Maternal Pre-Pregnancy Obesity and Gestational Diabetes Mellitus Increase the Risk of Childhood Obesity
Abstract
:1. Introduction
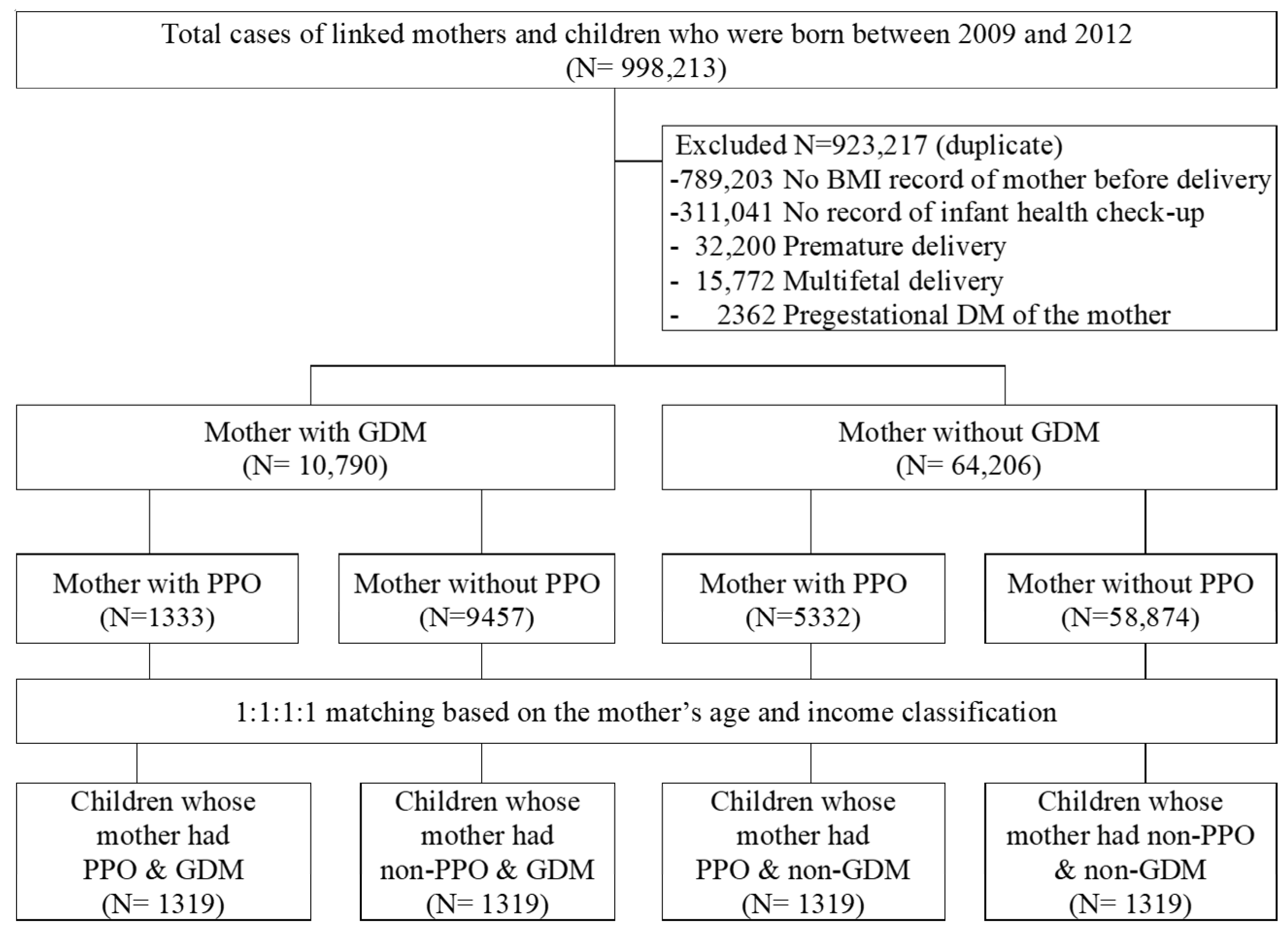
2. Materials and Methods
2.1. Data Source
2.2. Study Participants and Covariates
2.3. Data Analysis
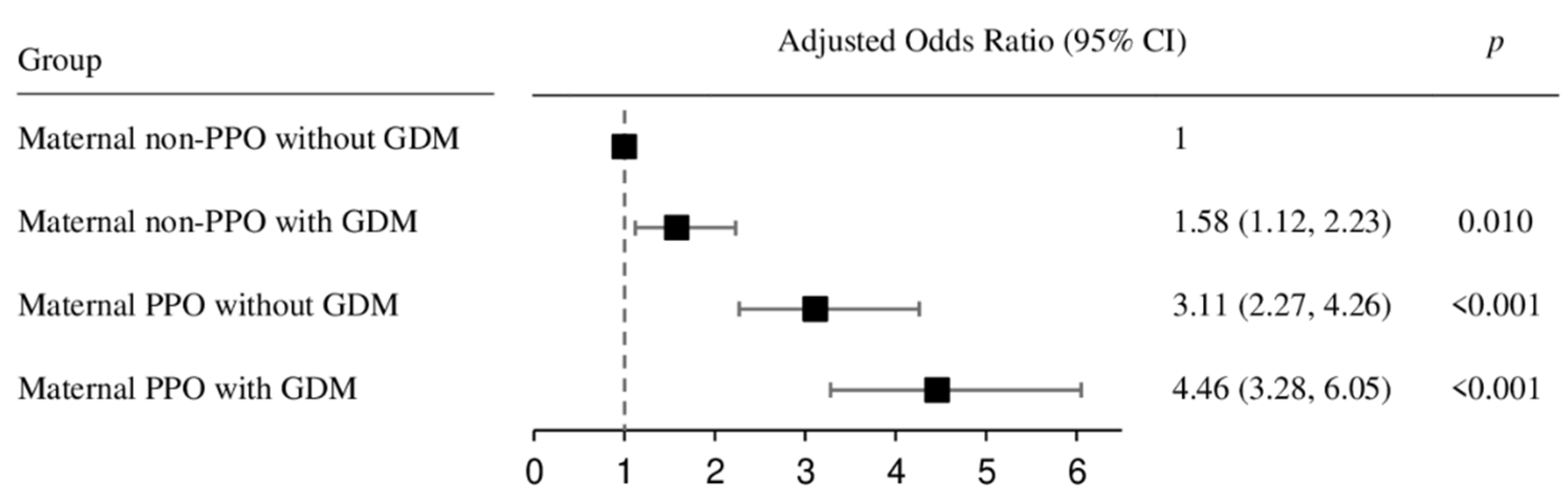
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Statistics 2021: Monitoring Health for the SDGs Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Nader, P.R.; O’Brien, M.; Houts, R.; Bradley, R.; Belsky, J.; Crosnoe, R.; Friedman, S.; Mei, Z.; Susman, E.J.; National Institute of Child Health and Human Development Early Child Care Research Network. Identifying Risk for Obesity in Early Childhood. Pediatrics 2006, 118, e594–e601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graversen, L.; Sørensen, T.I.; Petersen, L.; Sovio, U.; Kaakinen, M.; Sandbaek, A.; Laitinen, J.; Taanila, A.; Pouta, A.; Järvelin, M.R.; et al. Preschool Weight and Body Mass Index in Relation to Central Obesity and Metabolic Syndrome in Adulthood. PLoS ONE 2014, 9, e89986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swallen, K.C.; Reither, E.N.; Haas, S.A.; Meier, A.M. Overweight, Obesity, and Health-Related Quality of Life Among Adolescents: The National Longitudinal Study of Adolescent Health. Pediatrics 2005, 115, 340–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anzman, S.L.; Rollins, B.Y.; Birch, L.L. Parental Influence on Children’s Early Eating Environments and Obesity Risk: Implications for Prevention. Int. J. Obes. 2010, 34, 1116–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, M.K. Biological, Environmental, and Social Influences on Childhood Obesity. Pediatr. Res. 2016, 79, 205–211. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Report of the Commission on Ending Childhood Obesity; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Baranowski, T.; Motil, K.J.; Moreno, J.P. Multi-etiological Perspective on Child Obesity Prevention. Curr. Nutr. Rep. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Harrison, K.; Bost, K.K.; McBride, B.A.; Donovan, S.M.; Grigsby-Toussaint, D.S.; Kim, J.; Liechty, J.M.; Wiley, A.; Teran-Garcia, M.; Jacobsohn, G.C. Toward a Developmental Conceptualization of Contributors to Overweight and Obesity in Childhood: The Six-Cs Model. Child Dev. Perspect. 2011, 5, 50–58. [Google Scholar] [CrossRef]
- Trandafir, L.M.; Temneanu, O.R. Pre and Post-natal Risk and Determination of Factors for Child Obesity J. Med. Life 2016, 9, 386–391. [Google Scholar]
- Ornoy, A. Prenatal Origin of Obesity and Their Complications: Gestational Diabetes, Maternal Overweight and the Paradoxical Effects of Fetal Growth Restriction and Macrosomia. Reprod. Toxicol. 2011, 32, 205–212. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C. Infant Mortality, Childhood Nutrition, and Ischaemic Heart Disease in England and Wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef]
- Gordon-Larsen, P.; Adair, L.S.; Nelson, M.C.; Popkin, B.M. Five-Year Obesity Incidence in the Transition Period Between Adolescence and Adulthood: The National Longitudinal Study of Adolescent Health. Am. J. Clin. Nutr. 2004, 80, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic Syndrome in Childhood: Association with Birth Weight, Maternal Obesity, and Gestational Diabetes Mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzger, B.E. Long-Term Outcomes in Mothers Diagnosed with Gestational Diabetes Mellitus and Their Offspring. Clin. Obstet. Gynecol. 2007, 50, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, D.J.; Baird, H.R.; Aleck, K.A.; Bennett, P.H.; Knowler, W.C. Excessive Obesity in Offspring of Pima Indian Women with Diabetes During Pregnancy. N. Engl. J. Med. 1983, 308, 242–245. [Google Scholar] [CrossRef]
- Seong, W.J. Gestational Diabetes Mellitus and Long-Term Prognosis of the Offsprings. J. Korean Diabetes 2020, 21, 81–87. [Google Scholar] [CrossRef]
- Kim, S.Y.; England, J.L.; Sharma, J.A.; Njoroge, T. Gestational Diabetes Mellitus and Risk of Childhood Overweight and Obesity in Offspring: A Systematic Review. Exp. Diabetes Res. 2011, 2011, 541308. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, M.; Arata, N.; Miyazaki, C.; Mori, R.; Kikuchi, T.; Ogawa, Y.; Ota, E. Obesity and Abnormal Glucose Tolerance in Offspring of Diabetic Mothers: A Systematic Review and Meta-analysis. PLoS ONE 2018, 13, e0190676. [Google Scholar] [CrossRef] [Green Version]
- Fraser, A.; Lawlor, D.A. Long-Term Health Outcomes in Offspring Born to Women with Diabetes in Pregnancy. Curr. Diabetes Rep. 2014, 14, 489. [Google Scholar] [CrossRef] [Green Version]
- Burguet, A. Long-Term Outcome in Children of Mothers with Gestational Diabetes. Diabetes Metab. 2010, 36, 682–694. [Google Scholar] [CrossRef]
- Chu, S.Y.; Callaghan, W.M.; Kim, S.Y.; Schmid, C.H.; Lau, J.; England, L.J.; Dietz, P.M. Maternal Obesity and Risk of Gestational Diabetes Mellitus. Diabetes Care 2007, 30, 2070–2076. [Google Scholar] [CrossRef] [Green Version]
- Cheol Seong, S.; Kim, Y.Y.; Khang, Y.H.; Heon Park, J.; Kang, H.J.; Lee, H.; Do, C.H.; Song, J.S.; Hyon Bang, J.; Ha, S.; et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Standards of Medical Care in diabetes-2015 Abridged for Primary Care Providers. Clin. Diabetes 2015, 33, 97–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.S.; Lee, S.Y.; Nam, C.M. Korean National Growth Charts: Review of Developmental Process and an Outlook. Korean J. Pediatr. 2007, 51, 2008. [Google Scholar]
- Gillman, M.W.; Rifas-Shiman, S.; Berkey, C.S.; Field, A.E.; Colditz, G.A. Maternal Gestational Diabetes, Birth Weight, and Adolescent Obesity. Pediatrics 2003, 111, e221–e226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitaker, R.C.; Pepe, M.S.; Seidel, K.D.; Wright, J.A.; Knopp, R.H. Gestational Diabetes and the Risk of Offspring Obesity. Pediatrics 1998, 101, E9. [Google Scholar] [CrossRef] [Green Version]
- Philipps, L.H.; Santhakumaran, S.; Gale, C.; Prior, E.; Logan, K.M.; Hyde, M.J.; Modi, N. The Diabetic Pregnancy and Offspring BMI in Childhood: A Systematic Review and Meta-analysis. Diabetologia 2011, 54, 1957–1966. [Google Scholar] [CrossRef] [Green Version]
- Lawlor, D.A.; Fraser, A.; Lindsay, R.S.; Ness, A.; Dabelea, D.; Catalano, P.; Davey Smith, G.; Sattar, N.; Nelson, S.M. Association of Existing Diabetes, Gestational Diabetes and Glycosuria in Pregnancy with Macrosomia and Offspring Body Mass Index, Waist and Fat Mass in Later Childhood: Findings from a Prospective Pregnancy Cohort. Diabetologia 2010, 53, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Pirkola, J.; Pouta, A.; Bloigu, A.; Hartikainen, A.L.; Laitinen, J.; Järvelin, M.R.; Vääräsmäki, M. Risks of Overweight and Abdominal Obesity at Age 16 Years Associated with Prenatal Exposures to Maternal Prepregnancy Overweight and Gestational Diabetes Mellitus. Diabetes Care 2010, 33, 1115–1121. [Google Scholar] [CrossRef] [Green Version]
- Silverman, B.L.; Rizzo, T.; Green, O.C.; Cho, N.H.; Winter, R.J.; Ogata, E.S.; Richards, G.E.; Metzger, B.E. Long-Term Prospective Evaluation of Offspring of Diabetic Mothers. Diabetes 1991, 40, 121–125. [Google Scholar] [CrossRef]
- Vohr, B.R.; McGarvey, S.T.; Tucker, R. Effects of Maternal Gestational Diabetes on Offspring Adiposity at 4–7 Years of Age. Diabetes Care 1999, 22, 1284–1291. [Google Scholar] [CrossRef]
- Boerschmann, H.; Pflüger, M.; Henneberger, L.; Ziegler, A.G.; Hummel, S. Prevalence and Predictors of Overweight and Insulin Resistance in Offspring of Mothers with Gestational Diabetes Mellitus. Diabetes Care 2010, 33, 1845–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buffarini, R.; Barros, A.J.D.; Matijasevich, A.; Loret de Mola, C.; Santos, I.S. Gestational Diabetes Mellitus, Pre-gestational BMI and Offspring BMI z-Score During Infancy and Childhood: 2004 Pelotas Birth Cohort. BMJ 2019, 9, e024734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, P.M.; Ehrenberg, H.M. The Short- and Long-Term Implications of Maternal Obesity on the Mother and Her Offspring. BJOG 2006, 113, 1126–1133. [Google Scholar] [CrossRef]
- Catalano, P.M.; Farrell, K.; Thomas, A.; Huston-Presley, L.; Mencin, P.; de Mouzon, S.H.; Amini, S.B. Perinatal Risk Factors for Childhood Obesity and Metabolic Dysregulation. Am. J. Clin. Nutr. 2009, 90, 1303–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptiste-Roberts, K. Maternal Obesity and Implications for the Long-Term Health of the Offspring. In Obesity During Pregnancy in Clinical Practice; Nicholson, W., Baptiste-Roberts, K., Eds.; Springer: London, UK, 2014; pp. 259–295. [Google Scholar]
- Lawlor, D.A. The Society for Social Medicine John Pemberton Lecture 2011. Developmental Overnutrition—an Old Hypothesis with New Importance? Int. J. Epidemiol. 2013, 42, 7–29. [Google Scholar] [CrossRef]
- Monteiro, L.J.; Norman, J.E.; Rice, G.E.; Illanes, S.E. Fetal Programming and Gestational Diabetes Mellitus. Placenta 2016, 48, S54–S60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirabelli, M.; Chiefari, E.; Tocci, V.; Greco, E.; Foti, D.; Brunetti, A. Gestational diabetes: Implications for fetal growth, intervention timing, and treatment options. Curr. Opin. Pharmacol. 2021, 60, 1–10. [Google Scholar] [CrossRef]
- Gomes, D.; von Kries, R.; Delius, M.; Mansmann, U.; Nast, M.; Stubert, M.; Langhammer, L.; Haas, N.A.; Netz, H.; Obermeier, V.; et al. Late-pregnancy dysglycemia in obese pregnancies after negative testing for gestational diabetes and risk of future childhood overweight: An interim analysis from a longitudinal mother–child cohort study. PLOS Med. 2018, 15, e1002681. [Google Scholar] [CrossRef]
- Lain, K.Y.; Catalano, P.M. Metabolic Changes in Pregnancy. Clin Obstet Gynecol 2007, 50, 938–948. [Google Scholar] [CrossRef]
- Catalano, P.M.; Hauguel-De Mouzon, S. Is It Time to Revisit the Pedersen Hypothesis in the Face of the Obesity Epidemic? Am. J. Obstet. Gynecol. 2011, 204, 479–487. [Google Scholar] [CrossRef]
- Jensen, D.M.; Damm, P.; Sørensen, B.; Mølsted-Pedersen, L.; Westergaard, J.G.; Ovesen, P.; Beck-Nielsen, H. Pregnancy Outcome and Prepregnancy Body Mass Index in 2459 Glucose-Tolerant Danish Women. Am. J. Obstet. Gynecol. 2003, 189, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Salsberry, P.J.; Reagan, P.B. Dynamics of Early Childhood Overweight. Pediatrics 2005, 116, 1329–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, M.T.; Brubaker, K.; Pruett, K.; Caughey, A.B. Risk of Childhood Obesity in the Toddler Offspring of Mothers with Gestational Diabetes. Obstet. Gynecol. 2013, 121, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Taveras, E.M.; Gillman, M.W.; Oken, E. Intrauterine Exposure to Gestational Diabetes, Child Adiposity, and Blood Pressure. Am. J. Hypertens. 2009, 22, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillman, M.W.; Oakey, H.; Baghurst, P.A.; Volkmer, R.E.; Robinson, J.S.; Crowther, C.A. Effect of Treatment of Gestational Diabetes Mellitus on Obesity in the Next Generation. Diabetes Care 2010, 33, 964–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptiste-Roberts, K.; Nicholson, W.K.; Wang, N.Y.; Brancati, F.L. Gestational Diabetes and Subsequent Growth Patterns of Offspring: The National Collaborative Perinatal Project. Matern. Child Health J. 2012, 16, 125–132. [Google Scholar] [CrossRef] [Green Version]
| GDM | Non-GDM | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal PPO (BMI ≥ 25) | Maternal Non-PPO (BMI < 25) | Maternal PPO (BMI ≥ 25) | Maternal Non-PPO (BMI < 25) | ||||||||||
| Total | 1319 | 1319 | 1319 | 1319 | |||||||||
| Maternal characteristics (Matched variables) | |||||||||||||
| Age (years), mean (SD) | 33.0 | (4.0) | 33.0 | (4.0) | 33.0 | (4.0) | 33.0 | (4.0) | – | ||||
| Income classification | – | ||||||||||||
| High | 190 | (14.4) | 190 | (14.4) | 190 | (14.4) | 190 | (14.4) | |||||
| Middle | 685 | (51.9) | 685 | (51.9) | 685 | (51.9) | 685 | (51.9) | |||||
| Low | 444 | (33.7) | 444 | (33.7) | 444 | (33.7) | 444 | (33.7) | |||||
| BMI (kg/m2), mean (SD) | 27.6 | (2.5) | a | 20.7 | (2.1) | b | 27.3 | (2.2) | a | 20.6 | (2.0) | b | <0.001 |
| Children’s characteristics | |||||||||||||
| Birth weight (kg), mean (SD) | 3.30 | (0.52) | a | 3.20 | (0.44) | b | 3.29 | (0.49) | a | 3.19 | (0.42) | b | <0.001 |
| Sex (male) | 667 | (50.6) | 698 | (52.9) | 683 | (51.8) | 699 | (53.0) | 0.576 | ||||
| Breastfeeding | 0.087 | ||||||||||||
| Unknown | 740 | (56.1) | 735 | (55.7) | 752 | (57.0) | 695 | (52.7) | |||||
| Exclusive breastfeeding | 226 | (17.1) | 242 | (18.3) | 236 | (17.9) | 261 | (19.8) | |||||
| Mixed feeding | 133 | (10.1) | 135 | (10.2) | 104 | (7.9) | 147 | (11.1) | |||||
| Formula feeding | 220 | (16.7) | 207 | (15.7) | 227 | (17.2) | 216 | (16.4) | |||||
| Diet–sweetened drinks | 0.444 | ||||||||||||
| High consumption | 128 | (9.7) | 135 | (10.3) | 111 | (8.5) | 121 | (9.2) | |||||
| Low consumption | 1188 | (90.3) | 1181 | (89.7) | 1202 | (91.5) | 1197 | (90.8) | |||||
| Diet–high consumption of fast food | a | b | b | b | <0.001 | ||||||||
| Yes | 218 | (16.5) | 169 | (12.8) | 175 | (13.3) | 143 | (10.8) | |||||
| No | 1101 | (83.7) | 1150 | (87.4) | 1143 | (87.1) | 1176 | (89.2) | |||||
| TV or screen time | a | b | c | b | <0.001 | ||||||||
| >2 h/day | 518 | (39.3) | 410 | (31.1) | 464 | (35.2) | 379 | (28.8) | |||||
| ≤2 h/day | 800 | (60.7) | 909 | (68.9) | 853 | (64.8) | 939 | (71.2) | |||||
| Preference for exercise | 0.471 | ||||||||||||
| Yes | 983 | (74.6) | 953 | (72.3) | 952 | (72.2) | 963 | (73.0) | |||||
| No | 335 | (25.4) | 366 | (27.7) | 366 | (27.8) | 356 | (27.0) | |||||
| Children in the GDM Group | Children in the Non-GDM Group | p-Value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal PPO (BMI ≥ 25) | Maternal Non-PPO (BMI < 25) | Maternal PPO (BMI ≥ 25) | Maternal Non-PPO (BMI < 25) | |||||||||||
| 36 Months (30–36 Months) | ||||||||||||||
| N | 1319 | 1319 | 1319 | 1319 | ||||||||||
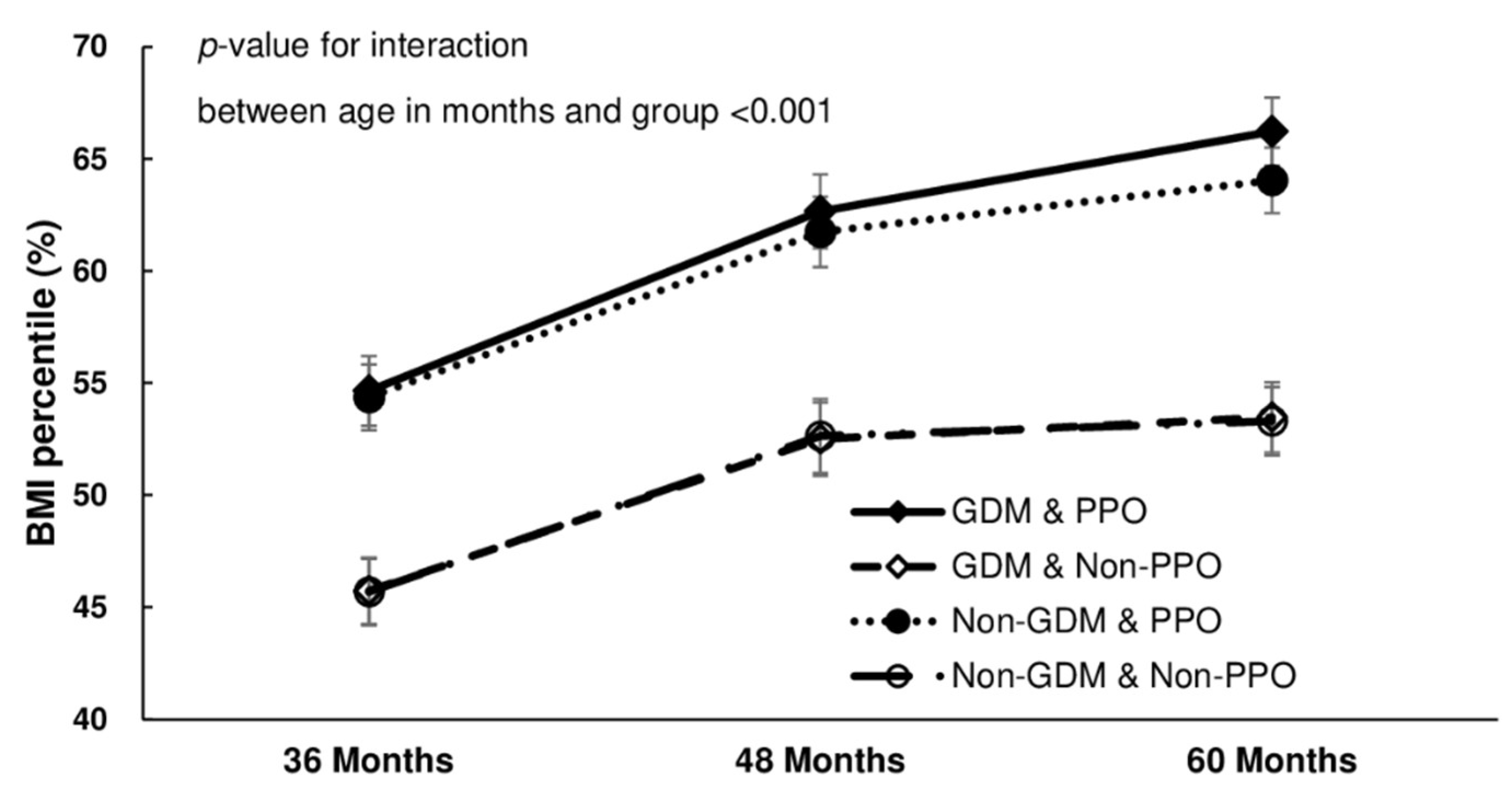
| Mean (SD) of BMI percentile * | 54.6 | (28.8) | a | 45.7 | (27.4) | b | 54.4 | (27.2) | a | 45.7 | (27.5) | b | <0.001 | |
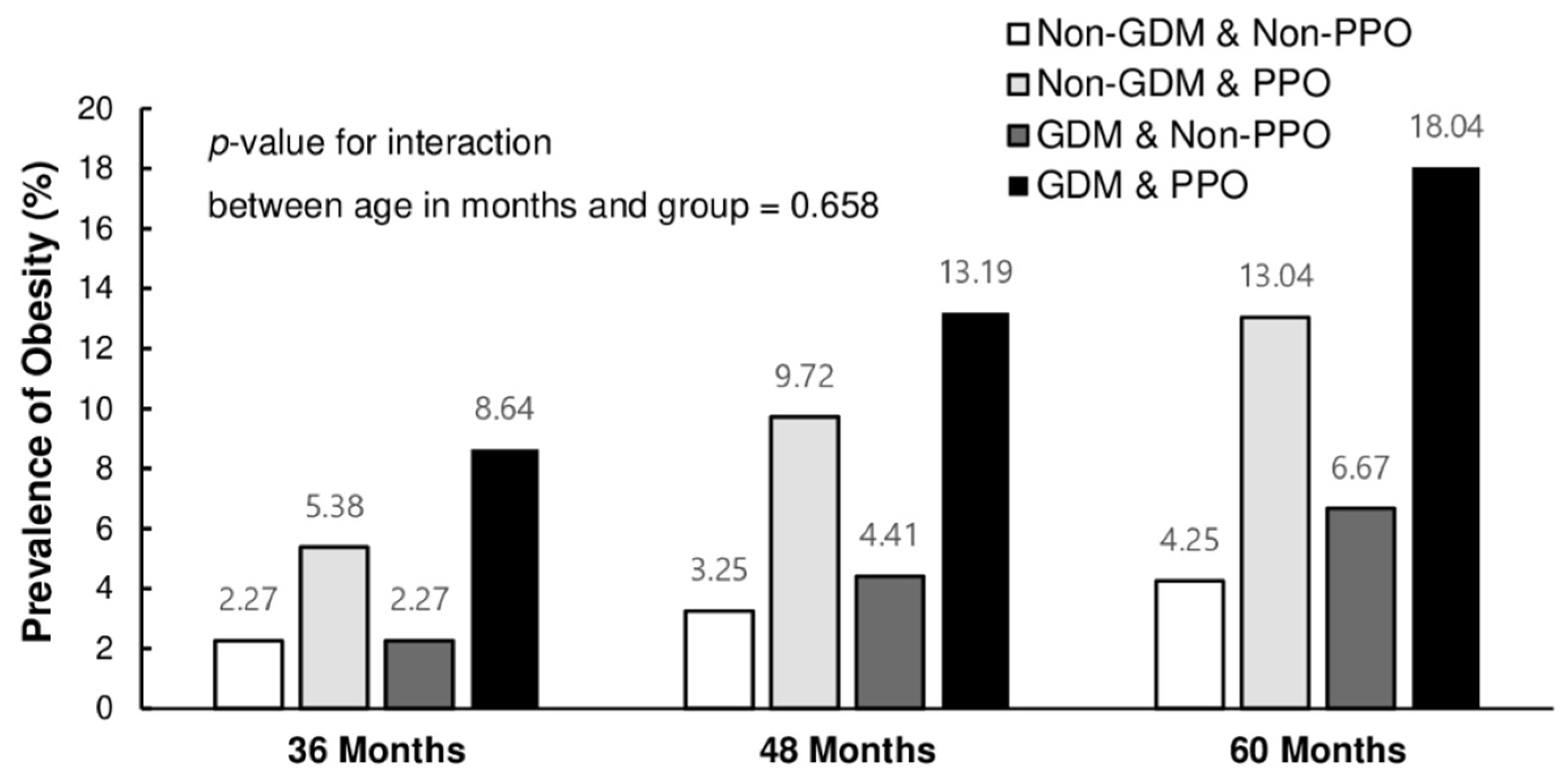
| Obesity **, n (%) | 114 | (8.6) | a | 30 | (2.3) | b | 71 | (5.4) | c | 30 | (2.3) | b | <0.001 | |
| 48 Months (42–48 Months) | ||||||||||||||
| N | 1152 | 1133 | 1142 | 1140 | ||||||||||
| Mean (SD) of BMI percentile * | 62.7 | (28.6) | a | 52.5 | (28.1) | b | 61.7 | (27.1) | a | 52.6 | (28.4) | b | <0.001 | |
| Obesity **, n (%) | 152 | (13.2) | a | 50 | (4.4) | b | 111 | (9.7) | c | 37 | (3.2) | b | <0.001 | |
| 60 Months (54–60 Months) | ||||||||||||||
| N | 1319 | 1319 | 1319 | 1319 | ||||||||||
| Mean (SD) of BMI percentile * | 66.2 | (28.2) | a | 53.5 | (29.1) | b | 64.0 | (27.1) | c | 53.3 | (28.2) | b | <0.001 | |
| Obesity **, n (%) | 238 | (18.0) | a | 88 | (6.7) | b | 172 | (13.0) | c | 56 | (4.2) | d | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.J.; Yu, J.; Choi, J. Maternal Pre-Pregnancy Obesity and Gestational Diabetes Mellitus Increase the Risk of Childhood Obesity. Children 2022, 9, 928. https://doi.org/10.3390/children9070928
Choi MJ, Yu J, Choi J. Maternal Pre-Pregnancy Obesity and Gestational Diabetes Mellitus Increase the Risk of Childhood Obesity. Children. 2022; 9(7):928. https://doi.org/10.3390/children9070928
Chicago/Turabian StyleChoi, Mi Jin, Juyoun Yu, and Jimi Choi. 2022. "Maternal Pre-Pregnancy Obesity and Gestational Diabetes Mellitus Increase the Risk of Childhood Obesity" Children 9, no. 7: 928. https://doi.org/10.3390/children9070928
APA StyleChoi, M. J., Yu, J., & Choi, J. (2022). Maternal Pre-Pregnancy Obesity and Gestational Diabetes Mellitus Increase the Risk of Childhood Obesity. Children, 9(7), 928. https://doi.org/10.3390/children9070928







