Gaps in Accessibility of Pediatric Formulations: A Cross-Sectional Observational Study of a Teaching Hospital in Northern Thailand
Abstract
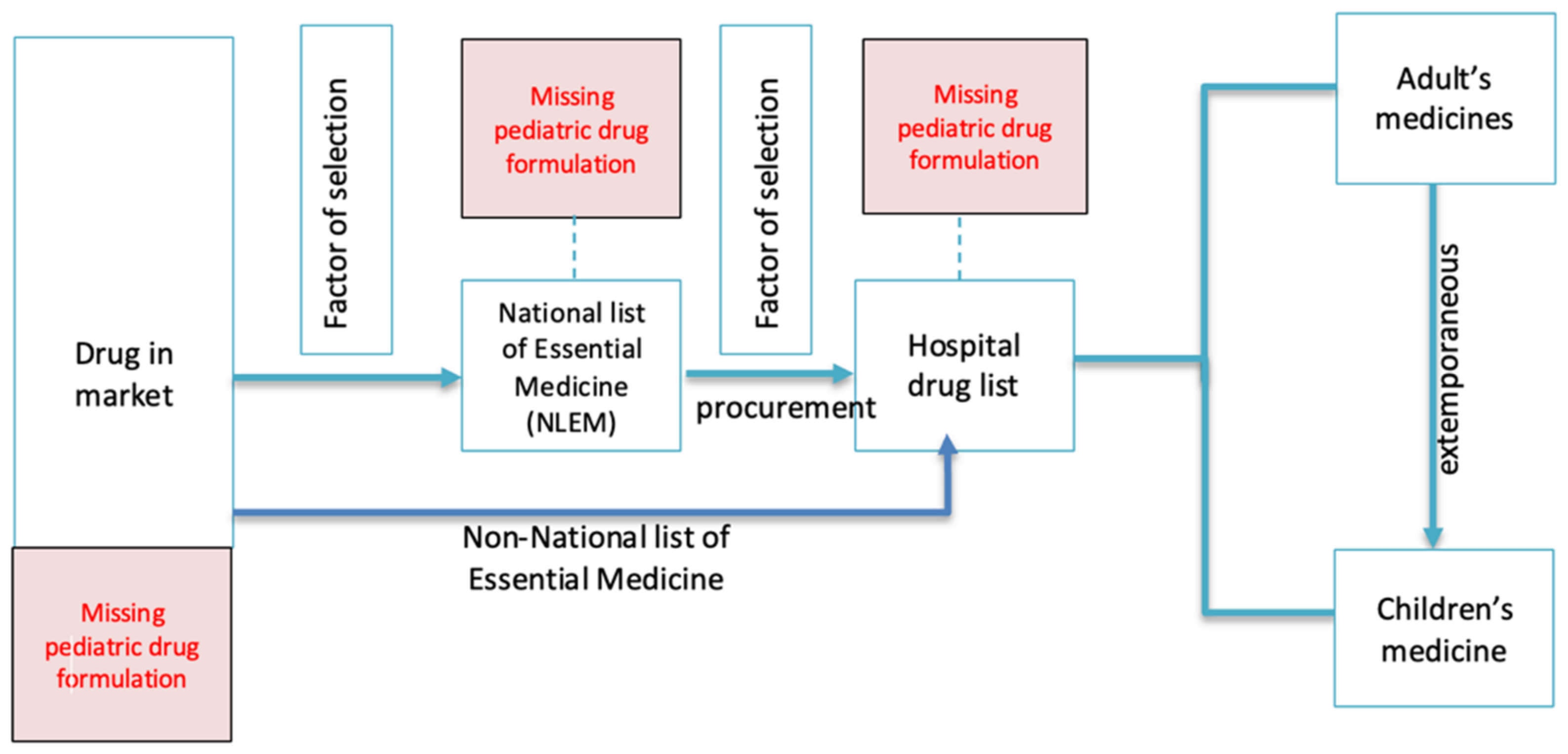
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coelho, H.L.L.; Rey, L.C.; de Medeiros, M.S.G.; Barbosa, R.A.; da Cruz Fonseca, S.G.; da Costa, P.Q. A critical comparison between the World Health Organization list of essential medicines for children and the Brazilian list of essential medicines (Rename). J. Pediatr. 2013, 89, 171–178. [Google Scholar] [CrossRef][Green Version]
- European Medicines Agency. Reflection Paper: Formulations of Choice for the Peadiatric Population; European Medicines Agency: London, UK, 2006. [Google Scholar]
- World Health Organization. Promoting Safety of Medicines for Children; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Allen, H.C.; Garbe, M.C.; Lees, J.; Aziz, N.; Chaaban, H.; Miller, J.L.; Johnson, P.; de Leon, S. Off-Label Medication use in Children, More Common than We Think: A Systematic Review of the Literature. J. Okla. State Med. Assoc. 2018, 111, 776–783. [Google Scholar] [PubMed]
- Lee, J.H.; Byon, H.J.; Choi, S.; Jang, Y.E.; Kim, E.H.; Kim, J.T.; Kim, H.S. Safety and efficacy of off-label and unlicensed medicines in children. J. Korean Med. Sci. 2018, 33, e227. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Avant, D.; Green, D.; Seo, S.; Fisher, J.; Mulberg, A.E.; McCune, S.K.; Burckart, G.J. A Survey of Neonatal Pharmacokinetic and Pharmacodynamic Studies in Pediatric Drug Development. Clin. Pharmacol. Ther. 2015, 98, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Field, M.J.; Boat, T.F. Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. In Safe and Effective Medicines for Children; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Venables, R.; Batchelor, H.; Hodson, J.; Stirling, H.; Marriott, J. Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int. J. Pharm. 2015, 480, 55–62. [Google Scholar] [CrossRef]
- Zahn, J.; Hoerning, A.; Trollmann, R.; Rascher, W.; Neubert, A. Manipulation of medicinal products for oral administration to paediatric patients at a German university hospital: An observational study. Pharmaceutics 2020, 12, 583. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC: College Station, TX, USA, 2019. [Google Scholar]
- Gore, R.; Chugh, P.K.; Tripathi, C.D.; Lhamo, Y.; Gautam, S. Pediatric off-label and unlicensed drug use and its implications. Curr. Clin. Pharmacol. 2017, 12, 18–25. [Google Scholar] [CrossRef]
- Moulis, F.; Durrieu, G.; Lapeyre-Mestre, M. Off-label and unlicensed drug use in children population. Therapie 2018, 73, 135–149. [Google Scholar] [CrossRef]
- Teigen, A.; Wang, S.; Truong, B.T.; Bjerknes, K. Off-label and unlicensed medicines to hospitalised children in Norway. J. Pharm. Pharmacol. 2017, 69, 432–438. [Google Scholar] [CrossRef]
- Arocas Casañ, V.; Escribano, B.C.; Garrido-Corro, B.; de la Cruz Murie, P.; Álvarez, J.B.B.; de la Rubia Nieto, A. Off-label and unlicensed drug use in a Spanish Neonatal Intensive Care Unit. Farm. Hosp. 2017, 41, 371–381. [Google Scholar]
- Aamir, M.; Khan, J.A.; Shakeel, F.; Shareef, R.; Shah, N. Drug utilization in neonatal setting of Pakistan: Focus on unlicensed and off label drug prescribing. BMC Pediatr. 2018, 18, 242. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, S.; Iffat, W.; Nesar, S.; Zaidi, H.; Jamshed, S. Exploratory findings of prescribing unlicensed and off-label medicines among children and neonates. Integr. Pharm. Res. Pract. 2020, 9, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Mukattash, T.; Hawwa, A.F.; Trew, K.; McElnay, J.C. Healthcare professional experiences and attitudes on unlicensed/off-label paediatric prescribing and paediatric clinical trials. Eur. J. Clin. Pharmacol. 2011, 67, 449–461. [Google Scholar] [CrossRef]
- Bjerknes, K.; Bøyum, S.; Kristensen, S.; Brustugun, J.; Wang, S. Manipulating tablets and capsules given to hospitalised children in Norway is common practice. Acta Paediatr. 2017, 106, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Richey, R.H.; Shah, U.U.; Peak, M.; Craig, J.V.; Ford, J.L.; Barker, C.E.; Nunn, A.J.; Turner, M.A. Manipulation of drugs to achieve the required dose is intrinsic to paediatric practice but is not supported by guidelines or evidence. BMC Pediatr 2013, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Van der Vossen, A.C.; Al-Hassany, L.; Buljac, S.; Brugma, J.D.; Vulto, A.G.; Hanff, L.M. Manipulation of oral medication for children by parents and nurses occurs frequently and is often not supported by instructions. Acta Paediatr. 2019, 108, 1475–1481. [Google Scholar] [CrossRef]
- Wensel, T.M. Administration of proton pump inhibitors in patients requiring enteral nutrition. Pharm. Ther. 2009, 34, 143–160. [Google Scholar]
- Zhang, L.; Hu, Y.; Pan, P.; Hong, C.; Fang, L. Estimated manipulation of tablets and capsules to meet dose requirements for Chinese children: A cross-sectional study. Front. Pediatr. 2021, 9, 747499. [Google Scholar] [CrossRef]
- Jitruknatee, A.; Tosanguan, K.; Doangjai, Y.; Theantawee, W.; Martro, K. A Review on the Selection of Drugs in Thai Heath Care at National, Pharmaceutical industries and Public Hospital Levels. J. Health Sci. 2020, 29, S31–S44. [Google Scholar]
- Plathong, J.P.; Sthapomnanon, N. Effect of Pediatric Pharmaceutical Care by Ward Based Clinical Pharmacist. Isan J. Pharm. Sci. 2017, 13, 311–321. [Google Scholar]
- García-López, I.; Vendrell, M.C.-M.; Romero, I.M.; de Noriega, I.; González, J.B.; Martino-Alba, R. Off-label and unlicensed drugs in pediatric palliative care: A prospective observational study. J. Pain Symptom Manag. 2020, 60, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Archary, M.; Zanoni, B.; Lallemant, M.; Suwannaprom, P.; Clarke, D.; Penazzato, M. Acceptability and feasibility of using Raltegravir oral granules for the treatment of neonates in a low-resource setting. Pediatr. Infect. Dis. J. 2020, 39, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Batchelor, H. Evidence of acceptability of oral paediatric medicines: A review. J. Pharm. Pharmacol. 2017, 69, 361–376. [Google Scholar] [CrossRef] [PubMed]
- University of Illinois at Chicago College of Pharmacy, Drug Information Group. Light-sensitive injectable prescription drugs. Hosp. Pharm. 2014, 49, 136–163. [Google Scholar] [CrossRef]
| Questionnaire for Physicians and Pharmacists | Questionnaire for Nurses |
|---|---|
| 1. Which needed medicines are unavailable in your hospital? | 1. Which are the most problematic medicines to prepare? |
| 2. Which off-label medicines do you frequently use among children? | 2. Which are the most problematic medicines in terms of acceptability? |
| 3. Which medicines require extemporaneous preparation? | 3. Which are the most problematic medicines to administer? |
| 4. Which are the most problematic medicines? | 4. Which are the most problematic medicines in terms of storage? |
| Specialization | Overall, n (%) (n = 223) |
|---|---|
| Physicians | 36 (16.1) |
| Pediatrician (general) | 12 |
| Pediatric hematology-oncology | 6 |
| Pediatric cardiology | 7 |
| Neonatal and perinatal medicine | 3 |
| Developmental and behavioral pediatrics | 3 |
| Pediatric neurology | 1 |
| Pediatric gastroenterology and hepatology | 1 |
| Pediatric allergy and immunology | 1 |
| Pediatric Nephrology | 1 |
| Pediatric Rheumatology | 1 |
| Pharmacists | 18 (8.1) |
| Inpatient services | 8 |
| Outpatient services | 4 |
| Extemporaneous preparations | 4 |
| Drug information services | 2 |
| Nurses | 169 (75.8) |
| Pediatric Nursing Section | 141 |
| Neonatal Intensive Unit: NICU | 20 |
| Nurse administration | 5 |
| Milk preparation department | 2 |
| Child development | 1 |
| Working Experience, n (%) (n = 223) | ||||
|---|---|---|---|---|
| <1 Year | 1–5 Years | 5–10 Years | >10 Years | |
| Physicians | 1 (0.4) | 13 (5.8) | 11 (4.9) | 11 (4.9) |
| Pharmacists | 4 (1.8) | 11 (4.9) | 1 (0.4) | 2 (0.9) |
| Nurses | 7 (3.1) | 48 (21.5) | 28 (12.6) | 86 (38.5) |
| Children’s Formulation | Number (%) of Responses |
|---|---|
| Missing formulations | |
| Omeprazole suspension | 9 (7.1) |
| Sildenafil suspension | 9 (7.1) |
| Prednisolone syrup | 8 (6.3) |
| Propranolol injection | 6 (4.7) |
| Eltrombopag syrup | 5 (3.9) |
| Spironolactone suspension | 5 (3.9) |
| Total | 127 (100.0) |
| Off-label use | |
| Clopidogrel | 5 (9.1) |
| Celecoxib | 4 (7.3) |
| Alectinib | 3 (5.5) |
| Aprepitant | 3 (5.5) |
| Risperidone | 3 (5.5) |
| Total | 55 (100.0) |
| Extemporaneous preparations | |
| Omeprazole suspension | 15 (10.9) |
| Sildenafil suspension | 13 (9.4) |
| Aspirin | 9 (6.5) |
| Ursodeoxycholic acid | 9 (6.5) |
| Calcium carbonate | 7 (5.1) |
| Prednisolone | 7 (5.1) |
| Total | 138 (100.0) |
| Problematic children’s medicines | |
| Warfarin | 13 (19.7) |
| Enoxaparin | 7 (10.6) |
| Heparin | 4 (6.1) |
| Cyclosporin A | 4 (6.1) |
| Aspirin | 3 (4.5) |
| Total | 66 (100.0) |
| Children’s Formulation | Number (%) of Responses |
|---|---|
| Missing preparation | |
| Erythromycin solution | 8 (14.8) |
| Cloxacillin solution | 6 (11.1) |
| Hyoscine solution | 6 (11.1) |
| Salbutamol syrup | 4 (7.4) |
| Total | 54 (100.0) |
| Off-label use | |
| Acetazolamide | 7 (26.9) |
| Topical nitroglycerine | 7 (26.9) |
| Cloxacillin | 4 (15.4) |
| Sulfamethoxazole and trimethoprim | 3 (11.5) |
| Total | 26 (100.0) |
| Extemporaneous preparation required | |
| Omeprazole suspension | 13 (18.3) |
| Furosemide suspension | 12 (16.9) |
| Sildenafil suspension | 8 (11.3) |
| Spironolactone suspension | 8 (11.3) |
| Vitamin D suspension | 8 (11.3) |
| Total | 71 (100.0) |
| Problematic children’s medicines | |
| Amoxicillin suspension | 9 (23.7) |
| Paracetamol syrup | 9 (23.7) |
| Aspirin (film-coated tablet) | 4 (10.5) |
| Azithromycin syrup | 2 (5.3) |
| Total | 38 (100.0) |
| Children’s Formulation | Number (%) of Responses |
|---|---|
| In medicine preparation | |
| 7.5% Sodium bicarbonate | 68 (13.1) |
| Furosemide injection | 30 (5.8) |
| Omeprazole capsule | 30 (5.8) |
| Calcium polystyrene sulfonate | 27 (5.2) |
| Calcium tablet | 25 (4.8) |
| Total | 519 (100.0) |
| In children’s acceptability to medicines | |
| Potassium chloride | 94 (21.1) |
| Multivitamin drops | 40 (9.1) |
| Chloral hydrate | 33 (7.5) |
| Sodium phosphate | 32 (7.3) |
| Ceftazidime | 21 (4.8) |
| Total | 441 (100.0) |
| In administration | |
| Potassium chloride | 79 (20.1) |
| Chloral hydrate | 37 (9.4) |
| Sodium phosphate | 24 (6.1) |
| Multivitamin drops | 21 (5.3) |
| Dipotassium phosphate | 20 (5.1) |
| Total | 393 (100.0) |
| Problematic children’s medicines | |
| Micafungin injection | 20 (11.3) |
| Insulin | 13 (7.3) |
| Amoxicillin/clavulanic acid injection | 12 (6.8) |
| Ampicillin | 11 (6.2) |
| Ganciclovir | 11 (6.2) |
| Total | 177 (100.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiengkate, P.; Lallemant, M.; Charoenkwan, P.; Angkurawaranon, C.; Kanjanarat, P.; Suwannaprom, P.; Borriharn, P. Gaps in Accessibility of Pediatric Formulations: A Cross-Sectional Observational Study of a Teaching Hospital in Northern Thailand. Children 2022, 9, 301. https://doi.org/10.3390/children9030301
Tiengkate P, Lallemant M, Charoenkwan P, Angkurawaranon C, Kanjanarat P, Suwannaprom P, Borriharn P. Gaps in Accessibility of Pediatric Formulations: A Cross-Sectional Observational Study of a Teaching Hospital in Northern Thailand. Children. 2022; 9(3):301. https://doi.org/10.3390/children9030301
Chicago/Turabian StyleTiengkate, Prangthong, Marc Lallemant, Pimlak Charoenkwan, Chaisiri Angkurawaranon, Penkarn Kanjanarat, Puckwipa Suwannaprom, and Phetlada Borriharn. 2022. "Gaps in Accessibility of Pediatric Formulations: A Cross-Sectional Observational Study of a Teaching Hospital in Northern Thailand" Children 9, no. 3: 301. https://doi.org/10.3390/children9030301
APA StyleTiengkate, P., Lallemant, M., Charoenkwan, P., Angkurawaranon, C., Kanjanarat, P., Suwannaprom, P., & Borriharn, P. (2022). Gaps in Accessibility of Pediatric Formulations: A Cross-Sectional Observational Study of a Teaching Hospital in Northern Thailand. Children, 9(3), 301. https://doi.org/10.3390/children9030301






