Diagnostic Values of Red Flags and a Clinical Prediction Score for Emergent Intracranial Lesions in Non-Traumatic Pediatric Headaches
Abstract
:1. Introductions
2. Methods
2.1. Red-Flag Clinical Predictors
2.2. Emergent Intracranial Lesions
2.3. Statistical Analyses
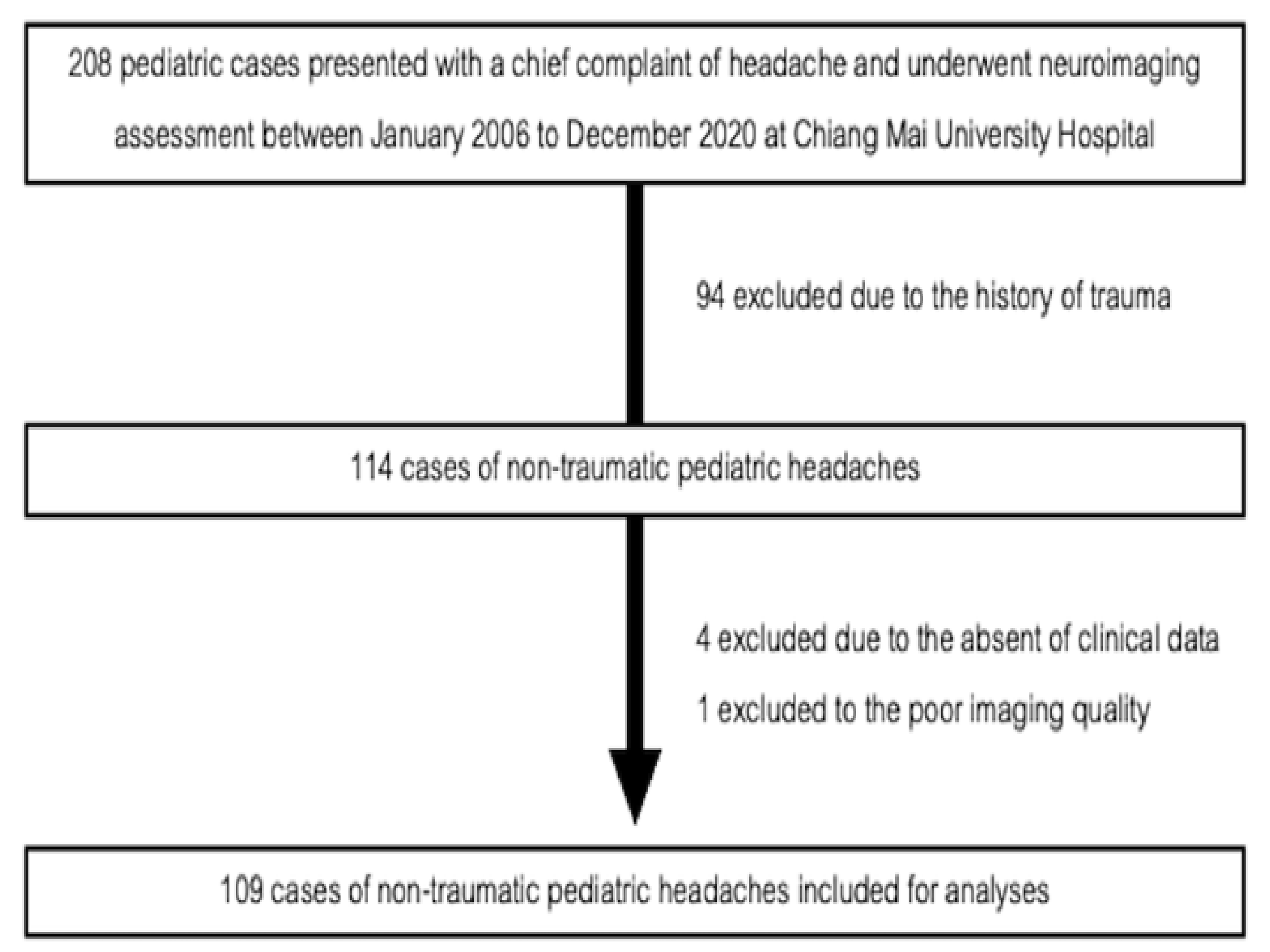
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Scheller, J.M. The history, epidemiology, and classification of headaches in childhood. Semin. Pediatr. Neurol. 1995, 2, 102–108. [Google Scholar] [CrossRef]
- Alfonzo, M.J.; Bechtel, K.; Babineau, S. Management of headache in the pediatric emergency department. Pediatr. Emerg. Med. Pract. 2013, 10, 1–25. [Google Scholar] [PubMed]
- Raucci, U.; Della Vecchia, N.; Ossella, C.; Paolino, M.C.; Villa, M.P.; Reale, A.; Parisi, P. Management of childhood headache in the emergency department. Review of the literature. Front. Neurol. 2019, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, G.A.; Argyropoulou, M.I. Neuroimaging in childhood headache: A systematic review. Pediatr. Radiol. 2013, 43, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Medina, L.S.; Kuntz, K.M.; Pomeroy, S. Children with headache suspected of having a brain tumor: A cost-effectiveness analysis of diagnostic strategies. Pediatrics 2001, 108, 255–263. [Google Scholar] [CrossRef]
- Smith-Bindman, R.; Lipson, J.; Marcus, R.; Kim, K.P.; Mahesh, M.; Gould, R.; De González, A.B.; Miglioretti, D.L. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 2009, 169, 2078–2086. [Google Scholar] [CrossRef]
- Roser, T.; Bonfert, M.; Ebinger, F.; Blankenburg, M.; Ertl-Wagner, B.; Heinen, F. Primary versus secondary headache in children: A frequent diagnostic challenge in clinical routine. Neuropediatrics 2013, 44, 34–39. [Google Scholar] [CrossRef]
- Uffman, J.C.; Tumin, D.; Raman, V.; Thung, A.; Adler, B.; Tobias, J.D. MRI utilization and the associated use of sedation and anesthesia in a pediatric ACO. J. Am. Coll. Radiol. 2017, 14, 924–930. [Google Scholar] [CrossRef]
- Trofimova, A.; Vey, B.L.; Mullins, M.E.; Wolf, D.S.; Kadom, N. Imaging of children with nontraumatic headaches. AJR Am. J. Roentgenol. 2018, 210, 8–17. [Google Scholar] [CrossRef]
- Tsze, D.S.; Ochs, J.B.; Gonzalez, A.E.; Dayan, P.S. Red flag findings in children with headaches: Prevalence and association with emergency department neuroimaging. Cephalalgia 2019, 39, 185–196. [Google Scholar] [CrossRef]
- Ratanakorn, D.; Keandoungchun, J.; Sittichanbuncha, Y.; Laothamatas, J.; Tegeler, C.H. Stroke fast track reduces time delay to neuroimaging and increases use of thrombolysis in an academic medical center in Thailand. J. Neuroimaging 2012, 22, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Areemit, R.; Manaboriboon, B. Adolescent health in Asia: Insights from Thailand. Int. J. Adolesc. Med. Health 2016, 28, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Gofshteyn, J.S.; Stephenson, D.J. Diagnosis and management of childhood headache. Curr. Probl. Pediatric Adolesc. Health Care 2016, 46, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Nye, B.L.; Ward, T.N. Clinic and emergency room evaluation and testing of headache. Headache J. Head Face Pain 2015, 55, 1301–1308. [Google Scholar] [CrossRef]
- Dodick, D. Diagnosing headache: Clinical clues and clinical rules. Adv. Stud. Med. 2003, 3, 87–92. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; Wiley: New York, NY, USA, 2013. [Google Scholar]
- Steyerberg, E.W. Statistical models for prediction. In Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating; Steyerberg, E.W., Ed.; Springer: New York, NY, USA, 2009; pp. 53–82. [Google Scholar]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef] [Green Version]
- Medina, L.S.; Pinter, J.D.; Zurakowski, D.; Davis, R.G.; Kuban, K.; Barnes, P.D. Children with headache: Clinical predictors of surgical space-occupying lesions and the role of neuroimaging. Radiology 1997, 202, 819–824. [Google Scholar] [CrossRef]
- Hsiao, H.-J.; Huang, J.-L.; Hsia, S.-H.; Lin, J.-J.; Huang, I.A.; Wu, C.-T. Headache in the pediatric emergency service: A medical center experience. Pediatrics Neonatol. 2014, 55, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Conicella, E.; Raucci, U.; Vanacore, N.; Vigevano, F.; Reale, A.; Pirozzi, N.; Valeriani, M. The child with headache in a pediatric emergency department. Headache 2008, 48, 1005–1011. [Google Scholar] [CrossRef]
- Burton, L.J.; Quinn, B.; Pratt-Cheney, J.L.; Pourani, M. Headache etiology in a pediatric emergency department. Pediatr. Emerg. Care 1997, 13, 1–4. [Google Scholar] [CrossRef]
- Rho, Y.I.; Chung, H.J.; Suh, E.S.; Lee, K.H.; Eun, B.L.; Nam, S.O.; Kim, W.S.; Eun, S.H.; Kim, Y.O. The role of neuroimaging in children and adolescents with recurrent headaches—Multicenter study. Headache J. Head Face Pain 2011, 51, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.W.; Ashwal, S. The quality standards subcommittee of the american academy of neurology, the practice committee of the child neurology society. Practice parameter: Evaluation of children and adolescents with recurrent headaches: Report of the quality standards subcommittee of the american academy of neurology and the practice committee of the child neurology society. Headache J. Head Face Pain 2003, 43, 424–425. [Google Scholar] [CrossRef]
- Lewis, D.W. Red flags in children who present with headache—How to recognize a serious problem. Nat. Clin. Pract. Neurol. 2008, 4, 412–413. [Google Scholar] [CrossRef] [PubMed]
- Button, K.; Capraro, A.; Monuteaux, M.; Mannix, R. Etiologies and yield of diagnostic testing in children presenting to the emergency department with altered mental status. J. Pediatr. 2018, 200, 218–224.e2. [Google Scholar] [CrossRef]
- Bear, J.J.; Gelfand, A.A.; Goadsby, P.J.; Bass, N. Occipital headaches and neuroimaging in children. Neurology 2017, 89, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Tantarattanapong, S.; Chalongkulasak, L. Predictive factors and clinical prediction score for serious intracranial causes in acute nontraumatic headache at an emergency department. Emerg. Med. Int. 2019, 2019, 4267825. [Google Scholar] [CrossRef]
- Locker, T.E.; Thompson, C.; Rylance, J.; Mason, S.M. The utility of clinical features in patients presenting with nontraumatic headache: An investigation of adult patients attending an emergency department. Headache 2006, 46, 954–961. [Google Scholar] [CrossRef]
- Morgan, M.; Jenkins, L.; Ridsdale, L. Patient pressure for referral for headache: A qualitative study of GPs’ referral behaviour. Br. J. Gen. Pract. 2007, 57, 29–35. [Google Scholar]
- Ngoya, P.S.; Muhogora, W.E.; Pitcher, R.D. Defining the diagnostic divide: An analysis of registered radiological equipment resources in a low-income African country. Pan Afr. Med. J. 2016, 25, 99. [Google Scholar] [CrossRef]
- Ridsdale, L.; Clark, L.V.; Dowson, A.J.; Goldstein, L.H.; Jenkins, L.; McCrone, P.; Morgan, M.; Seed, P.T. How do patients referred to neurologists for headache differ from those managed in primary care? Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2007, 57, 388–395. [Google Scholar]

| Demographics | Total (n = 109) | Emergent Intracranial Lesion | ||
|---|---|---|---|---|
| Negative Lesions (n = 58) | Positive Lesion (n = 51) | p-Value | ||
| Mean Age (SD) | 10.6 (3.4) | 11.2 (3.1) | 10.1 (3.7) | 0.09 |
| Age < 5 (n, col %) | 6 (5.5) | 2 (3.5) | 4 (7.8) | 0.32 |
| Male (n, col %) | 46 (42.2) | 27 (46.6) | 19 (37.3) | 0.33 |
| Presence of high-risk comorbidities; (hematologic malignancy, SLE, and HIV infection). | 26 (23.8) | 11 (19.0) | 15 (29.4) | 0.20 |
| Diagnosis | Frequency | Percentage |
|---|---|---|
| Emergent intracranial lesion (n = 51) | ||
| Brain tumor | 13 | 25.5% |
| Intracranial infection | 13 | 25.5% |
| Intracranial hemorrhage | 7 | 13.7% |
| Non-emergent intracranial lesion (n = 58) | ||
| Headache disorders | 26 | 44.8% |
| 20 | 34.5% |
| 6 | 10.3% |
| Inconclusive diagnosis | 14 | 24.8% |
| Others, such as dengue fever, epilepsy, chronic sinusitis, and hypertension | 11 | 19.0% |
| Common Red-Flags (n, col %) | Total (n = 109) | Emergent Intracranial Lesion | ||
|---|---|---|---|---|
| Negative Lesions (n = 58) | Positive Lesion (n = 51) | p-Value | ||
| Acute onset (<3 months) | 72 (66.1) | 30 (51.7) | 42 (82.4) | <0.01 |
| Severe vomiting | 65 (59.6) | 31 (53.5) | 34 (66.7) | 0.16 |
| High-risk underlying comorbidities | 26 (23.8) | 11 (19.0) | 15 (29.4) | 0.20 |
| Fever | 21 (19.3) | 8 (13.8) | 13 (25.5) | 0.12 |
| Focal motor abnormality | 20 (18.4) | 3 (5.2) | 17 (33.3) | <0.01 |
| Changes in mood or personality over days or weeks | 20 (18.4) | 5 (8.6) | 15 (29.4) | <0.01 |
| Altered conscious state | 19 (17.4) | 4 (6.9) | 15 (29.4) | <0.01 |
| Seizures | 16 (14.7) | 5 (8.6) | 11 (21.6) | 0.06 |
| Abnormal ocular movements, squint, pathological pupillary responses | 16 (14.7) | 2 (3.5) | 14 (27.5) | <0.01 |
| Increase in severity or characteristics of the headache | 14 (12.8) | 5 (8.6) | 9 (17.7) | 0.16 |
| Pain that wakes the child from sleep or occurs on waking | 13 (11.9) | 8 (13.8) | 5 (9.8) | 0.52 |
| Ataxia, gait abnormalities, impaired coordination | 13 (11.9) | 1 (1.7) | 12 (23.4) | <0.01 |
| Meningism | 12 (11.0) | 3 (3.5) | 10 (19.6) | <0.01 |
| Occipital headache | 11 (10.1) | 8 (13.8) | 3 (5.9) | 0.17 |
| Red-Flags | Odds Ratio (95% CI) | p-Value | Coefficient | Weight |
|---|---|---|---|---|
| Acute onset (<3 months) | 5.24 (1.60 to 17.1) | <0.01 | 1.656654 | 1 |
| Altered conscious state | 3.07(0.80 to 11.79) | 0.10 | 1.120896 | 1 |
| Focal motor abnormality | 10.06 (2.34 to 43.22 | <0.01 | 2.308371 | 2 |
| Abnormal ocular movements, squint, pathologic pupillary responses | 19.87 (3.54 to 111.58) | <0.01 | 2.989329 | 3 |
| Cut-Point | Sensitivity | Specificity | Correctly Classified | Positive Likelihood Ratio | Negative Likelihood Ratio |
|---|---|---|---|---|---|
| ≥1 | 96.1% | 44.8% | 68.8% | 1.74 | 0.08 |
| ≥2 | 68.6% | 86.2% | 78.0% | 4.98 | 0.36 |
| ≥3 | 51.0% | 93.1% | 73.4% | 7.39 | 0.53 |
| ≥4 | 33.3% | 98.3% | 67.9% | 19.3 | 0.68 |
| ≥5 | 5.9% | 98.3% | 55.1% | 3.41 | 0.96 |
| ≥6 | 3.9% | 100% | 55.5% | 0.96 | |
| ≥7 | 2.0% | 100% | 54.1% | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manoyana, A.; Angkurawaranon, S.; Katib, S.; Wiwattanadittakul, N.; Sirikul, W.; Angkurawaranon, C. Diagnostic Values of Red Flags and a Clinical Prediction Score for Emergent Intracranial Lesions in Non-Traumatic Pediatric Headaches. Children 2022, 9, 863. https://doi.org/10.3390/children9060863
Manoyana A, Angkurawaranon S, Katib S, Wiwattanadittakul N, Sirikul W, Angkurawaranon C. Diagnostic Values of Red Flags and a Clinical Prediction Score for Emergent Intracranial Lesions in Non-Traumatic Pediatric Headaches. Children. 2022; 9(6):863. https://doi.org/10.3390/children9060863
Chicago/Turabian StyleManoyana, Atipat, Salita Angkurawaranon, Sumintra Katib, Natrujee Wiwattanadittakul, Wachiranun Sirikul, and Chaisiri Angkurawaranon. 2022. "Diagnostic Values of Red Flags and a Clinical Prediction Score for Emergent Intracranial Lesions in Non-Traumatic Pediatric Headaches" Children 9, no. 6: 863. https://doi.org/10.3390/children9060863
APA StyleManoyana, A., Angkurawaranon, S., Katib, S., Wiwattanadittakul, N., Sirikul, W., & Angkurawaranon, C. (2022). Diagnostic Values of Red Flags and a Clinical Prediction Score for Emergent Intracranial Lesions in Non-Traumatic Pediatric Headaches. Children, 9(6), 863. https://doi.org/10.3390/children9060863







