Tuberculosis in Adolescents in Bulgaria for a Three-Year Period: 2018–2020
Abstract
:1. Introduction
- Prevalence of secondary forms;
- “Pubertal phthisis”—evidence of both primary and secondary TB;
- Physiologically lower natural immunity;
- The higher frequency of TB incidence among adolescents in most countries has medium and increased morbidity.
- Exposure to Mycobacterium tuberculosis as a part of the investigation for contact tracing;
- Manifestation of clinical symptoms or signs indicating the diagnosis of tuberculosis;
- Presence of results positive for tuberculosis–X-ray changes or positive blood immunological tests such as Interferon Gamma Release Assay (IGRA) or Tuberculin Skin Test (TST) [5].
2. Materials and Methods
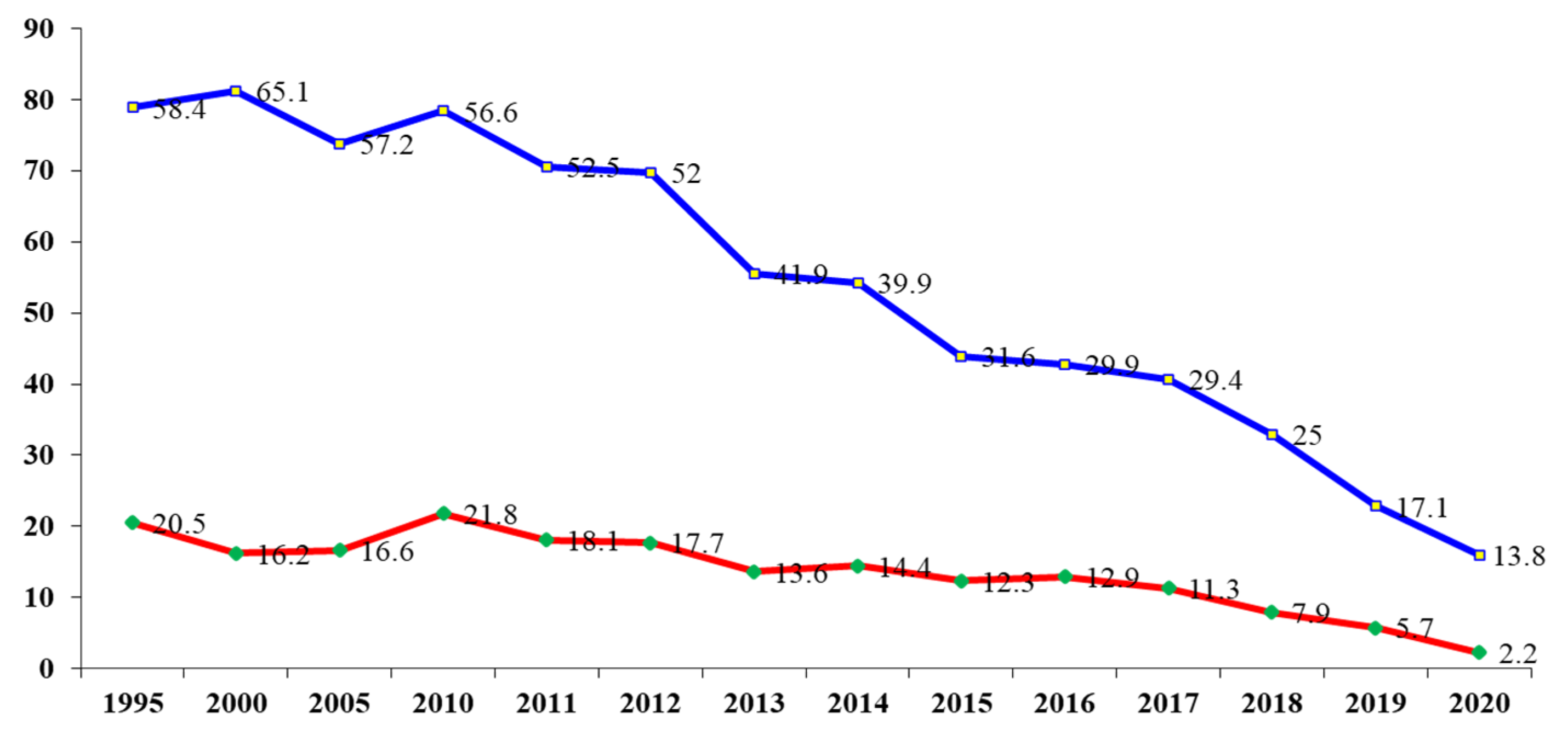
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seddon, J.A.; Chiang, S.S.; Esmail, H.; Coussens, A.K. The Wonder Years: What Can Primary School Children Teach Us about Immunity to Mycobacterium tuberculosis? Front. Immunol. 2018, 9, 2946. [Google Scholar] [CrossRef] [PubMed]
- ECDS. Tuberculosis Surveillance and Monitoring in Europe. Available online: https://www.ecdc.europa.eu/en/publications-data/tuberculosis-surveillance-and-monitoring-europe-2022-2020-data (accessed on 20 March 2022).
- WHO. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 18 May 2022).
- Snow, K.J.; Sismanidis, C.; Denholm, J.; Sawyer, S.M.; Graham, S. The incidence of tuberculosis among adolescents and young adults: A global estimate. Eur. Respir. J. 2018, 51, 1702352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snow, K.J.; Cruz, A.T.; Seddon, J.A.; Ferrand, R.A.; Chiang, S.S.; Hughes, J.A.; Kampmann, B.; Graham, S.M.; Dodd, P.J.; Houben, R.M.G.J.; et al. Adolescent tuberculosis. Lancet Child Adolesc. Health 2020, 4, 68–79. [Google Scholar] [CrossRef]
- Cruz, A.T.; Hwang, K.M.; Birnbaum, G.D.; Starke, J.R. Adolescents with tuberculosis: A review of 145 cases. Pediatr. Infect. Dis. J. 2013, 32, 937–941. [Google Scholar] [CrossRef]
- Pontual, L.; Balu, L.; Ovetchkine, P.; Maury-Tisseron, B.; Lachassinne, E.; Cruaud, P.; Jeantils, V.; Valeyre, D.; Fain, O.; Gaudelus, J. Tuberculosis in adolescents: A French retrospective study of 52 cases. Pediatr. Infect. Dis. J. 2006, 25, 930–932. [Google Scholar] [CrossRef]
- Furin, J.; Cox, H.; Pai, M. Tuberculosis. Lancet 2019, 393, 1642–1656. [Google Scholar] [CrossRef]
- Jenkins, H.E.; Plesca, V.; Ciobanu, A.; Crudu, V.; Galusca, I.; Soltan, V.; Serbulenco, A.; Zignol, M.; Dadu, A.; Dara, M.; et al. Assessing spatial heterogeneity of multidrug-resistant tuberculosis in a high-burden country. Eur. Respir. J. 2012, 42, 1291–1301. [Google Scholar] [CrossRef]
- Skrahina, A.; Hurevich, H.; Zalutskaya, A.; Sahalchyk, E.; Astrauko, A.; Hoffner, S.; Rusovich, V.; Dadu, A.; De Colombani, P.; Dara, M.; et al. Multidrug-resistant tuberculosis in Belarus: The size of the problem and associated risk factors. Bull. World Health Organ. 2012, 91, 36–45. [Google Scholar] [CrossRef]
- García-Basteiro, A.L.; Schaaf, H.S.; Diel, R.; Migliori, G.B. Adolescents and young adults: A neglected population group for tuberculosis surveillance. Eur. Respir. J. 2018, 51, 1800176. [Google Scholar] [CrossRef] [Green Version]
- National Statistical Institute (NSI). Healthcare. Available online: https://www.nsi.bg/bg/content/14521 (accessed on 20 March 2022).
- Snow, K.; Hesseling, A.C.; Naidoo, P.; Graham, S.M.; Denholm, J.; du Preez, K. Tuberculosis in adolescents and young adults: Epidemiology and treatment outcomes in the Western Cape. Int. J. Tuberc. Lung Dis. 2017, 21, 651–657. [Google Scholar] [CrossRef]
- Mulongeni, P.; Hermans, S.; Caldwell, J.; Bekker, L.-G.; Wood, R.; Kaplan, R. HIV prevalence and determinants of loss-to-follow-up in adolescents and young adults with tuberculosis in Cape Town. PLoS ONE 2019, 14, e0210937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, S.S.; Dolynska, M.; Rybak, N.R.; Cruz, A.T.; Aibana, O.; Sheremeta, Y.; Petrenko, V.; Mamotenko, A.; Terleieva, I.; Horsburgh, R.C., Jr.; et al. Clinical manifestations and epidemiology of adolescent tuberculosis in Ukraine. ERJ Open Res. 2020, 6, 003082020. [Google Scholar] [CrossRef] [PubMed]
- Donald, P.R.; Marais, B.J.; Barry, E.C. Age and the epidemiology and pathogenesis of tuberculosis. Lancet 2010, 375, 1852–1854. [Google Scholar] [CrossRef]
- Shingadia, D.; Seddon, A.J. Epidemiology and disease burden of tuberculosis in children: A global perspective. Infect. Drug Resist. 2014, 7, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Middelkoop, K.; Bekker, L.-G.; Myer, L.; Dawson, R.; Wood, R. Rates of tuberculosis transmission to children and adolescents in a community with a high prevalence of HIV infection among adults. Clin. Infect. Dis. 2008, 47, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Mumpe-Mwanja, D.; Verver, S.; Yeka, A.; Etwom, A.; Waako, J.; Ssengooba, W.; Matovu, J.K.; Wanyenze, R.K.; Musoke, P.; Mayanja-Kizza, H. Prevalence and risk factors of latent Tuberculosis among adolescents in rural Eastern Uganda. Afr. Health Sci. 2015, 15, 851–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Velez, C.M.; Marais, B.J. Tuberculosis in children. N. Engl. J. Med. 2012, 367, 348–361. [Google Scholar] [CrossRef] [Green Version]
- Marais, B.J.; Tadolini, M.; Zignol, M.; Migliori, G.B. Paediatric tuberculosis in Europe: Lessons from Denmark and inclusive strategies to consider. Eur. Respir. J. 2014, 43, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Du Preez, K.; Schaaf, H.S.; Dunbar, R.; Swartz, A.; Bissell, K.; Enarson, D.A.; Hesseling, A.C. Incomplete registration and reporting of culture-confirmed childhood tuberculosis diagnosed in hospital. Public Health Action 2011, 1, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Sant’Anna, C.C.; Schmidt, C.M.; March, M.F.B.P.; Pereira, S.M.; Barreto, M.L. Tuberculose em adolescentes em duas capitais brasileiras (Port) [Tuberculosis among adolescents in Brazilian capitals]. Cad. Saúde Pública 2013, 29, 111–116. [Google Scholar]
- WHO. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children, 2nd ed.; World Health Organization: Geneva, Switzerland, 2014.
- Weber, H.C.; Beyers, N.; Gie, R.P.; Schaaf, H.S.; Fish, T.; Donald, P.R. The clinical and radiological features of tuberculosis in adolescents. Ann. Trop. Paediatr. 2000, 20, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, C.C.; Pombo March, D.F.B.; Aurílio, R.B. Diagnosis of Tuberculosis among Children and Adolescents; Ribón, W., Ed.; Mycobacterium—Research and Development, IntechOpen: London, UK, 2017. [Google Scholar]
- Grahan, S. The Union’s Desk Guide for Diagnosis and Management of Tuberculosis in Children, 3rd ed.; IUATLD: Paris, France, 2016. [Google Scholar]
| Group I | Group II | Group III | Statistical Test | Between Group Comparison (Statistical Significance–p-Value) | ||||
|---|---|---|---|---|---|---|---|---|
| 8–11 | 12–14 | Above 15 | Total | I vs. II | I vs. III | II vs. III | ||
| Diagnosis | ||||||||
| TB of the intrathoracic lymph nodes | 28 (82.4%) | 22 (75.9%) | 3 (17.6%) | 53 (66.2%) | Chi-square/ Fisher’s exact test | 0.526 | <0.001 | <0.001 |
| Others: | 6 (17.6%) | 7 (24.1%) | 14 (82.4%) | 27 (33.8%) | ||||
| Infiltrative-pneumonic TB | 2 (5.9%) | 5 (17.2%) | 12 (70.7%) | 19 (23.8%) | ||||
| Pleuritis | 3 (8.8%) | 1 (3.4%) | 1 (5.9%) | 5 (6.3%) | ||||
| Primary TB complex | 1 (2.9%) | 1 (3.4%) | 0 | 2 (2.5%) | ||||
| Extrapulmonary TB | 0 | 0 | 1 (5.9%) | 1 (1.3%) | ||||
| Sex | ||||||||
| Male | 19 (55.9%) | 16 (55.2%) | 10 (58.8%) | 45 (56.3%) | Chi-square/ Fisher’s exact test | 0.955 | 0.842 | 0.809 |
| Female | 15 (44.1%) | 13 (44.8%) | 7 (41.2%) | 35 (43.7%) | ||||
| Intoxication | ||||||||
| None | 22 (64.7%) | 19 (65.5%) | 3 (17.6%) | 44 (55%) | Mann–Whitney U-test | 0.909 | <0.001 | 0.002 |
| Mild | 7 (20.6%) | 4 (13.8%) | 4 (23.5%) | 15 (18.8%) | ||||
| Severe | 5 (14.7%) | 6 (20.7%) | 10 (58.8%) | 21 (26.3%) | ||||
| BCG scar | ||||||||
| Yes | 11 (32.4%) | 16 (55.2%) | 5 (29.4%) | 32 (40%) | Chi-square/ Fisher’s exact test | 0.068 | 0.831 | 0.090 |
| No | 23 (67.6%) | 13 (44.8%) | 12 (70.6%) | 48 (60%) | ||||
| Known contact | ||||||||
| None | 9 (26.5%) | 5 (17.2%) | 7 (41.2%) | 21 (26.3%) | Chi-square/ Fisher’s exact test | 0.380 | 0.286 | 0.093 |
| Present | 25 (73.5%) | 24 (82.8%) | ||||||
| In the family | 22 (75.9%) | 8 (47.1%) | 55 (68.8%) | |||||
| At school | 0 | 0 | 1 (5.9%) | 1 (1.3%) | ||||
| Distant | 0 | 2 (6.9%) | 1 (5.9%) | 3 (3.8%) | ||||
| BK spreading | ||||||||
| Negative | 31 (91.2%) | 24 (82.8%) | 9 (52.9%) | 64 (80%) | Chi-square/ Fisher’s exact test | 0.453 | 0.002 | 0.044 |
| Positive | 3 (8.8%) | 5 (17.2%) | 8 (47.1%) | 16 (20%) | ||||
| TST | ||||||||
| 0–5 mm | 4 (12.9%) | 4 (14.2%) | 3 (14.3%) | 11 (13.8%) | Chi-square | 0.354 | 0.367 | 0.411 |
| 6–14 mm | 5 (16.2%) | 3 (10.8%) | 3 (14.3%) | 11 (13.8%) | ||||
| >15 mm | 22 (70.9%) | 21 (75%) | 15 (71.4%) | 58 (72.5%) | ||||
| X-ray–changes | ||||||||
| Bronchadenitis | 27 (79.4%) | 21 (72.4%) | 4 (23.5%) | 52 (65%) | ||||
| Infiltrate with bronchadenitis | 1 (2.9%) | 1 (3.4%) | 8 (47.1%) | 10 (12.5%) | ||||
| Infiltrate with cavity | 2 (5.9%) | 4 (13.8%) | 3 (17.6%) | 9 (11.3%) | ||||
| Pleuritis | 3 (8.8%) | 2 (6.9%) | 2 (11.8%) | 7 (8.8%) | ||||
| Primary TB complex | 1 (2.9%) | 1 (3.4%) | 0 | 2 (2.5%) | ||||
| Location of the findings | ||||||||
| Unilateral | 16 (47.1%) | 16 (55.2%) | 9 (52.9%) | 41 (51.2%) | Chi-square/ Fisher’s exact test | 0.521 | 0.692 | 0.883 |
| left | 7 (20.6%) | 9 (31%) | 3 (17.6%) | 19 (23.8%) | ||||
| right | 9 (26.5%) | 7 (24.1%) | 6 (35.3%) | 22 (27.6%) | ||||
| Bilateral | 18 (52.9%) | 13 (44.8%) | 8 (47.1%) | 39 (48.8%) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrovska, N.; Spasova, A.; Galacheva, A.; Kostadinov, D.; Yanev, N.; Milanov, V.; Gabrovski, K.; Velizarova, S. Tuberculosis in Adolescents in Bulgaria for a Three-Year Period: 2018–2020. Children 2022, 9, 785. https://doi.org/10.3390/children9060785
Gabrovska N, Spasova A, Galacheva A, Kostadinov D, Yanev N, Milanov V, Gabrovski K, Velizarova S. Tuberculosis in Adolescents in Bulgaria for a Three-Year Period: 2018–2020. Children. 2022; 9(6):785. https://doi.org/10.3390/children9060785
Chicago/Turabian StyleGabrovska, Natalia, Albena Spasova, Anabela Galacheva, Dimitar Kostadinov, Nikolay Yanev, Vladimir Milanov, Kaloyan Gabrovski, and Svetlana Velizarova. 2022. "Tuberculosis in Adolescents in Bulgaria for a Three-Year Period: 2018–2020" Children 9, no. 6: 785. https://doi.org/10.3390/children9060785
APA StyleGabrovska, N., Spasova, A., Galacheva, A., Kostadinov, D., Yanev, N., Milanov, V., Gabrovski, K., & Velizarova, S. (2022). Tuberculosis in Adolescents in Bulgaria for a Three-Year Period: 2018–2020. Children, 9(6), 785. https://doi.org/10.3390/children9060785






