Vitamin D Levels in Pregnant Women Do Not Affect Neonatal Bone Strength
Abstract
1. Introduction
2. Materials and Methods
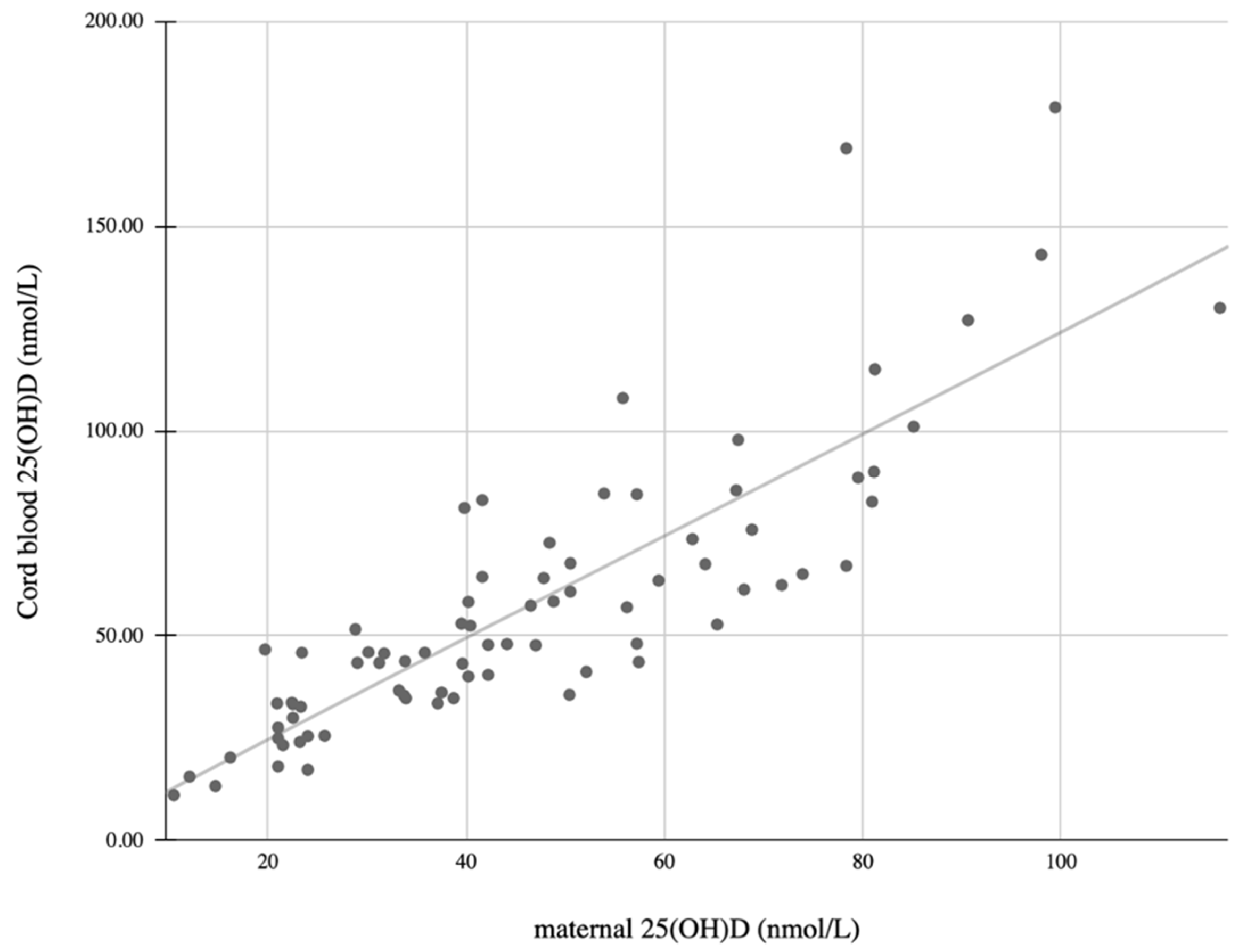
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uday, S.; Högler, W. Nutritional Rickets and Osteomalacia in the Twenty-first Century: Revised Concepts, Public Health, and Prevention Strategies. Curr. Osteoporos. Rep. 2017, 15, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Marshall, I.; Mehta, R.; Petrova, A. Vitamin D in the maternal–fetal–neonatal interface: Clinical implications and requirements for supplementation. J. Matern. Fetal Neonatal Med. 2013, 26, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol. Rev. 2016, 96, 449–547. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. Bone Development and Mineral Homeostasis in the Fetus and Neonate: Roles of the Calciotropic and Phosphotropic Hormones. Physiol. Rev. 2014, 94, 1143–1218. [Google Scholar] [CrossRef]
- Verhaeghe, J.; Bouillon, R. Calciotropic hormones during reproduction. J. Steroid Biochem. Mol. Biol. 1992, 41, 469–477. [Google Scholar] [CrossRef]
- Fudge, N.J.; Kovacs, C.S. Pregnancy Up-Regulates Intestinal Calcium Absorption and Skeletal Mineralization Independently of the Vitamin D Receptor. Endocrinology 2010, 151, 886–895. [Google Scholar] [CrossRef]
- Gillies, B.R.; Ryan, B.A.; Tonkin, B.A.; Poulton, I.J.; Ma, Y.; Kirby, B.J.; St-Arnaud, R.; Sims, N.A.; Kovacs, C.S. Absence of calcitriol causes increased lactational bone loss and lower milk calcium but does not impair post-lactation bone recovery in Cyp27b1 null mice: Effects of calcitriol during pregnancy and lactation. J. Bone Miner. Res. 2018, 33, 16–26. [Google Scholar] [CrossRef]
- Halloran, B.P.; DeLuca, H.F. Calcium transport in small intestine during pregnancy and lactation. Am. J. Physiol. Endocrinol. Metab. 1980, 239, E64–E68. [Google Scholar] [CrossRef]
- Brommage, R.; Baxter, D.C.; Gierke, L.W. Vitamin D-independent intestinal calcium and phosphorus absorption during reproduction. Am. J. Physiol. Gastrointest. Liver Physiol. 1990, 259, G631–G638. [Google Scholar] [CrossRef]
- Ward, L.M.; Gaboury, I.; Ladhani, M.; Zlotkin, S. Vitamin D-deficiency rickets among children in Canada. Can. Med. Assoc. J. 2007, 177, 161–166. [Google Scholar] [CrossRef]
- Cooper, C.; Harvey, N.C.; Bishop, N.J.; Kennedy, S.; Papageorghiou, A.T.; Schoenmakers, I.; Fraser, R.; Gandhi, S.V.; Carr, A.; D’Angelo, S.; et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): A multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016, 4, 393–402. [Google Scholar] [CrossRef]
- Viljakainen, H.T.; Saarnio, E.; Hytinantti, T.; Miettinen, M.; Surcel, H.; Mäkitie, O.; Andersson, S.; Laitinen, K.; Lamberg-Allardt, C. Maternal Vitamin D Status Determines Bone Variables in the Newborn. J. Clin. Endocrinol. Metab. 2010, 95, 1749–1757. [Google Scholar] [CrossRef]
- Mahon, P.; Harvey, N.; Crozier, S.; Inskip, H.; Robinson, S.; Arden, N.; Swaminathan, R.; Cooper, C.; Godfrey, K. Low maternal vitamin D status and fetal bone development: Cohort study. J. Bone Miner. Res. 2010, 25, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, C.; Javaid, M.K.; Mahon, P.; Yaqub, M.K.; Harvey, N.C.; Godfrey, K.M.; Noble, J.A.; Cooper, C.; Papageorghiou, A.T. The Effect of Maternal Vitamin D Concentration on Fetal Bone. J. Clin. Endocrinol. Metab. 2012, 97, E2070–E2077. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.C.; Sheppard, A.; Godfrey, K.M.; McLean, C.; Garratt, E.; Ntani, G.; Davies, L.; Murray, R.; Inskip, H.M.; Gluckman, P.D.; et al. Childhood Bone Mineral Content Is Associated with Methylation Status of the RXRA Promoter at Birth. J. Bone Miner. Res. 2014, 29, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Nemet, D.; Dolfin, T.; Wolach, B.; Eliakim, A. Quantitative ultrasound measurements of bone speed of sound in premature infants. Eur. J. Pediatr. 2001, 160, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.L.; Glade, M.J.; Gopal, J. The Use of Transmission Ultrasonics to Assess Bone Status in the Human Newborn. Pediatr. Res. 1987, 22, 541–544. [Google Scholar] [CrossRef]
- Gursoy, T.; Yurdakok, M.; Hayran, M.; Korkmaz, A.; Yigit, S.; Tekinalp, G. Bone Speed of Sound Curves of Twin and Singleton Neonates. J. Pediatric Endocrinol. Metab. 2008, 21, 1065–1071. [Google Scholar] [CrossRef]
- Regev, R.H.; Dolfin, T.; Eliakim, A.; Arnon, S.; Bauer, S.; Nemet, D.; Litmanovitz, I. Bone Speed of Sound in Infants of Mothers with Gestational Diabetes Mellitus. J. Pediatric Endocrinol. Metab. 2004, 17, 1083–1088. [Google Scholar] [CrossRef]
- Litmanovitz, I.; Dolfin, T.; Friedland, O.; Arnon, S.; Regev, R.; Shainkin-Kestenbaum, R.; Lis, M.; Eliakim, A. Early Physical Activity Intervention Prevents Decrease of Bone Strength in Very Low Birth Weight Infants. Pediatrics 2003, 112, 15–19. [Google Scholar] [CrossRef]
- Mukamel, M.N.; Weisman, Y.; Somech, R.; Eisenberg, Z.; Landman, J.; Shapira, I.; Spirer, Z.; Jurgenson, U. Vitamin D deficiency and insufficiency in Orthodox and non-Orthodox Jewish mothers in Israel. Isr. Med. Assoc. J. 2001, 3, 419–421. [Google Scholar] [PubMed]
- Fouda, M.A.; Turkestani, I.Z.; Almusharraf, S.; Al-Ajlan, A.; Angkaya-Bagayawa, F.F.; Sabico, S.; Mohammed, A.G.; Hassanato, R.; Al-Serehi, A.; Alshingetti, N.M.; et al. Extremely High Prevalence of Maternal and Neonatal Vitamin D Deficiency in the Arab Population. Neonatology 2017, 112, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Simhan, H.N.; Powers, R.W.; Frank, M.P.; Cooperstein, E.; Roberts, J.M. High Prevalence of Vitamin D Insufficiency in Black and White Pregnant Women Residing in the Northern United States and Their Neonates. J. Nutr. 2007, 137, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Sharifi, F.; Jafari, N.; Mousavinasab, N. High Prevalence of Vitamin D Deficiency among Pregnant Women and their Newborns in an Iranian Population. J. Women’s Health 2009, 18, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Shany, S.; Biale, Y.; Zuili, I.; Yankowitz, N.; Berry, J.L.; Mawer, E.B. Feto-maternal relationships between vitamin D metabolites in Israeli Bedouins and Jews. Am. J. Clin. Nutr. 1984, 40, 1290–1294. [Google Scholar] [CrossRef]
- Karras, S.; Bili, H.; Goulis, D.; Papadopoulou, F.; Harizopoulou, V.; Kintiraki, E.; Gkastaris, K.; Badis, K.; Tarlatzis, B. High prevalence of vitamin d deficiency among pregnant women at term and their neonates in Thessaloniki, Northern Greece. Bone 2012, 50 (Suppl. S1), S104. [Google Scholar] [CrossRef]
- Esmeraldo, C.U.P.; Martins, M.E.P.; Maia, E.R.; Leite, J.L.A.; Ramos, J.L.S.; Goncalves, J., Jr.; Neta, C.M.; Suano-Souza, F.I.; Sarni, R.O.S. Vitamin D in Term Newborns: Relation with Maternal Concentrations and Birth Weight. Ann. Nutr. Metab. 2019, 75, 39–46. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Avila, E.; Durand-Carbajal, M.; Díaz, L. Regulation of Calcitriol Biosynthesis and Activity: Focus on Gestational Vitamin D Deficiency and Adverse Pregnancy Outcomes. Nutrients 2015, 7, 443–480. [Google Scholar] [CrossRef]
- Karras, S.N.; Wagner, C.L.; Castracane, V.D. Understanding vitamin D metabolism in pregnancy: From physiology to pathophysiology and clinical outcomes. Metabolism 2018, 86, 112–123. [Google Scholar] [CrossRef]
- Evans, K.N.; Bulmer, J.N.; Kilby, M.D.; Hewison, M. Vitamin D and Placental-Decidual Function. J. Soc. Gynecol. Investig. 2004, 11, 263–271. [Google Scholar] [CrossRef]
- Velkavrh, M.; Paro-Panjan, D.; Benedik, E.; Mis, N.F.; Godnov, U.; Salamon, A.S. The Influence of Maternal Levels of Vitamin D and Adiponectin on Anthropometrical Measures and Bone Health in Offspring. PRILOZI 2019, 40, 91–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liao, X.P.; Zhang, W.L.; Yan, C.H.; Zhou, X.J.; Wang, P.; Sun, J.H.; Yu, X.D.; Wu, M.Q. Reduced tibial speed of sound in Chinese infants at birth compared with Caucasian peers: The effects of race, gender, and vitamin D on fetal bone development. Osteoporos. Int. 2010, 21, 2003–2011. [Google Scholar] [CrossRef]
- Masztalerz-Kozubek, D.; Zielinska-Pukos, M.A.; Hamulka, J. Maternal Diet, Nutritional Status, and Birth-Related Factors Influencing Offspring’s Bone Mineral Density: A Narrative Review of Observational, Cohort, and Randomized Controlled Trials. Nutrients 2021, 13, 2302. [Google Scholar] [CrossRef] [PubMed]
- Hyde, N.K.; Brennan-Olsen, S.L.; Wark, J.D.; Hosking, S.M.; Pasco, J.A. Gestational Vitamin D and Offspring Bone Measures: Is the Association Independent of Maternal Bone Quality? Calcif. Tissue Int. 2021, 108, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.K.; Crozier, S.R.; Harvey, N.C.; Gale, C.R.; Dennison, E.M.; Boucher, B.J.; Arden, N.K.; Godfrey, K.M.; Cooper, C. Maternal Vitamin D Status During Pregnancy and Childhood Bone Mass at Age 9 Years: A Longitudinal Study. Obstet. Gynecol. Surv. 2006, 61, 305–307. [Google Scholar] [CrossRef]
- Sayers, A.; Tobias, J.H. Estimated maternal ultraviolet B exposure levels in pregnancy influence skeletal development of the child. J. Clin. Endocrinol. Metab. 2009, 94, 765–771. [Google Scholar] [CrossRef]
- Cole, Z.A.; Gale, C.R.; Javaid, M.K.; Robinson, S.M.; Law, C.; Boucher, B.J.; Crozier, S.R.; Godfrey, K.M.; Dennison, E.M.; Cooper, C. Maternal dietary patterns during pregnancy and childhood bone mass: A longitudinal study. J. Bone Miner. Res. 2009, 24, 663–668. [Google Scholar] [CrossRef]
- Ryan, B.A.; Kovacs, C.S. Maternal and fetal vitamin D and their roles in mineral homeostasis and fetal bone development. J. Endocrinol. Investig. 2021, 44, 643–659. [Google Scholar] [CrossRef]
- Wierzejska, R.; Jarosz, M.; Klemińska-Nowak, M.; Tomaszewska, M.; Sawicki, W.; Bechanek, M.; Siuba-Strzelinska, M. Maternal and Cord Blood Vitamin D Status and Anthropometric Measurements in Term Newborns at Birth. Front. Endocrinol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Miliku, K.; Vinkhuyzen, A.; Blanken, L.M.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.; Hofman, A.; Tiemeier, H.; Steegers, E.A.; Gaillard, R.; et al. Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am. J. Clin. Nutr. 2016, 103, 1514–1522. [Google Scholar] [CrossRef]
- Leffelaar, E.R.; Vrijkotte, T.G.M.; van Eijsden, M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: Results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br. J. Nutr. 2010, 104, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, L.; Catling-Paull, C.; Diamond, T.; Homer, C.; Davis, G.; Craig, M.E. Vitamin D, PTH and calcium levels in pregnant women and their neonates. Clin. Endocrinol. 2009, 70, 372–377. [Google Scholar] [CrossRef] [PubMed]
- De-Regil, L.M.; Palacios, C.; Lombardo, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2016, CD008873. [Google Scholar] [CrossRef]
- Harvey, N.C.; Holroyd, C.; Ntani, G.; Javaid, K.; Cooper, P.; Moor, R.; Cole, Z.; Tinati, T.; Godfrey, K.; Dennison, E.; et al. Vitamin D supplementation in pregnancy: A systematic review. Health Technol. Assess. 2014, 18, 1–190. [Google Scholar] [CrossRef]
- Theodoratou, E.; Tzoulaki, I.; Zgaga, L.; Ioannidis, J.P.A. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014, 348, g2035. [Google Scholar] [CrossRef]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef]
- Thorne-Lyman, A.; Fawzi, W.W. Vitamin D During Pregnancy and Maternal, Neonatal and Infant Health Outcomes: A Systematic Review and Meta-analysis: Vitamin D and health outcomes. Paediatr. Perinat. Epidemiol. 2012, 26, 75–90. [Google Scholar] [CrossRef]
- Al-Faris, N. High Prevalence of Vitamin D Deficiency among Pregnant Saudi Women. Nutrients 2016, 8, 77. [Google Scholar] [CrossRef]
- Hajizadeh, S.; Rankin Shary, J.; Gayle Reed, S.; Lynn Wagner, C. The prevalence of hypovitaminosis D and its risk factors in pregnant women and their newborns in the Middle East: A systematic review. Int. J. Reprod. Biomed. (IJRM) 2019, 17, 685–708. [Google Scholar] [CrossRef]
- Buyukuslu, N.; Esin, K.; Hizli, H.; Sunal, N.; Yigit, P.; Garipagaoglu, M. Clothing preference affects vitamin D status of young women. Nutr. Res. 2014, 34, 688–693. [Google Scholar] [CrossRef]
- Kasirer, Y.; Reichman, B.; Zaslavsky-Paltiel, I.; Bin-Nun, A.; Lerner-Geva, L.; Mimouni, F.B.; in collaboration with the Israel Neonatal Network. Short-term outcomes of Jewish and Arab preterms: A population-based comparison. J. Perinatol. 2021, 41, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, M.; Khorsandi, A.; Haghollahi, F. Comparison of sun exposure versus vitamin D supplementation for pregnant women with vitamin D deficiency. J. Matern. Fetal Neonatal Med. 2019, 32, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Shalof, H.; Dimitri, P.; Shuweihdi, F.; Offiah, A.C. Which skeletal imaging modality is best for assessing bone health in children and young adults compared to DXA? A systematic review and meta-analysis. Bone 2021, 150, 116013. [Google Scholar] [CrossRef] [PubMed]
- Pezzuti, I.L.; Kakehasi, A.M.; Filgueiras, M.T.; de Guimarães, J.A.; de Lacerda, I.A.C.; Silva, I.N. Imaging methods for bone mass evaluation during childhood and adolescence: An update. J. Pediatr. Endocrinol. Metab. 2017, 30, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Wang, K.C.; Amirabadi, A.; Cheung, E.; Uleryk, E.; Moineddin, R.; Doria, A.S. Evidence-based outcomes on diagnostic accuracy of quantitative ultrasound for assessment of pediatric osteoporosis—A systematic review. Pediatr. Radiol. 2014, 44, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
| Maternal | Value |
|---|---|
| Age (years) | 30.7 ± 5.1 |
| Education (years) | 15.6 ± 2.2 |
| Origin: Jewish | 69 (86%) |
| Nulliparous | 47 (59%) |
| Singleton | 75 (94%) |
| Daily sun exposure | |
| Less than 15 min | 31 (40%) |
| More than 30 min | 47 (60%) |
| Sun skin protection | 29 (37%) |
| Vitamin D supplementation | 36 (45%) |
| Vitamin D supplementation dose (IU) | 1127 ± 894 |
| Vitamin D from nutrition (IU) | 239.3 ± 140.6 |
| Total Vitamin D consumption (IU) | 748 ± 824 |
| Infant | |
| Gestational age (weeks) | 39.5 ± 1.26 |
| Birth weight (grams) | 3223 ± 476 |
| Length (centimeters) | 50.7 ± 2.9 |
| Head circumference (cm) | 34.3 ± 1.3 |
| Male sex | 35 (44%) |
| Speed of sound (m/s) | 3042 ± 130 |
| 25(OH)D (nmol/L) | N | SOS (m/s) (Mean ± SD) | p-Value |
|---|---|---|---|
| Cord blood | |||
| <25 | 6 | 3040 ± 161 | 0.97 |
| 25–50 | 29 | 3049 ± 137 | |
| >50 | 38 | 3042 ± 123 | |
| Maternal | |||
| <25 | 13 | 3053 ± 119 | 0.15 |
| 25–50 | 29 | 3076 ± 143 | |
| >50 | 31 | 3011 ± 118 | |
| Continuous Variables | Pearson Correlation | Significance |
| Infant 25(OH)D levels | 0.85 | <0.001 |
| Infant SOS | −0.1 | 0.4 |
| Vitamin D intake–total | 0.36 | 0.001 |
| Vitamin D intake from food only | 0.026 | 0.82 |
| Vitamin D intake from supplements | 0.21 | 0.002 |
| Infant length at birth | 0.009 | 0.94 |
| Infant weight at birth | 0.082 | 0.47 |
| Infant head circumference | 0.212 | 0.063 |
| Maternal age | 0.11 | 0.32 |
| Non-continuous variables (MW test) | Significance | |
| Maternal ethnicity | <0.0001 | |
| Maternal sunlight exposure | 0.62 | |
| Maternal skin exposure | 0.12 | |
| Maternal use of sunscreen | 0.08 | |
| Season of the year | 0.76 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levkovitz, O.; Lagerev, E.; Bauer-Rusak, S.; Litmanovitz, I.; Grinblatt, E.; Sirota, G.L.; Shalit, S.; Arnon, S. Vitamin D Levels in Pregnant Women Do Not Affect Neonatal Bone Strength. Children 2022, 9, 883. https://doi.org/10.3390/children9060883
Levkovitz O, Lagerev E, Bauer-Rusak S, Litmanovitz I, Grinblatt E, Sirota GL, Shalit S, Arnon S. Vitamin D Levels in Pregnant Women Do Not Affect Neonatal Bone Strength. Children. 2022; 9(6):883. https://doi.org/10.3390/children9060883
Chicago/Turabian StyleLevkovitz, Orly, Elena Lagerev, Sofia Bauer-Rusak, Ita Litmanovitz, Eynit Grinblatt, Gisela Laura Sirota, Shachar Shalit, and Shmuel Arnon. 2022. "Vitamin D Levels in Pregnant Women Do Not Affect Neonatal Bone Strength" Children 9, no. 6: 883. https://doi.org/10.3390/children9060883
APA StyleLevkovitz, O., Lagerev, E., Bauer-Rusak, S., Litmanovitz, I., Grinblatt, E., Sirota, G. L., Shalit, S., & Arnon, S. (2022). Vitamin D Levels in Pregnant Women Do Not Affect Neonatal Bone Strength. Children, 9(6), 883. https://doi.org/10.3390/children9060883






