Structural Changes in Primary Teeth of Diabetic Children: Composition and Ultrastructure Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation and SEM–EDX Analysis
3. Results
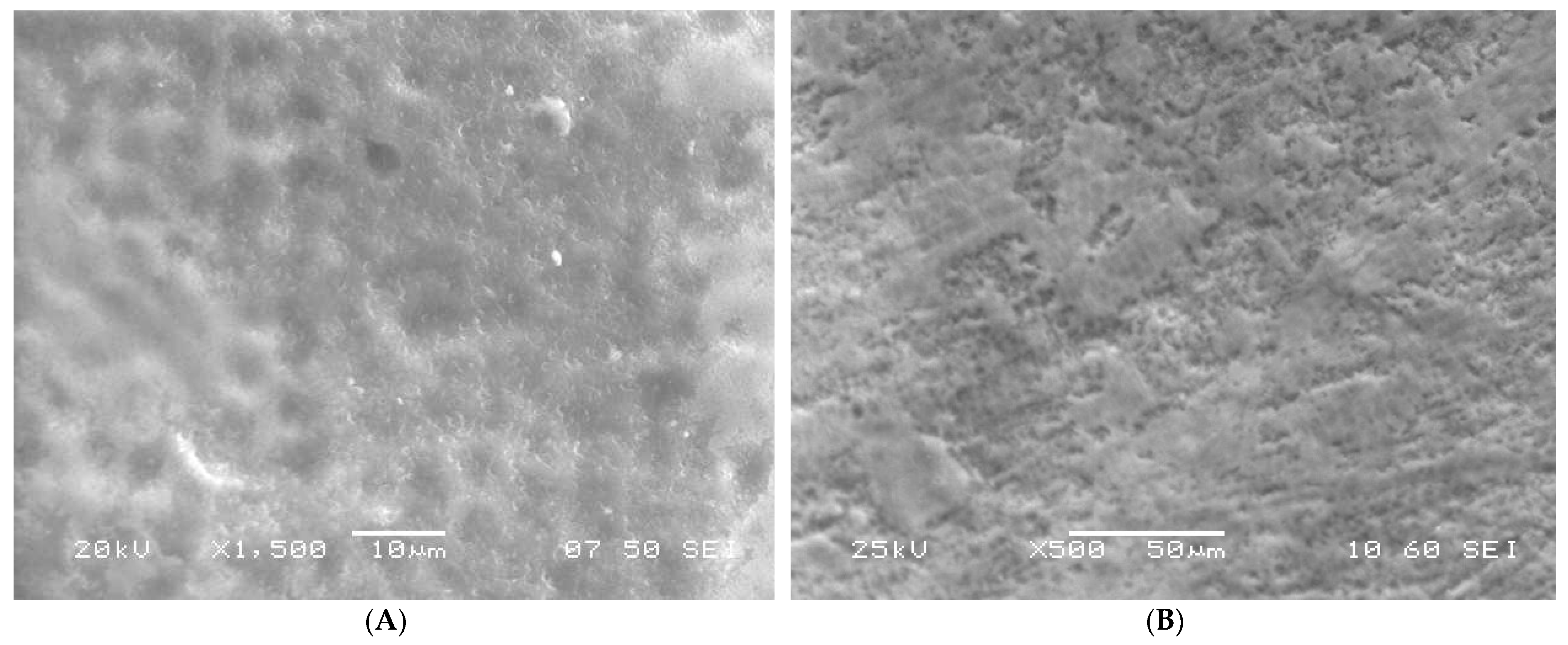
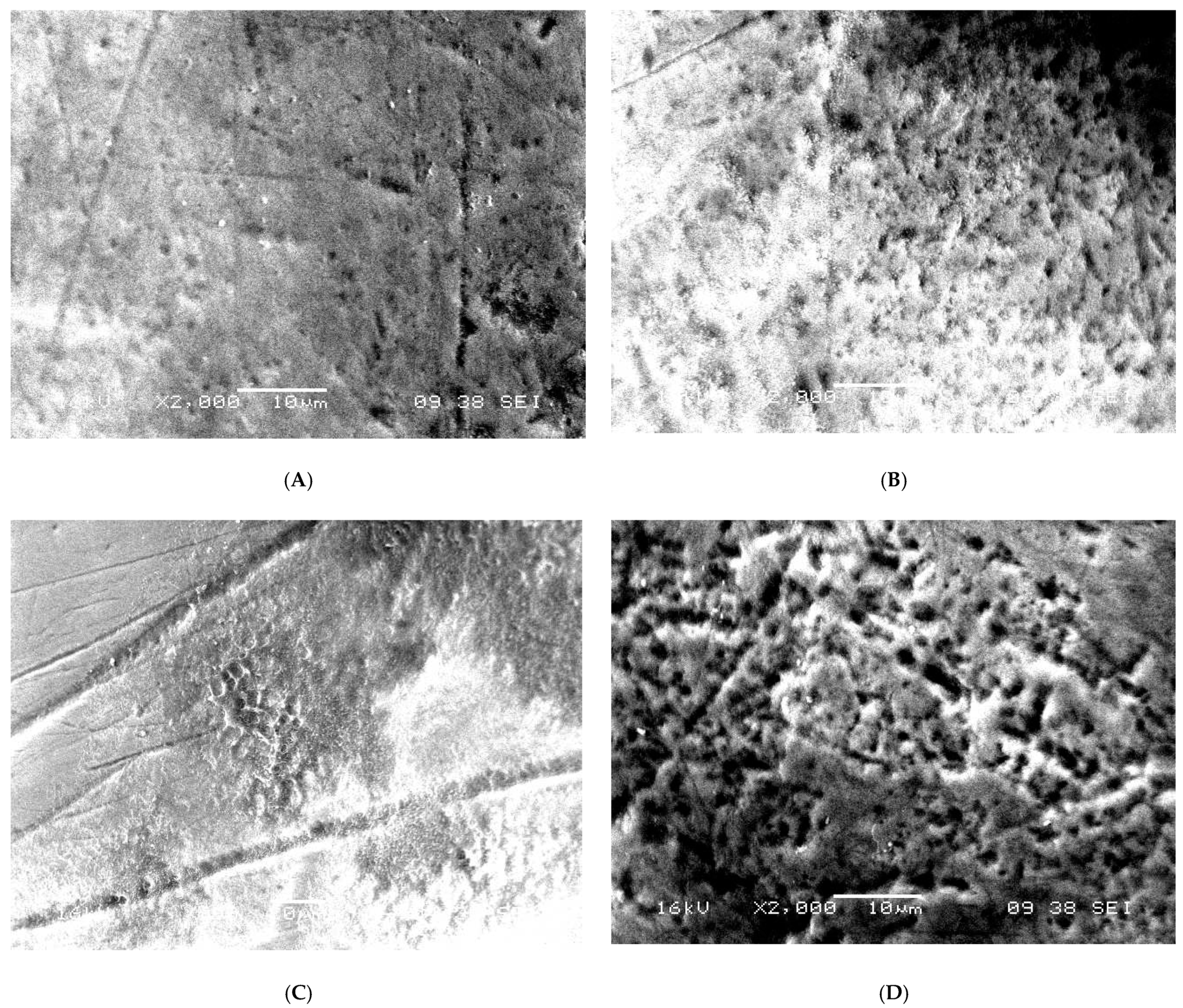
3.1. SEM Analysis
3.2. EDX Analysis
4. Discussion
- The effect of hyperglycemia on the mineralization leads to a low concentration of Ca and P in the mature enamel. Decreased levels of Ca and P could be a direct reason for the enamel defects. The work of Atar et al. [31] supports this hypothesis of ours.
- In addition to the impact on elemental composition, the influence of oxidative stress on the expression of MMP-20 and KLK4 during the secretory stage can lead to impaired development of HAP crystals in the rod and inter-rod enamel. The exact effect of diabetes on MMP-20 and KLK4 is still unreported, however, our assumption in this regard is based on the literature published by Bartlett JD [24].
- Primary teeth of diabetic children should be screened for macroscopic deficiencies as soon as they erupt.
- Diabetic children should be considered high caries risk children and they should be clinically monitored every three months.
- Primary and secondary prevention protocols specific to the teeth of diabetic children should be developed and implemented to curtail the initiation of dental caries.
- The parents of diabetic children should be made aware of the increased risk of dental caries and its consequences at an early stage.
- The parents should be educated to ensure these children maintain good oral hygiene and a healthy non-cariogenic diet.
- Finally, parents should be counseled to help maintain healthy blood glycemic levels to prevent the undesired oral and systemic consequences of diabetes.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liese, A.D.; D’Agostino, R.B.; Hamman, R.F.; Kilgo, P.D.; Lawrence, J.M.; Liu, L.L.; Loots, B.; Linder, B.; Marcovina, S.; Rodriguez, B. The burden of diabetes mellitus among US youth: Prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics 2006, 118, 1510–1518. [Google Scholar] [PubMed]
- Dabelea, D.; Bell, R.A.; D’Agostino, R.B.; Imperatore, G.; Johansen, J.M.; Linder, B.; Liu, L.L.; Loots, B.; Marcovina, S.; Mayer-Davis, E.J. Incidence of diabetes in youth in the United States. JAMA 2007, 297, 2716–2724. [Google Scholar] [PubMed] [Green Version]
- El-Bialy, T.; Aboul-Azm, S.F.; El-Sakhawy, M. Study of craniofacial morphology and skeletal maturation in juvenile diabetics (Type I). Am. J. Orthod. Dentofac. Orthop. 2000, 118, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.R.; Schwartz, G. Epidemiology of Skeletal Health in Type 1 Diabetes. Curr. Osteoporos. Rep. 2016, 14, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Malvania, E.A.; Sheth, S.A.; Sharma, A.S.; Mansuri, S.; Shaikh, F.; Sahani, S. Dental caries prevalence among type II diabetic and nondiabetic adults attending a hospital. J. Int Soc. Prev. Community Dent. 2016, 6 (Suppl. S3), S232–S236. [Google Scholar] [CrossRef] [Green Version]
- Ofoego, A.; Obi, A.; Ihentuge, C.; Fawehinmi, H. Abnormalities of the single-rooted anerior teeth: An index for early detection of diabetes mellitus. Int. J. Community Res. 2013, 2, 50–56. [Google Scholar]
- Chałas, R.; Rudzka, O.; Wójcik-Chęcińska, I.; Vodanović, M. The impact of type 1 diabetes on the development of the craniofacial mineralised tissues (bones and teeth): Literature review. Folia Morphol. 2016, 75, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Silva, B.L.L.; Medeiros, D.L.; Soares, A.P.; Line, S.R.P.; Pinto, M.D.G.F.; Soares, T.D.J.; Santo, A.R.D.E. Type 1 diabetes mellitus effects on dental enamel formation revealed by microscopy and microanalysis. J. Oral Pathol. Med. 2018, 47, 306–313. [Google Scholar] [CrossRef]
- Yamunadevi, A.; Basandi, P.S.; Madhushankari, G.S.; Selvamani, M.; Puneeth, H.; Basandi, P.S.; Donoghue, M. Morphological alterations in the dentition of type I diabetes mellitus patients. J. Pharm. Bioallied Sci. 2014, 6 (Suppl. S1), S122–S126. [Google Scholar] [CrossRef]
- Bensch, L.; Braem, M.; Van Acker, K.; Willems, G. Orthodontic treatment considerations in patients with diabetes mellitus. Am. J. Orthod. Dentofac. Orthop. 2003, 123, 74–78. [Google Scholar] [CrossRef]
- Balint, E.; Szabo, P.; Marshall, C.F.; Sprague, S.M. Glucose-induced inhibition of in vitro bone mineralization. Bone 2001, 28, 21–28. [Google Scholar] [CrossRef]
- Bender, I.B.; Bender, A.B. Diabetes mellitus and the dental pulp. J. Endod. 2003, 29, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Norén, J.G. Microscopic study of enamel defects in deciduous teeth of infants of diabetic mothers. Acta Odontol. Scand. 1984, 42, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sousa, Y.T.C.; Peres, L.C.; Foss, M.C. Enamel hypoplasia in a litter of rats with alloxan-induced diabetes mellitus. Braz. Dent. J. 2003, 14, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Sousa, Y.T.C.; Peres, L.C.; Foss, M.C. Are there structural alterations in the enamel organ of offspring of rats with alloxan-induced diabetes mellitus? Braz. Dent. J. 2003, 14, 162–167. [Google Scholar] [CrossRef]
- Ferizi, L.; Dragidella, F.; Spahiu, L.; Begzati, A.; Kotori, V. The Influence of Type 1 Diabetes Mellitus on Dental Caries and Salivary Composition. Int. J. Dent. 2018, 2018, 5780916. [Google Scholar] [CrossRef]
- Atar, M.; Atar-Zwillenberg, D.R.; Verry, P.; Spornitz, U.M. Defective enamel ultrastructure in diabetic rodents. Int. J. Paediatr. Dent. 2004, 14, 301–307. [Google Scholar] [CrossRef]
- Gutowska, I.; Baranowska-Bosiacka, I.; Rybicka, M.; Noceń, I.; Dudzińska, W.; Marchlewicz, M.; Wiszniewska, B.; Chlubek, D. Changes in the concentration of microelements in the teeth of rats in the final stage of type 1 diabetes, with an absolute lack of insulin. Biol. Trace Elem. Res. 2011, 139, 332–340. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry. Caries-Risk Assessment and Management for Infants, Children, and Adolescents. The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2019; pp. 220–224. [Google Scholar]
- Jefferies, C.; Rhodes, E.; Rachmiel, M.; Chizo, A.J.; Kapellen, T.; Abdulla, M.A.; Hofer, S.E. ISPAD Clinical Practice Consensus Guidelines 2018: Management of children and adolescents with diabetes requiring Surgery. Pediatr Diabetes 2019, 20, 137. [Google Scholar] [CrossRef]
- Eli, I.; Sarnat, H.; Talmi, E. Effect of the birth process on the neonatal line in primary tooth enamel. Pediatr Dent. 1989, 11, 220–223. [Google Scholar]
- Nanci, A. (Ed.) Ten Cate’s Oral Histology, 9th ed.; Elsevier Ltd.: St. Louis, MO, USA, 2017. [Google Scholar]
- Eastoe, J.E. Organic Matrix of Tooth Enamel. Nature 1960, 187, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D. Dental enamel development: Proteinases and their enamel matrix substrates. ISRN Dent 2013, 2013, 684607. [Google Scholar] [CrossRef]
- Brings, S.; Zhang, S.; Choong, Y.S.; Hogl, S.; Middleditch, M.; Kamalov, M.; Brimble, M.A.; Gong, D.; Cooper, G.J. Diabetes-induced alterations in tissue collagen and carboxymethyllysine in rat kidneys: Association with increased collagen-degrading proteinases and amelioration by Cu(II)-selective chelation. Biochim. Biophys. Acta-Mol. Basis Dis. 2015, 1852, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Uemura, S.; Matsushita, H.; Li, W.; Glassford, A.J.; Asagami, T.; Lee, K.H.; Harrison, D.G.; Tsao, P.S. Diabetes mellitus enhances vascular matrix metalloproteinase activity: Role of oxidative stress. Circ. Res. 2001, 88, 1291–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodaka, T.; Nakajima, F.; Higashi, S. Structure of the so-called “prismless” enamel in human deciduous teeth. Caries Res. 1989, 23, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Maridonneau, I.; Braquet, P.; Garay, R.P. Na+ and K+ transport damage induced by oxygen free radicals in human red cell membranes. J. Biol. Chem. 1983, 258, 3107–3113. [Google Scholar] [CrossRef]
- Xu, H.H.; Smith, D.T.; Jahanmir, S.; Romberg, E.; Kelly, J.; Thompson, V.P.; Rekow, E. Indentation damage and mechanical properties of human enamel and dentin. J. Dent. Res. 1998, 77, 472–480. [Google Scholar] [CrossRef]
- Armstrong, W.; Brekhus, P.J. Chemical constitution of enamel and dentin: 1. Principal components. J. Biol. Chem. 1937, 120, 677–687. [Google Scholar] [CrossRef]
- Atar, M.; Davis, G.R.; Verry, P.; Wong, F.S.L. Enamel mineral concentration in diabetic rodents. Eur Arch. Paediatr. Dent. 2007, 8, 195–200. [Google Scholar] [CrossRef]


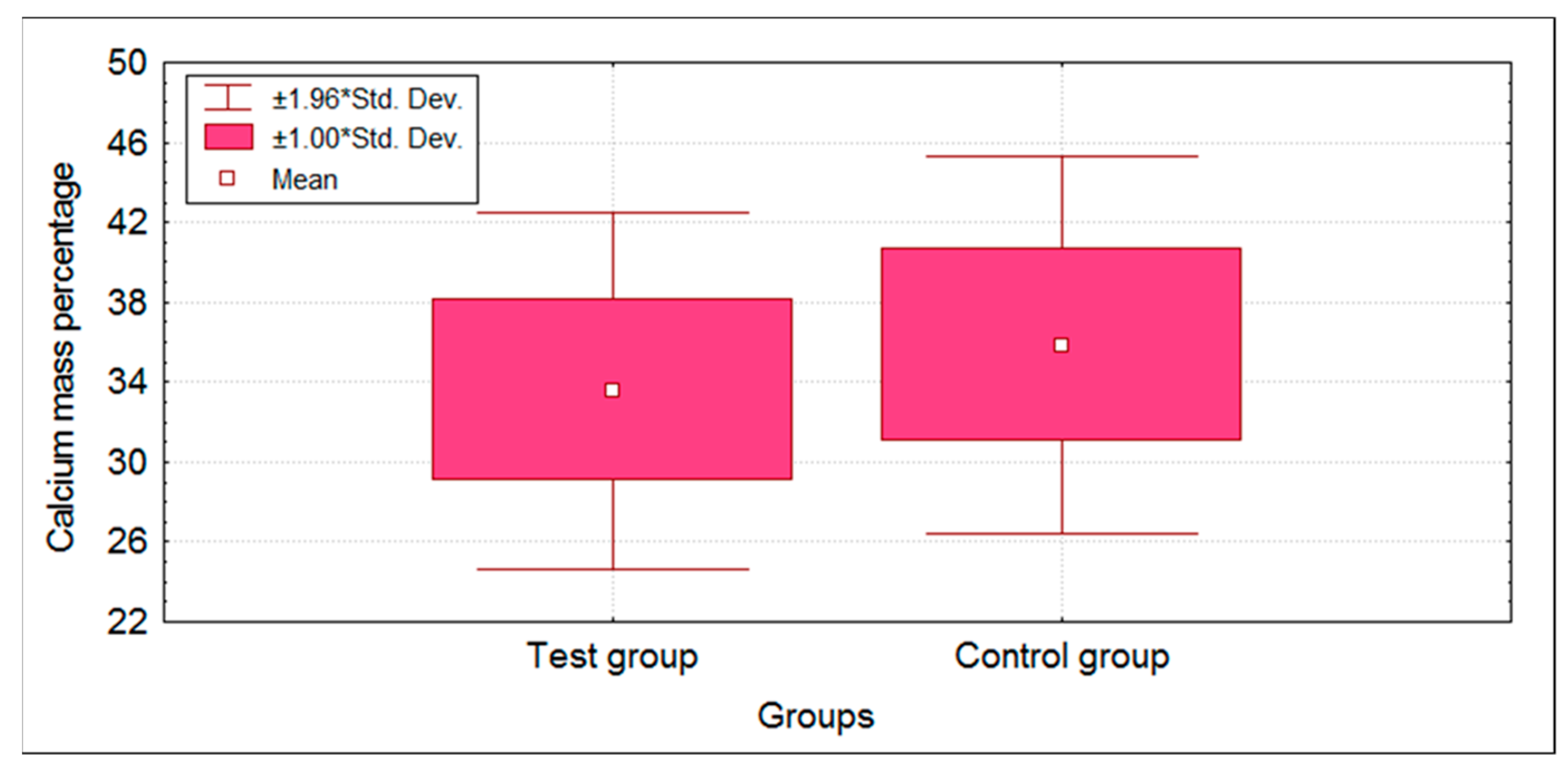

| Groups | n | Mean | SD | SE | t-Value | p-Value | |
|---|---|---|---|---|---|---|---|
| Ca | Test group | 50 | 33.57 | 4.56 | 0.65 | −2.4232 | 0.0172 * |
| Control group | 50 | 35.84 | 4.83 | 0.68 | |||
| P | Test group | 50 | 16.76 | 1.55 | 0.22 | −4.4569 | 0.0001 * |
| Control group | 50 | 17.94 | 1.05 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed, S.; Yassin, S.M.; Almalki, A.Y.; Ali, S.A.A.; Alqarni, A.M.M.; Moadi, Y.M.; Alkhaldi, A.M.; Alqahtani, N.M.; Hosmani, J.; Heboyan, A.; et al. Structural Changes in Primary Teeth of Diabetic Children: Composition and Ultrastructure Analysis. Children 2022, 9, 317. https://doi.org/10.3390/children9030317
Syed S, Yassin SM, Almalki AY, Ali SAA, Alqarni AMM, Moadi YM, Alkhaldi AM, Alqahtani NM, Hosmani J, Heboyan A, et al. Structural Changes in Primary Teeth of Diabetic Children: Composition and Ultrastructure Analysis. Children. 2022; 9(3):317. https://doi.org/10.3390/children9030317
Chicago/Turabian StyleSyed, Sadatullah, Syed M. Yassin, Abdulrahman Yahya Almalki, Salma Abubaker Abbas Ali, Abdulaziz M. Maken Alqarni, Yousef M. Moadi, Abdulrahman Masoud Alkhaldi, Nasser M. Alqahtani, Jagadish Hosmani, Artak Heboyan, and et al. 2022. "Structural Changes in Primary Teeth of Diabetic Children: Composition and Ultrastructure Analysis" Children 9, no. 3: 317. https://doi.org/10.3390/children9030317
APA StyleSyed, S., Yassin, S. M., Almalki, A. Y., Ali, S. A. A., Alqarni, A. M. M., Moadi, Y. M., Alkhaldi, A. M., Alqahtani, N. M., Hosmani, J., Heboyan, A., & Patil, S. (2022). Structural Changes in Primary Teeth of Diabetic Children: Composition and Ultrastructure Analysis. Children, 9(3), 317. https://doi.org/10.3390/children9030317








