Disordered Breathing on Sleep Macro- and Micro-Architecture in Children with Down Syndrome
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Protocol
2.3. Sleep and Respiratory Analysis
2.4. Sleep Macro-Architecture Analysis
2.5. Sleep Micro-Architecture—Spectral Analysis
2.6. Statistical Analysis
3. Results
3.1. The Impact of Treatment of OSA on Sleep Macro-Architecture and Respiratory Parameters
3.2. The Impact of Treatment of OSA on Sleep Micro-Architecture
3.3. The Impact of Treatment of OSA on Behavior, Daytime Functioning, and Quality of Life
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horne, R.S.; Wijayaratne, P.; Nixon, G.M.; Walter, L.M. Sleep and sleep disordered breathing in children with down syndrome: Effects on behaviour, neurocognition and the cardiovascular system. Sleep Med. Rev. 2018, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.L.; Brooks, L.J.; Ward, S.D.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Lehmann, C.; Schechter, M.S.; Sheldon, S.; et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, e714–e755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shott, S.R.; Amin, R.; Chini, B.; Heubi, C.; Hotze, S.; Akers, R. Obstructive sleep apnea: Should all children with Down syndrome be tested? Arch. Otolaryngol. Head Neck Surg. 2006, 132, 432–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanyam, R.; Fleck, R.; McAuliffe, J.; Radhakrishnan, R.; Jung, D.; Patino, M.; Mahmoud, M. Upper airway morphology in Down Syndrome patients under dexmedetomidine sedation. Braz. J. Anesthesiol. 2016, 66, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggs, S.N.; Nixon, G.M.; Horne, R.S.C. The conundrum of primary snoring in children: What are we missing in regards to cognitive and behavioural morbidity? Sleep Med. Rev. 2014, 18, 463–475. [Google Scholar] [CrossRef]
- Chen, C.C.; Spano, G.; Edgin, J.O. The impact of sleep disruption on executive function in Down syndrome. Res. Dev. Disabil. 2013, 34, 2033–2039. [Google Scholar] [CrossRef]
- Churchill, S.S.; Kieckhefer, G.M.; Bjornson, K.F.; Herting, J.R. Relationship between sleep disturbance and functional outcomes in daily life habits of children with Down syndrome. Sleep 2015, 38, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Breslin, J.; Spanò, G.; Bootzin, R.; Anand, P.; Nadel, L.; Edgin, J. Obstructive sleep apnea syndrome and cognition in Down syndrome. Dev. Med. Child. Neurol. 2014, 56, 657–664. [Google Scholar] [CrossRef]
- Brooks, L.; Olsen, M.; Bacevice, A.; Beebe, A.; Konstantinopoulou, S.; Taylor, H. Relationship between sleep, sleep apnea, and neuropsychological function in children with Down syndrome. Int. J. Sci. Pract. Sleep Med. 2015, 19, 197–204. [Google Scholar] [CrossRef]
- Lee, N.-C.; Hsu, W.-C.; Chang, L.-M.; Chen, Y.-C.; Huang, P.-T.; Chien, C.-C.; Chien, Y.-H.; Chen, C.-L.; Hwu, W.-L.; Lee, P.-L. REM sleep and sleep apnea are associated with language function in Down syndrome children: An analysis of a community sample. J. Formos. Med. Assoc. 2020, 119, 516–523. [Google Scholar] [CrossRef]
- Joyce, A.; Dimitriou, D. Sleep-Disordered Breathing and Cognitive Functioning in Preschool Children with and without Down Syndrome. J. Intellect. Disabil. Res. 2017, 61, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Joyce, A.; Elphick, H.; Farquhar, M.; Gringras, P.; Evans, H.; Bucks, R.S.; Kreppner, J.; Kingshott, R.; Martin, J.; Reynolds, J.; et al. Obstructive Sleep Apnoea Contributes to Executive Function Impairment in Young Children with Down Syndrome. Behav. Sleep Med. 2020, 18, 611–621. [Google Scholar] [CrossRef]
- Beebe, D.W.; Gozal, D. Obstructive sleep apnea and the prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J. Sleep Res. 2002, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Nicholas, C.L.; Nixon, G.M.; Davey, M.J.; Anderson, V.; Walker, A.M.; Trinder, J.A.; Horne, R.S. Determining sleep quality in children with sleep disordered breathing: EEG spectral analysis compared with conventional polysomnography. Sleep 2010, 33, 1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, L.M.; Tamanyan, K.; Weichard, A.J.; Biggs, S.N.; Davey, M.J.; Nixon, G.M.; Horne, R.S.C. Age and autonomic control, but not cerebral oxygenation, are significant determinants of EEG spectral power in children. Sleep 2019, 42, zsz118. [Google Scholar] [CrossRef]
- Prerau, M.J.; Brown, R.E.; Bianchi, M.T.; Ellenbogen, J.M.; Purdon, P.L. Sleep Neurophysiological Dynamics Through the Lens of Multitaper Spectral Analysis. Physiology 2017, 32, 60–92. [Google Scholar] [CrossRef] [Green Version]
- Sibarani, C.R.; Walter, L.M.; Davey, M.J.; Nixon, G.M.; Horne, R.S.C. Sleep-disordered breathing and sleep macro- and micro-architecture in children with Down syndrome. Pediatr. Res. 2021, 91, 1248–1256. [Google Scholar] [CrossRef]
- Connolly, H.V.; Tomaselli, L.T.; McKenna Benoit, M.K. Adenotonsillectomy for pediatric obstructive sleep apnea: How to predict those at risk for postoperative complications. J. Clin. Sleep Med. 2020, 16, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Marcus, C.L.; Moore, R.H.; Rosen, C.L.; Giordani, B.; Garetz, S.L.; Taylor, H.G.; Mitchell, R.B.; Amin, R.; Katz, E.S.; Arens, R.; et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N. Engl. J. Med. 2013, 368, 2366–2376. [Google Scholar] [CrossRef] [Green Version]
- Ingram, D.G.; Ruiz, A.G.; Gao, D.; Friedman, N.R. Success of Tonsillectomy for Obstructive Sleep Apnea in Children with Down Syndrome. J. Clin. Sleep Med. 2017, 13, 975–980. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, L.F.; Shott, S.R.; LaRose, C.R.; Chini, B.A.; Amin, R.S. Causes of persistent obstructive sleep apnea despite previous tonsillectomy and adenoidectomy in children with down syndrome as depicted on static and dynamic cine MRI. Am. J. Roentgenol. 2004, 183, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogden, C.L.; Kuczmarski, R.J.; Flegal, K.M.; Mei, Z.; Guo, S.; Wei, R.; Grummer-Strawn, L.M.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002, 109, 45–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, R.A., Jr.; Rosenfeld, R.M.; Rao, M. Quality of life for children with obstructive sleep apnea. Otolaryngol. Head Neck Surg. 2000, 123, 9–16. [Google Scholar] [CrossRef]
- Goldstein, N.A.; Fatima, M.; Campbell, T.F.; Rosenfeld, R.M. Child behavior and quality of life before and after tonsillectomy and adenoidectomy. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 770–775. [Google Scholar] [CrossRef] [Green Version]
- Achenbach, T.M. The child behavior checklist and related instruments. In The Use of Psychological Testing for Treatment Planning and Outcomes Assessment, 2nd ed.; Mahwah, N.J., Ed.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 1999; pp. 429–466. [Google Scholar]
- Eisenhower, A.S.; Baker, B.L.; Blacher, J. Preschool children with intellectual disability: Syndrome specificity, behaviour problems, and maternal well-being. J. Intellect. Disabil. Res. 2005, 49, 657–671. [Google Scholar] [CrossRef] [Green Version]
- Marino, M.; Scala, I.; Scicolone, O.; Strisciuglio, P.; Bravaccio, C. Distribution and age of onset of psychopathological risk in a cohort of children with Down syndrome in developmental age. Ital. J. Pediatr. 2019, 45, 92. [Google Scholar] [CrossRef] [Green Version]
- Oakland, T.; Harrison, P. Adaptive Behavior Assessment System-II; Western Psychological Services: Los Angeles, CA, USA, 2008. [Google Scholar]
- Biggs, S.N.; Kennedy, J.D.; Martin, A.J.; van den Heuvel, C.J.; Lushington, K. Psychometric properties of an omnibus sleep problems questionnaire for school-aged children. Sleep Med. 2012, 13, 390–395. [Google Scholar] [CrossRef]
- Janssen, K.C.; Phillipson, S.; O’Connor, J.; Johns, M.W. Validation of the Epworth Sleepiness Scale for Children and Adolescents using Rasch analysis. Sleep Med. 2017, 33, 30–35. [Google Scholar] [CrossRef]
- Patel, V.P.; Patroneva, A.; Glaze, D.G.; Davis Ms, K.; Merikle, E.; Revana, A. Establishing the content validity of the Epworth Sleepiness Scale for Children and Adolescents in Prader-Willi syndrome. J. Clin. Sleep Med. 2022, 18, 485–496. [Google Scholar] [CrossRef]
- Nation, J.; Brigger, M. The Efficacy of Adenotonsillectomy for Obstructive Sleep Apnea in Children with Down Syndrome: A Systematic Review. Otolaryngol. Head Neck Surg. 2017, 157, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Kheirandish-Gozal, L.; Spruyt, K.; Mitchell, R.B.; Promchiarak, J.; Simakajornboon, N.; Kaditis, A.G.; Splaingard, D.; Splaingard, M.; Brooks, L.J.; et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: A multicenter retrospective study. Am. J. Respir. Crit. Care Med. 2010, 182, 676–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shete, M.M.; Stocks, R.M.; Sebelik, M.E.; Schoumacher, R.A. Effects of adeno-tonsillectomy on polysomnography patterns in Down syndrome children with obstructive sleep apnea: A comparative study with children without Down syndrome. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Mims, M.; Thottam, P.J.; Kitsko, D.; Shaffer, A.; Choi, S. Characterization of Sleep Architecture in Down Syndrome Patients Pre and Post Airway Surgery. Cureus 2017, 9, e983. [Google Scholar] [CrossRef] [Green Version]
- Nerfeldt, P.; Sundelin, A. Obstructive sleep apnea in children with down syndrome—Prevalence and evaluation of surgical treatment. Int. J. Pediatr. Otorhinolaryngol. 2020, 133, 109968. [Google Scholar] [CrossRef]
- Morisson, F.; Lavigne, G.; Petit, D.; Nielsen, T.; Malo, J.; Montplaisir, J. Spectral analysis of wakefulness and REM sleep EEG in patients with sleep apnoea syndrome. Eur. Respir. J. 1998, 11, 1135–1140. [Google Scholar] [CrossRef] [Green Version]
- Marcus, C.L.; Radcliffe, J.; Konstantinopoulou, S.; Beck, S.E.; Cornaglia, M.A.; Traylor, J.; DiFeo, N.; Karamessinis, L.R.; Gallagher, P.R.; Meltzer, L.J. Effects of positive airway pressure therapy on neurobehavioral outcomes in children with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2012, 185, 998–1003. [Google Scholar] [CrossRef] [Green Version]
- Esbensen, A.J.; Hoffman, E.K.; Shaffer, R.; Chen, E.; Patel, L.; Jacola, L. Reliability of parent report measures of behaviour in children with Down syndrome. J. Intellect. Disabil. Res. 2018, 62, 785–797. [Google Scholar] [CrossRef]
- Garetz, S.L.; Mitchell, R.B.; Parker, P.D.; Moore, R.H.; Rosen, C.L.; Giordani, B.; Muzumdar, H.; Paruthi, S.; Elden, L.; Willging, P.; et al. Quality of life and obstructive sleep apnea symptoms after pediatric adenotonsillectomy. Pediatrics 2015, 135, e477–e486. [Google Scholar] [CrossRef] [Green Version]
- Biggs, S.N.; Bourke, R.; Anderson, V.; Jackman, A.R.; Killedar, A.; Nixon, G.M.; Davey, M.J.; Walker, A.M.; Trinder, J.; Horne, R.S. Working memory in children with sleep-disordered breathing: Objective versus subjective measures. Sleep Med. 2011, 12, 887–891. [Google Scholar] [CrossRef]
- Sudarsan, S.S.; Paramasivan, V.K.; Arumugam, S.V.; Murali, S.; Kameswaran, M. Comparison of treatment modalities in syndromic children with obstructive sleep apnea--a randomized cohort study. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Nixon, G.M.; Biggs, S.N.; Jitpiriyaroj, S.; Horne, R.S. The Relationship Between Sleep-Disordered Breathing Severity and Daytime Adaptive Functioning in Children with Down Syndrome. CNS Neurosci. Ther. 2016, 22, 936–937. [Google Scholar] [CrossRef] [PubMed]
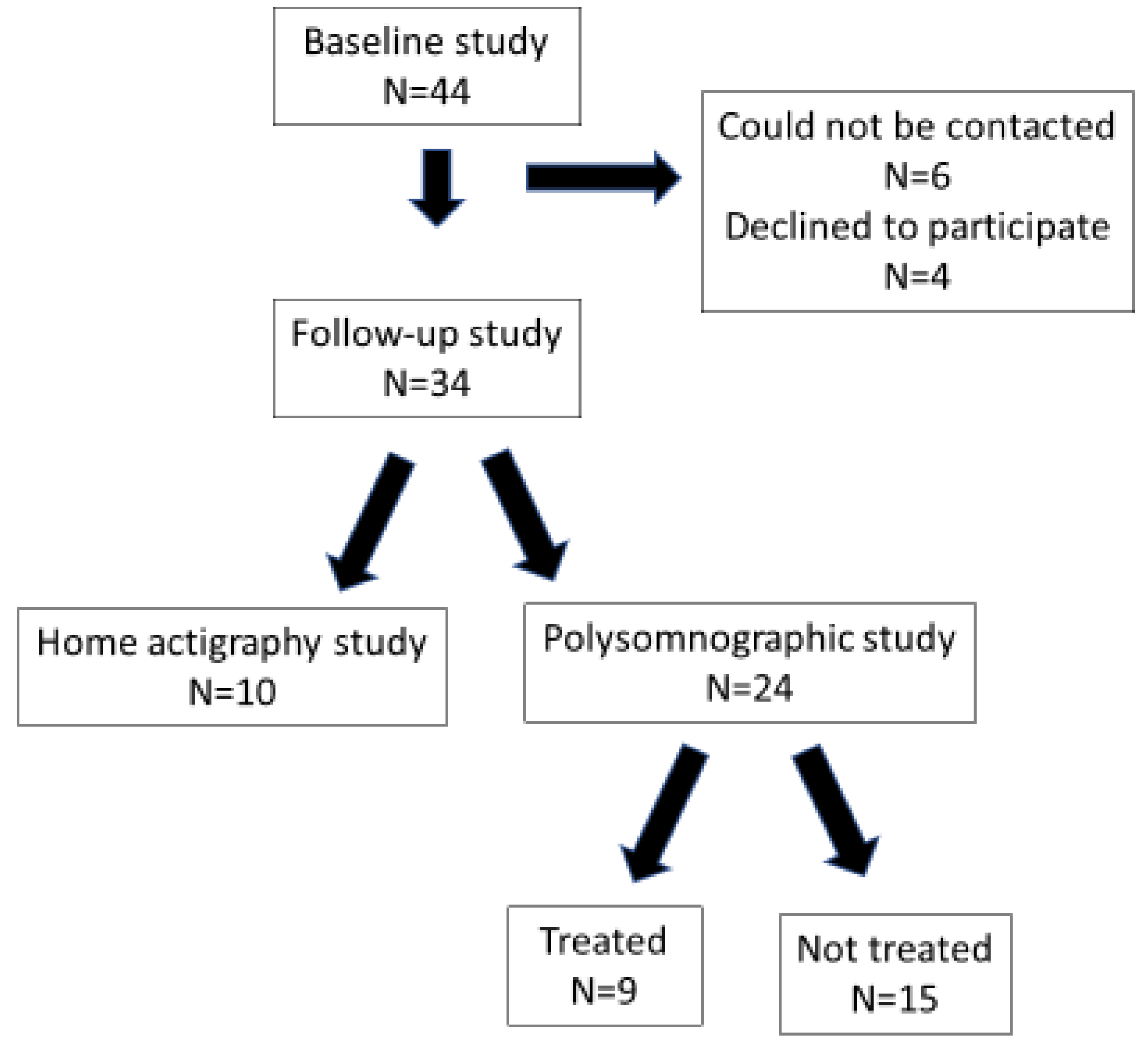
| No Follow-Up PSG (n = 20) | Follow-Up PSG (n = 24) | p-Value | |
|---|---|---|---|
| Females/Males | 11/9 | 13/11 | NS |
| Age (years) | 5.5 (4.2, 12.0) | 8.8 (6.8, 14.5) | <0.05 |
| BMI z-score | 0.8 (0.1, 2.1) | 1.1 (0.6, 1.8) | NS |
| Neck circumference (cm) | 28.5 (26.3, 33.0) | 31.0 (28.0, 38.0) | NS |
| Waist circumference (cm) | 59.0 (53.0, 72.0) | 64.0 (57.0, 80.0) | NS |
| Hip circumference (cm) | 58.0 (56.0, 77.0) | 72.0 (64.0, 90.0) | <0.05 |
| OAHI (events/h) | 1.8 (0.2, 9.0) | 5.3 (1.8, 20.7) | NS |
| RDI (events/h) | 4.9 (2.8, 12.8) | 9.4 (4.3, 26.1) | NS |
| REM RDI (events/h) | 13.6 (5.3, 24.9) | 19.1 (9.4, 31.6) | NS |
| CAHI (events/h) | 1.2 (0.4, 2.6) | 1.8 (1.5, 4.0) | NS |
| SpO2 nadir (%) | 89.0 (85.3, 90.8) | 88.0 (86.0, 89.8) | NS |
| Average SpO2 drop | 4.0 (3.0, 5.0) | 4.0 (3.0, 5.0) | NS |
| SpO2 < 90%/h | 0.1 (0.0, 0.6) | 0.2 (0.1, 0.4) | NS |
| SpO2 > 4% drop/h | 2.0 (1.0, 5.0) | 3.9 (1.7, 8.0) | NS |
| Arousal index (events/h) | 12.0 (9.7, 19.2) | 14.7 (13.0, 18.2) | NS |
| Average TcCO2 TST | 42.6 (40.1, 46.8) | 48.0 (43.7, 49.9) | <0.05 |
| Treatment and SDB Severity Characteristics | ||||
|---|---|---|---|---|
| Treatment before Baseline Study | Baseline SDB Severity Group (OAHI) | Treatment after Baseline Study | Follow Up SDB Severity Group (OAHI) | Improved |
| Children Treated at Follow-up | ||||
| No treatment | Severe (13.9) | Adenotonsillectomy | Severe (16.5) | No |
| No treatment | Severe (154) | Lingual tonsillectomy | Severe (77.0) | No |
| No treatment | Severe (30.0) | Adenotonsillectomy | Mild (2.3) | Yes |
| No treatment | Severe (13.0) | Adenotonsillectomy | Severe (47.9) | No |
| No treatment | Severe (39.0) | Adenotonsillectomy | Severe (13.7) | No |
| No treatment | Severe (62.0) | CPAP | PS (0.0) | Yes |
| No treatment | Severe (42.0) | CPAP | Mild (1.4) | Yes |
| No treatment | Mod (5.9) | Tonsillectomy | Mild (1.9) | Yes |
| No treatment | Mild (2.5) | Adenotonsillectomy | PS (0.5) | Yes |
| Children Un-treated at Follow-up | ||||
| No treatment | Severe (22.9) | No treatment | Severe (54.6) | No |
| No treatment | Moderate (5.7) | No treatment | Severe (27.1) | No |
| Adenotonsillectomy | Moderate (8.3) | No treatment | Mild (1.3) | Yes |
| Adenotonsillectomy | Moderate (6.4) | No treatment | Moderate (6.8) | No |
| No treatment | Mild (3.5) | No treatment | Mild (3.1) | No |
| Adenotonsillectomy | Mild (4.9) | No treatment | Mild (4.8) | No |
| No treatment | Mild (2.3) | No treatment | PS (0.6) | Yes |
| Adenotonsillectomy | Mild (1.2) | No treatment | Mild (3.3) | No |
| Tonsillectomy | Mild (1.6) | No treatment | PS (0.6) | Yes |
| Adenotonsillectomy | Mild (3.5) | No treatment | Mild (1.2) | No |
| No treatment | Mild (3.1) | No treatment | PS (0.1) | Yes |
| Tonsillectomy | PS (0.6) | No treatment | Mild (2.2) | No |
| No treatment | PS (0.4) | No treatment | PS (0.3) | No |
| No treatment | PS (0.1) | No treatment | Mild (1.3) | No |
| Adenotonsillectomy | PS (0.0) | No treatment | Mild (2.8) | No |
| Untreated | Treated | |||
|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | |
| N | 15 | 15 | 9 | 9 |
| Time in bed (min) | 531 ± 45 | 509 ± 28 | 518 ± 22 | 506 ± 42 |
| Sleep period time (min) | 485 ± 59 | 480 ± 36 | 465 ± 71 | 458 ± 54 |
| Total sleep time (min) | 444 ± 56 | 424 ± 46 | 369 ± 83 ** | 398 ± 62 |
| Wake after sleep onset (%) | 9 ± 6 | 11 ± 7 | 20 ± 15 ** | 13 ± 7 † |
| Sleep efficiency (%) | 84 ± 9 | 84 ± 9 | 71 ± 16 ** | 79 ± 10 |
| Sleep latency (min) | 42 ± 48 | 23 ± 17 | 35 ± 25 | 37 ± 27 |
| REM latency (min) | 164 ± 63 | 188 ± 78 | 223 ± 94 * | 307 ± 86 |
| N1 (%) | 5 ± 4 | 6 ± 5 | 9 ± 7 | 6 ± 4 |
| N2 (%) | 48 ± 9 | 48 ± 6 | 50 ± 8 | 49 ± 10 |
| N3 (%) | 31 ± 17 | 30 ± 6 | 28 ± 10 | 29 ± 5 |
| NREM (%) | 81 ± 6 | 84 ± 6 | 87 ± 8 | 84 ± 6 |
| REM (%) | 19 ± 6 | 16 ± 5 | 13 ± 8 | 16 ± 6 |
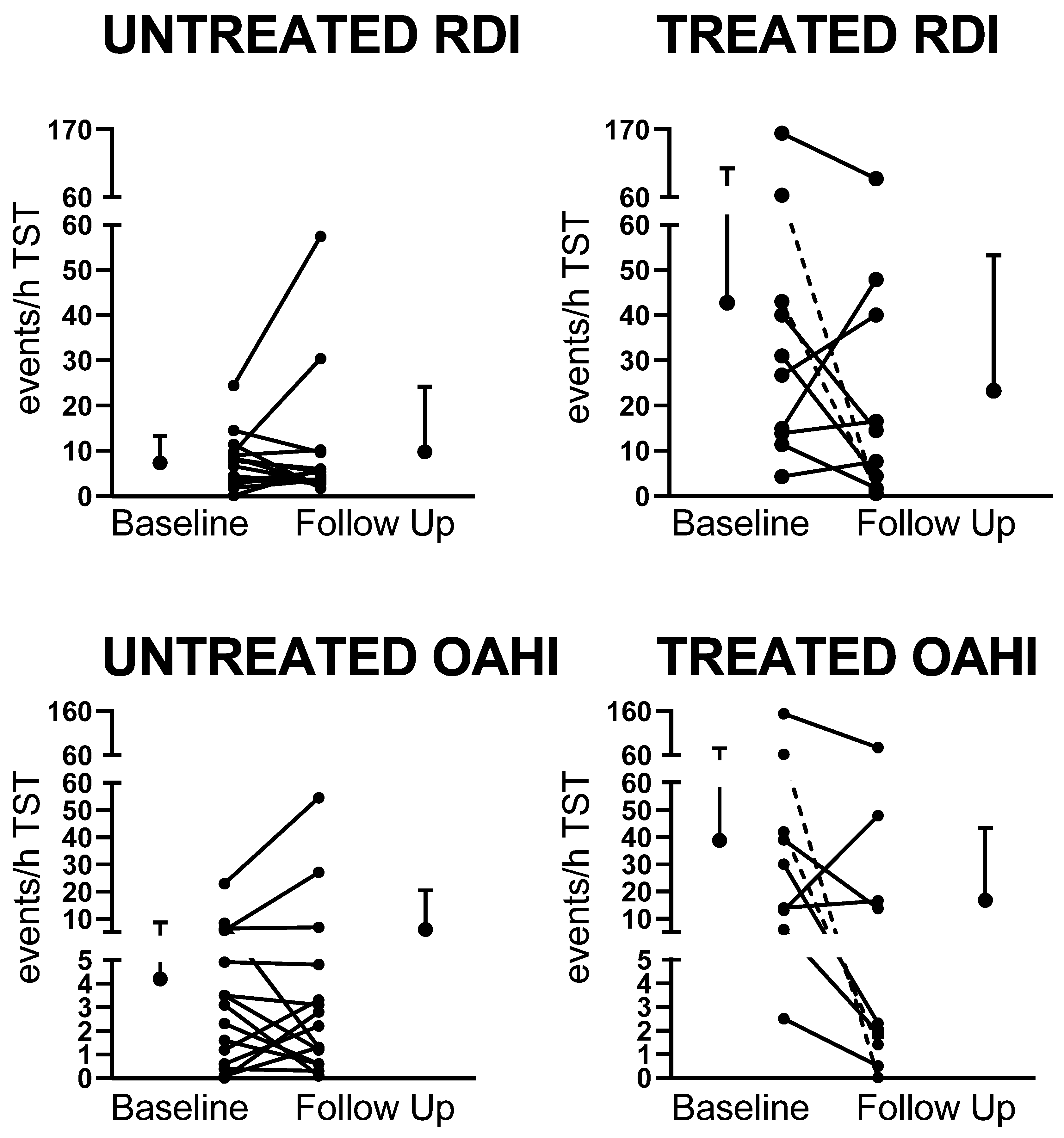
| OAHI (events/h) | 4.3 ± 5.7 | 7.3 ± 14.7 | 40.3 ± 46.9 ** | 17.9 ±26.9 †† |
| RDI (events/h) | 7.7 ± 6.1 | 10.2 ± 14.8 | 44.6 ± 48.2 ** | 24.8 ± 29.6 † |
| REM RDI (events/h) | 14.1 ± 10.2 | 19.8 ± 22.9 | 56.0 ± 50.7 * | 47.5 ± 65.0 |
| CAHI (events/h) | 2.5 ± 1.4 | 2.3 ± 0.9 | 4.3 ± 6.9 | 6.4 ± 12.2 |
| Arousal index (events/h) | 13.9 ± 9.7 | 14.9 ± 6.9 | 31.4 ± 28.0 ** | 20.8 ± 17.0 † |
| SpO2 nadir (%) | 88.91± 2.9 | 86.9 ± 5.0 | 81.6 ± 10.4 ** | 87.0 ± 6.3 † |
| Average SpO2 drop | 3.9 ± 0.8 # | 4.4 ± 0.9 | 4.9 ± 1.8 | 4.7 ± 1.7 |
| SpO2 < 90%/h | 0.2 ± 0.3 | 0.5 ± 0.6 | 10.2 ± 25.6 | 4.0 ± 10.2 |
| SpO2 > 4% drop/h | 2.9 ± 2.1 | 5.2 ± 7.1 | 25.7 ± 41.2 * | 14.1 ± 23.1 † |
| Average TcCO2 TST | 46.2 ± 5.3 | 42.9 ± 4.4 | 47.4 ± 4.4 | 43.3 ± 3.8 |
| Untreated | Treated | |||
|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | |
| N2 | ||||
| Total Power (µV2) | 905.2 ± 377.2 | 673.8 ± 183.4 | 671.5 ± 395.3 | 783.9 ± 598.1 |
| SEF (µV2) | 10.0 ± 2.4 ### | 11.2 ± 2.1 | 10.0 ± 2.1 | 10.8 ± 1.7 |
| Delta Power (µV2) | 677.3 ± 284.0 | 501.9 ± 135.9 | 538.5 ± 346.6 | 638.7 ± 506.6 |
| Theta Power (µV2) | 159.2 ± 87.8 ### | 106.9 ± 55.9 | 91.8 ± 60.4 * | 89.3 ± 70.4 |
| Alpha Power (µV2) | 27.5 ± 10.5 | 24.5 ± 8.4 | 16.9 ± 6.0 * | 22.0 ± 13.8 |
| Sigma Power (µV2) | 13.9 ± 8.9 | 14.9 ± 9.1 | 6.8 ± 3.2 | 10.3 ± 6.3 |
| Beta Power (µV2) | 18.8 ± 8.4 | 19.6 ± 8.7 | 12.4 ± 4.8 | 17.9 ± 13.0 |
| N3 | ||||
| Total Power (µV2) | 4274.3 ± 1541.5 | 3740.4 ± 972.5 | 3820.0 ± 1864.6 | 3348.5 ± 2859.8 |
| SEF (µV2) | 5.3 ± 0.6 | 5.3 ± 0.6 | 4.9 ± 0.5 | 5.5 ± 1.1 † |
| Delta Power (µV2) | 3920.5 ± 1401.4 | 3453.0 ± 901.7 | 3538.1 ± 1712.0 | 3090.3 ± 2650.1 |
| Theta Power (µV2) | 283.3 ± 122.4 # | 223.1 ± 70.6 | 229.6 ± 131.8 | 194.2 ± 157.6 |
| Alpha Power (µV2) | 34.6 ± 15.9 | 30.4 ± 9.2 | 24.5 ± 10.9 | 25.0 ± 15.1 |
| Sigma Power (µV2) | 8.4 ± 4.3 | 8.2 ± 3.8 | 5.6 ± 2.2 | 7.2 ± 3.6 |
| Beta Power (µV2) | 11.1 ± 5.2 | 12.1 ± 9.9 | 8.7 ± 3.8 | 20.0 ± 29.2 |
| REM | ||||
| Total Power (µV2) | 799.1 ± 507.4 ## | 523.8 ± 254.2 | 428.5 ± 276.1 | 510.0 ± 525.8 |
| SEF (µV2) | 9.5 ± 2.9 ## | 10.6 ± 2.7 | 10.6 ± 2.3 | 11.4 ± 2.8 |
| Delta Power (µV2) | 594.5 ± 409.4 ## | 383.2 ± 205.1 | 325.0 ± 219.1 | 400.4 ± 456.6 |
| Theta Power (µV2) | 152.8 ± 84.9 ### | 97.4 ± 46.3 | 71.6 ± 47.9 * | 75.1 ± 64.3 |
| Alpha Power (µV2) | 20.4 ± 9.2 ## | 16.6 ± 7.3 | 12.7 ± 5.9 * | 13.4 ± 6.3 |
| Sigma Power (µV2) | 5.1 ± 1.8 | 5.1 ± 2.1 | 3.2 ± 1.5 | 3.8 ± 1.3 |
| Beta Power (µV2) | 15.5 ± 6.5 | 15.0 ± 5.7 | 11.0 ± 5.2 | 12.1 ± 5.4 |
| Untreated | Treated | |||
|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | |
| CBCL internalizing problems | 55.4 ± 10.4 | 55.7 ± 10.9 | 54.3 ± 10.3 | 56.1 ± 9.4 |
| CBCL externalizing problems | 54.6 ± 10.5 | 57.3 ± 7.7 | 53.6 ± 7.1 | 55.0 ± 9.9 |
| CBCL total problems | 56.4 ± 10.7 | 58.2 ± 9.5 | 55.0 ± 9.5 | 57.0 ± 8.0 |
| ABAS GAC composite score | 55.4 ± 14.1 | 57.4 ± 12.2 | 53.8 ± 9.2 | 53.7 ± 13.1 |
| ABAS conceptual composite score | 57.8 ± 11.5 | 60.4 ± 8.8 | 55.0 ± 7.0 | 58.1 ± 10.8 |
| ABAS social composite score | 73.1 ± 13.9 | 73.2 ± 13.2 | 69.5 ± 11.0 | 69.2 ± 12.7 |
| ABAS practical composite score | 53.0 ± 17.7 | 54.3 ± 15.1 | 51.8 ± 15.3 | 51.2 ± 13.1 |
| OSA-18 sleep disturbances sub-scale | 12.2 ± 5.3 | 11.7 ± 5.7 | 14.2 ± 5.9 | 9.7 ± 1.2 |
| OSA-18 physical symptoms sub-scale | 13.7 ± 5.6 # | 9.1 ± 3.7 | 14.2 ± 6.9 | 9.2 ± 3.0 |
| OSA-18 emotional symptoms sub-scale | 8.3 ± 4.5 | 8.2 ± 4.0 | 10.4 ± 3.1 | 6.5 ± 4.2 |
| OSA-18 daytime function sub-scale | 9.3 ± 4.6 | 7.4 ± 4.3 | 9.2 ± 5.2 | 5.5 ± 1.3 |
| OSA-18 care giver concerns sub-scale | 13.0 ± 7.2 | 11.3 ± 5.8 | 12.8 ± 8.2 | 9.0 ± 2.1 |
| OSA-18 total symptoms | 56.5 ± 23.0 | 47.6 ± 17.1 | 60.8 ± 27.8 | 39.8 ± 3.8 † |
| PSSI sleep routine | 54.5 ± 10.8 | 53.4 ± 11.2 | 51.4 ± 12.5 | 51.0 ± 6.9 |
| PSSI bed time anxiety | 52.2 ± 9.6 | 54.6 ± 14.4 | 62.7 ± 15.9 | 56.0 ± 10.4 |
| PSSI morning tiredness | 52.2 ± 13.8 | 53.2 ± 12.6 | 52.7 ± 10.5 | 48.7 ± 7.2 |
| PSSI night arousal | 55.5 ± 14.6 | 59.9 ± 13.7 | 55.9 ± 17.2 | 58.0 ± 14.1 |
| PSSI sleep disordered breathing | 68.3 ± 13.0 | 70.9 ± 13.7 | 78.0 ± 15.2 ** | 70.2 ± 13.4 |
| PSSI restless sleep | 59.8 ± 11.8 | 55.6 ± 12.8 | 57.4 ± 6.4 | 55.3 ± 14.1 |
| ESS-CHAD | 4.0 ± 3.9 | 5.7 ± 4.9 | 6.0 ± 4.9 | 3.3 ± 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betavani, V.M.P.; Davey, M.J.; Nixon, G.M.; Walter, L.M.; Horne, R.S.C. Effects of Treatment of Sleep Disordered Breathing on Sleep Macro- and Micro-Architecture in Children with Down Syndrome. Children 2022, 9, 984. https://doi.org/10.3390/children9070984
Betavani VMP, Davey MJ, Nixon GM, Walter LM, Horne RSC. Effects of Treatment of Sleep Disordered Breathing on Sleep Macro- and Micro-Architecture in Children with Down Syndrome. Children. 2022; 9(7):984. https://doi.org/10.3390/children9070984
Chicago/Turabian StyleBetavani, Viecky M. P., Margot J. Davey, Gillian M. Nixon, Lisa M. Walter, and Rosemary S. C. Horne. 2022. "Effects of Treatment of Sleep Disordered Breathing on Sleep Macro- and Micro-Architecture in Children with Down Syndrome" Children 9, no. 7: 984. https://doi.org/10.3390/children9070984
APA StyleBetavani, V. M. P., Davey, M. J., Nixon, G. M., Walter, L. M., & Horne, R. S. C. (2022). Effects of Treatment of Sleep Disordered Breathing on Sleep Macro- and Micro-Architecture in Children with Down Syndrome. Children, 9(7), 984. https://doi.org/10.3390/children9070984





