Role of Cross-Sectional Imaging in Pediatric Interventional Cardiac Catheterization
Abstract
:1. Background
2. Case Examples
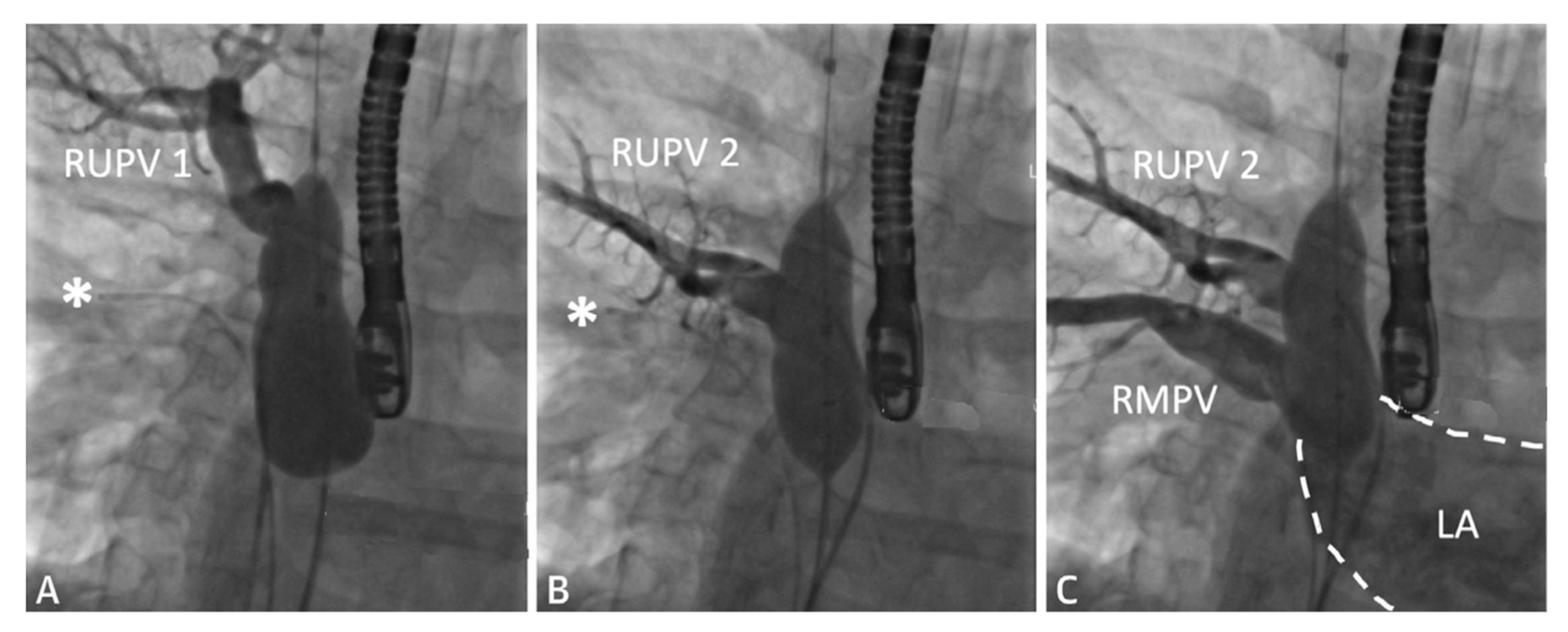
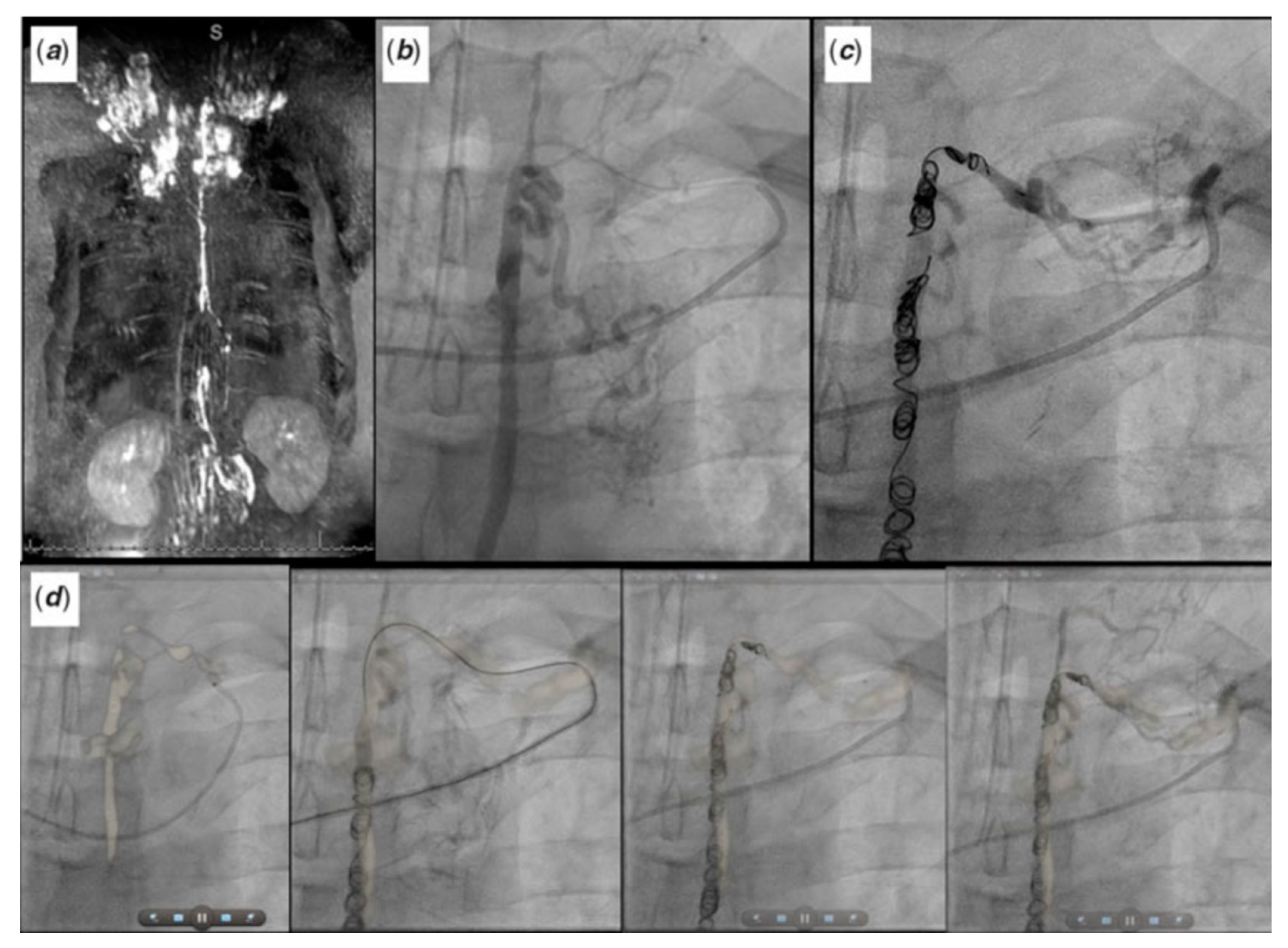
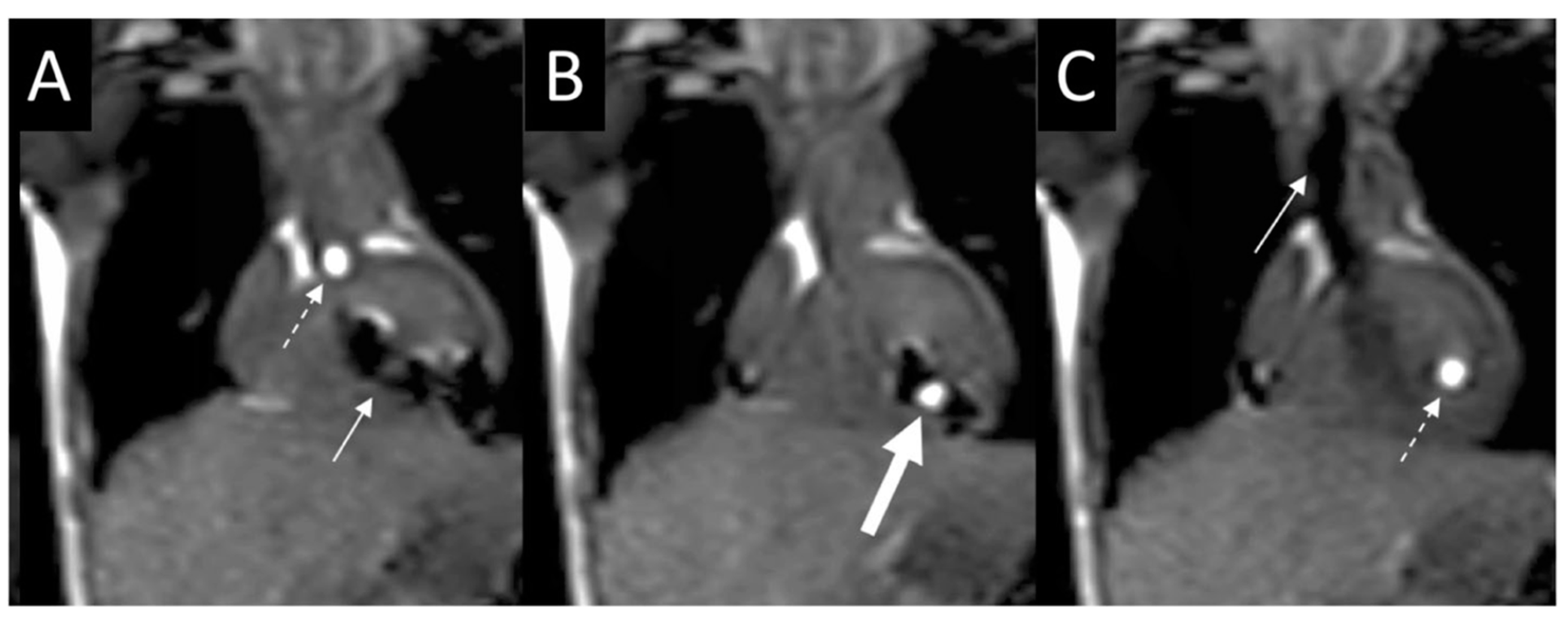
2.1. Sinus Venosus Atrial Septal Defect (SVASD)
2.2. Patent Ductus Arteriosus (PDA) Stenting for Ductal-Dependent Pulmonary Blood Flow
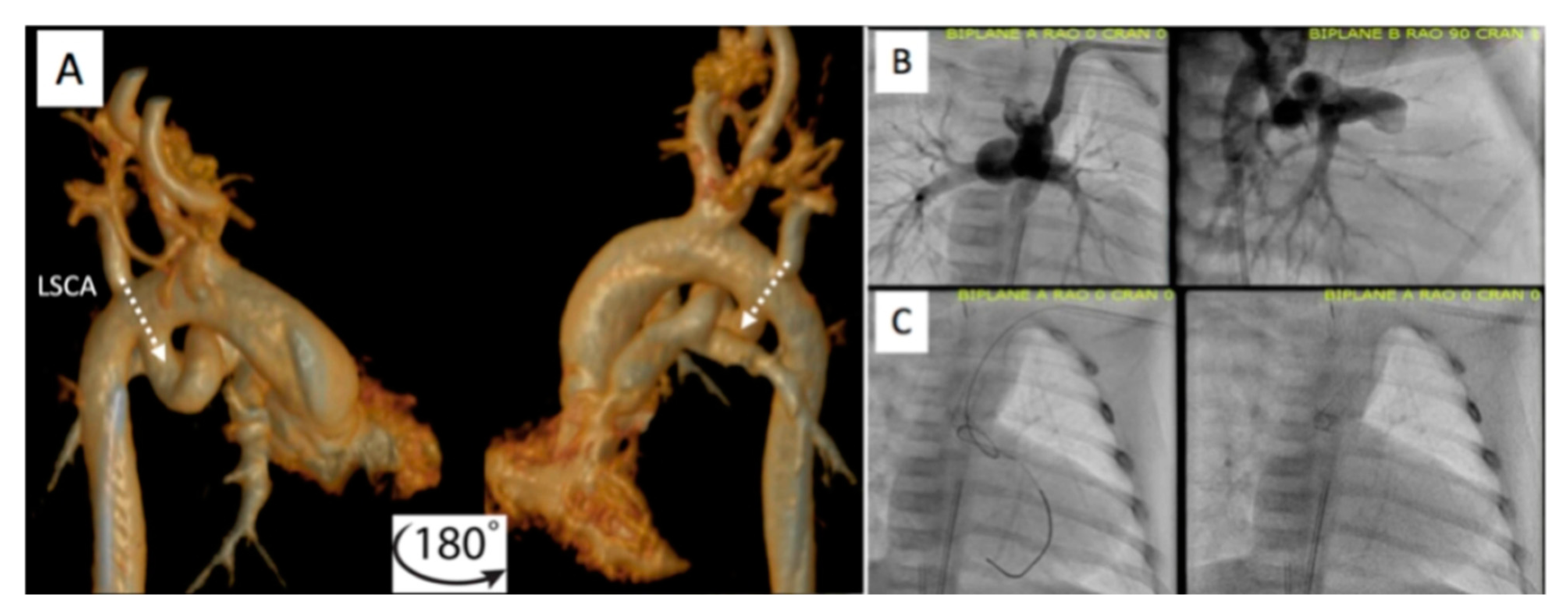
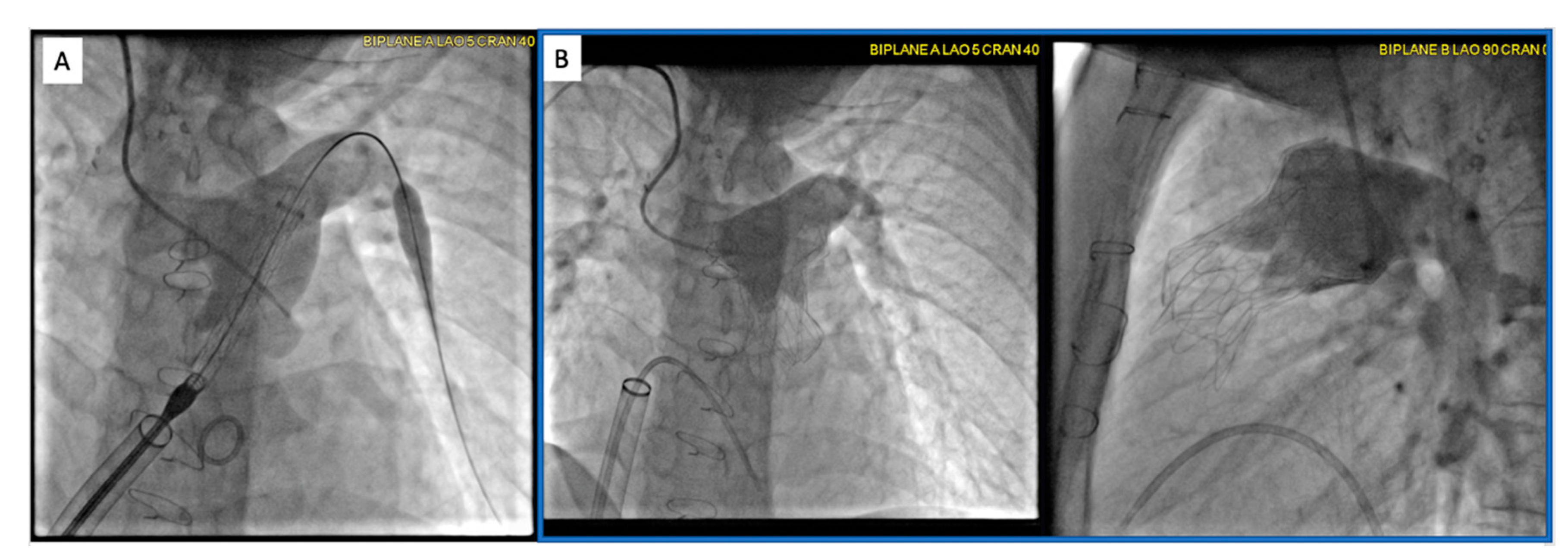
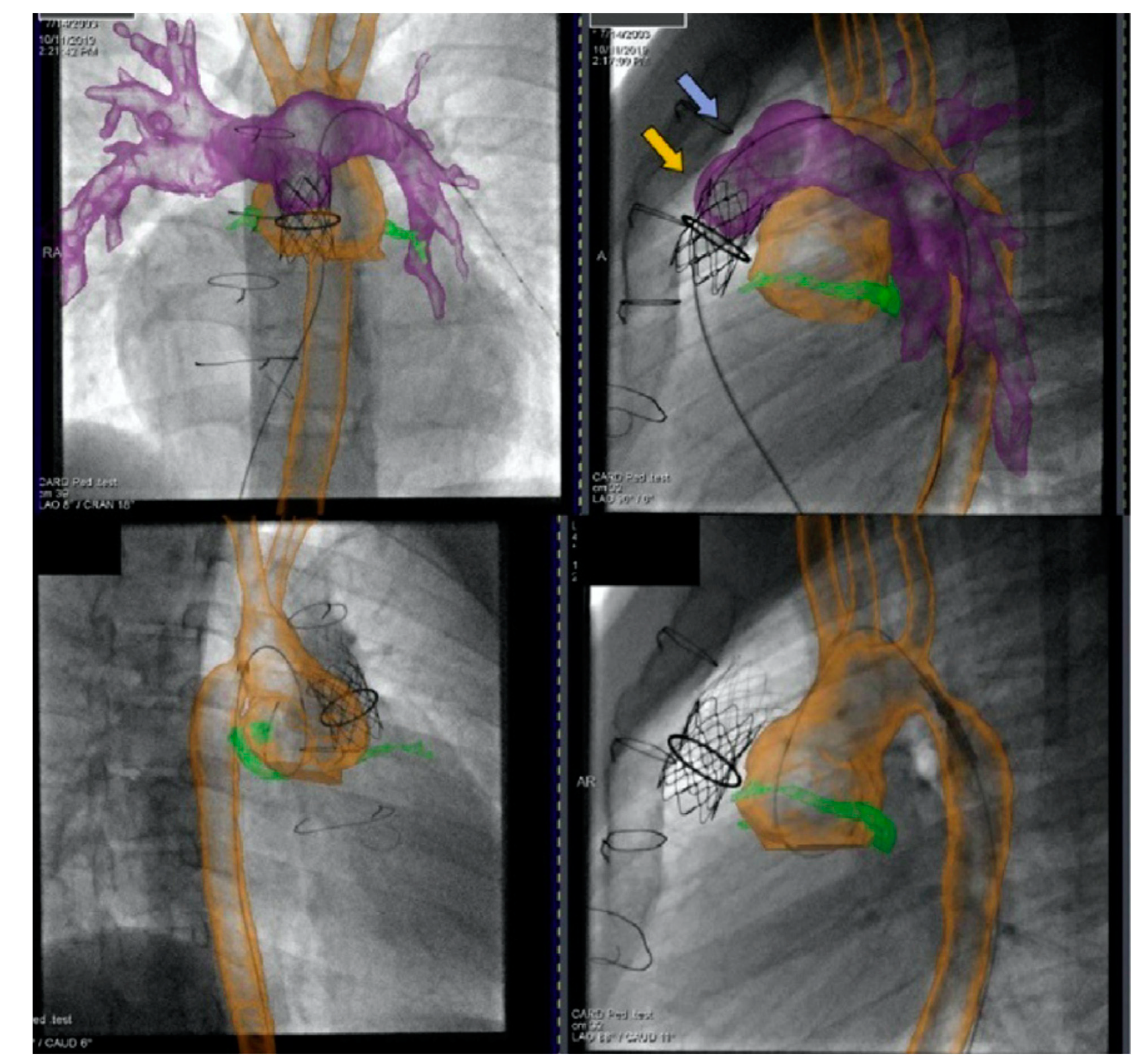
2.2.1. Pre-Procedural
2.2.2. Post-Procedural
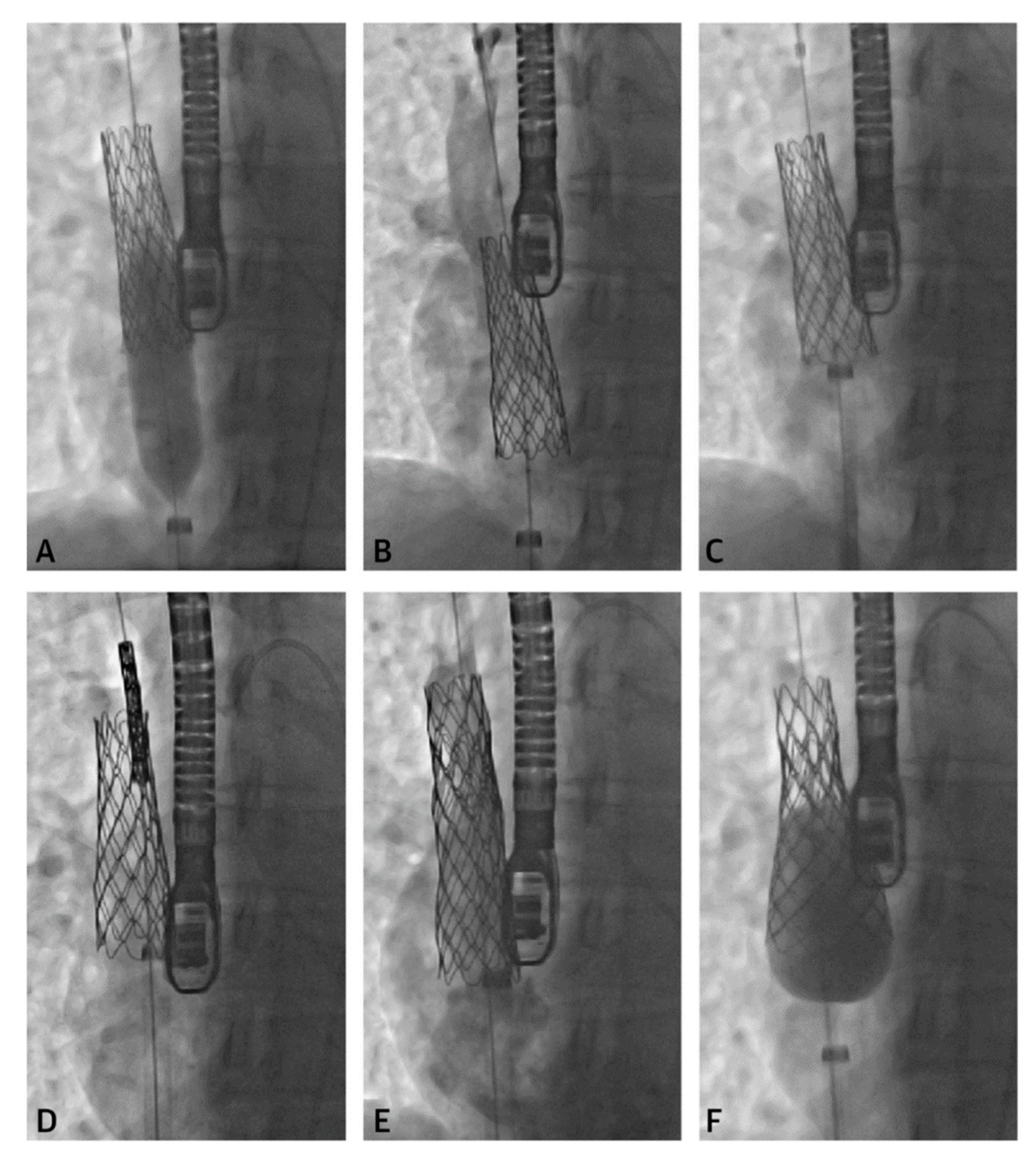
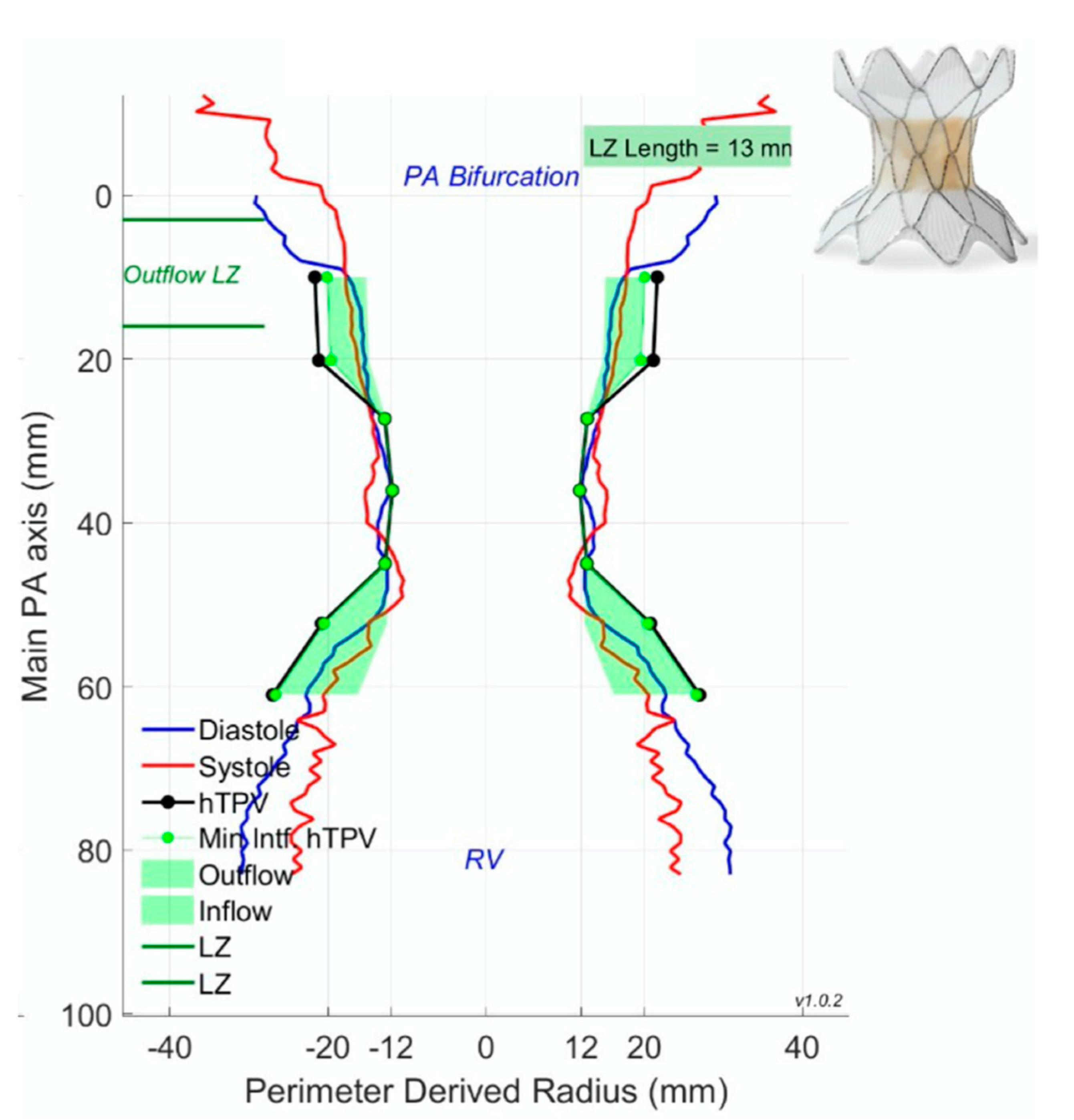
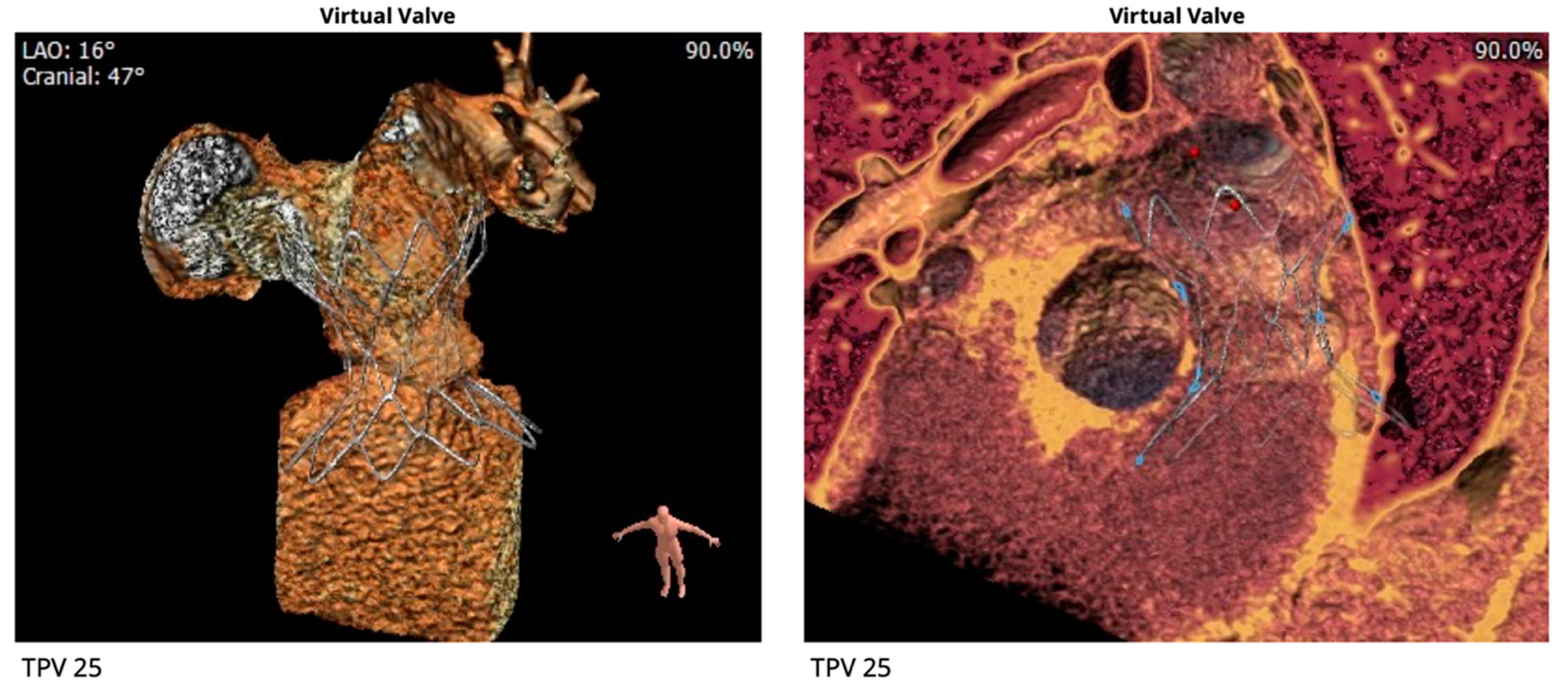
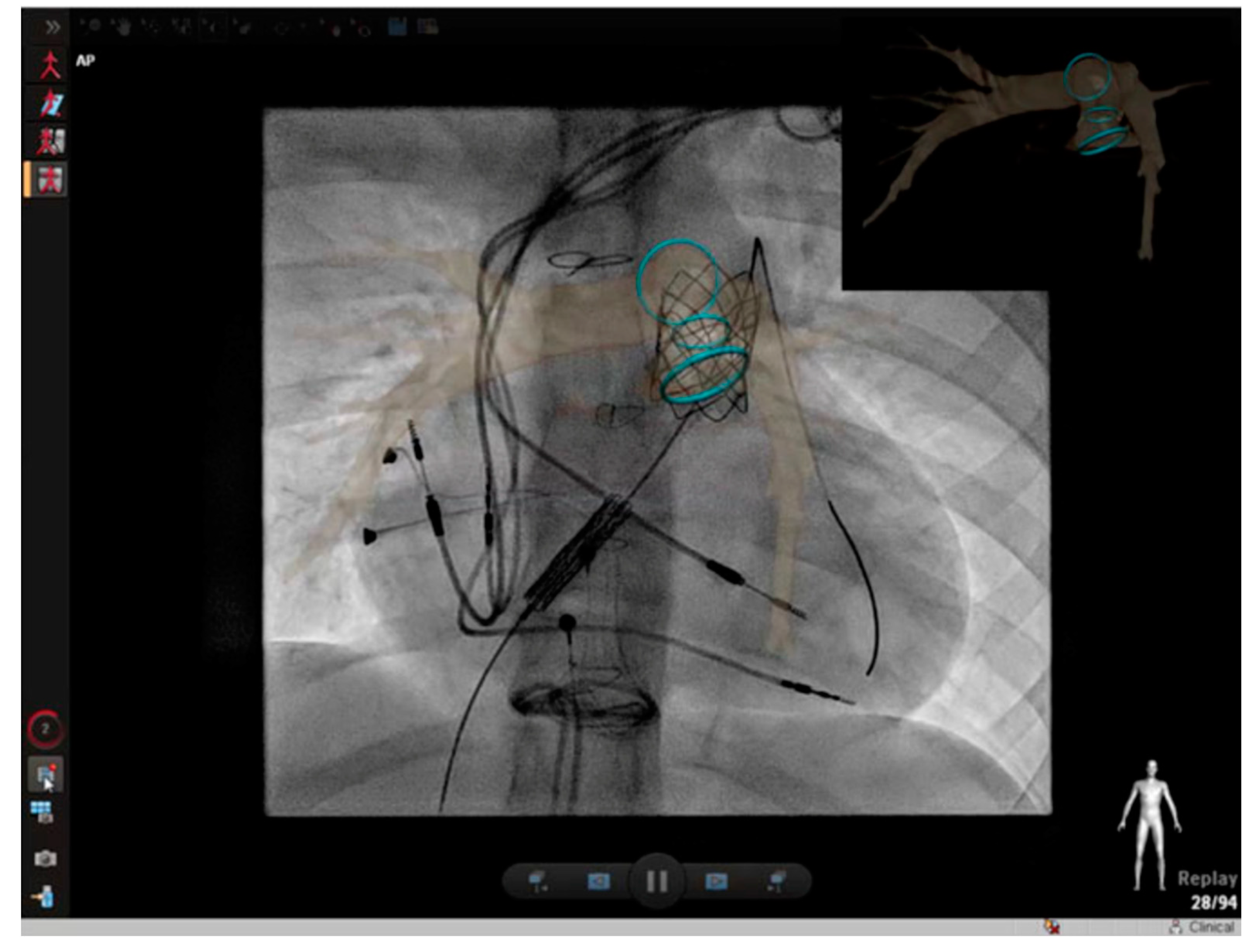
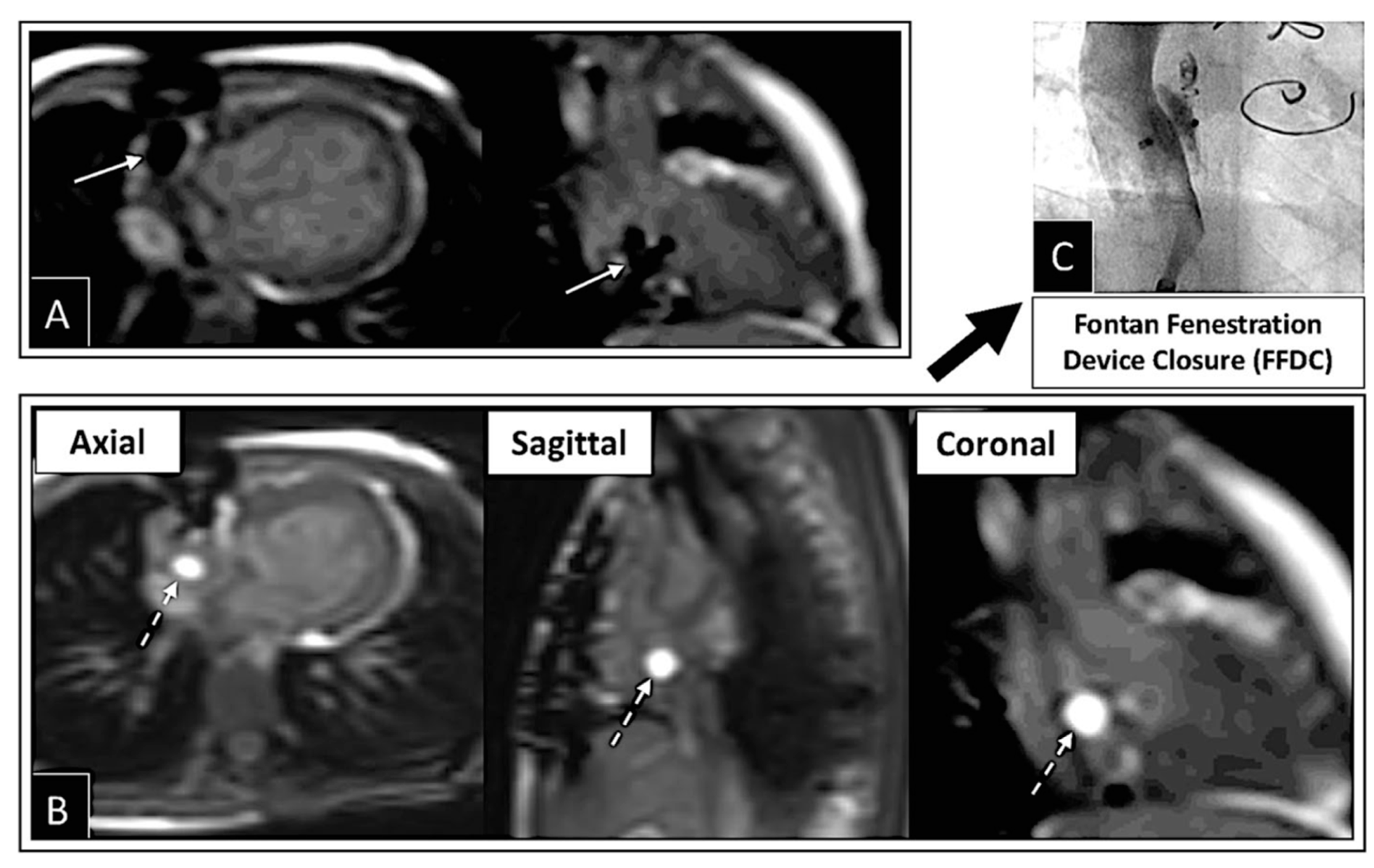
2.3. Percutaneous Pulmonary Valve Placement
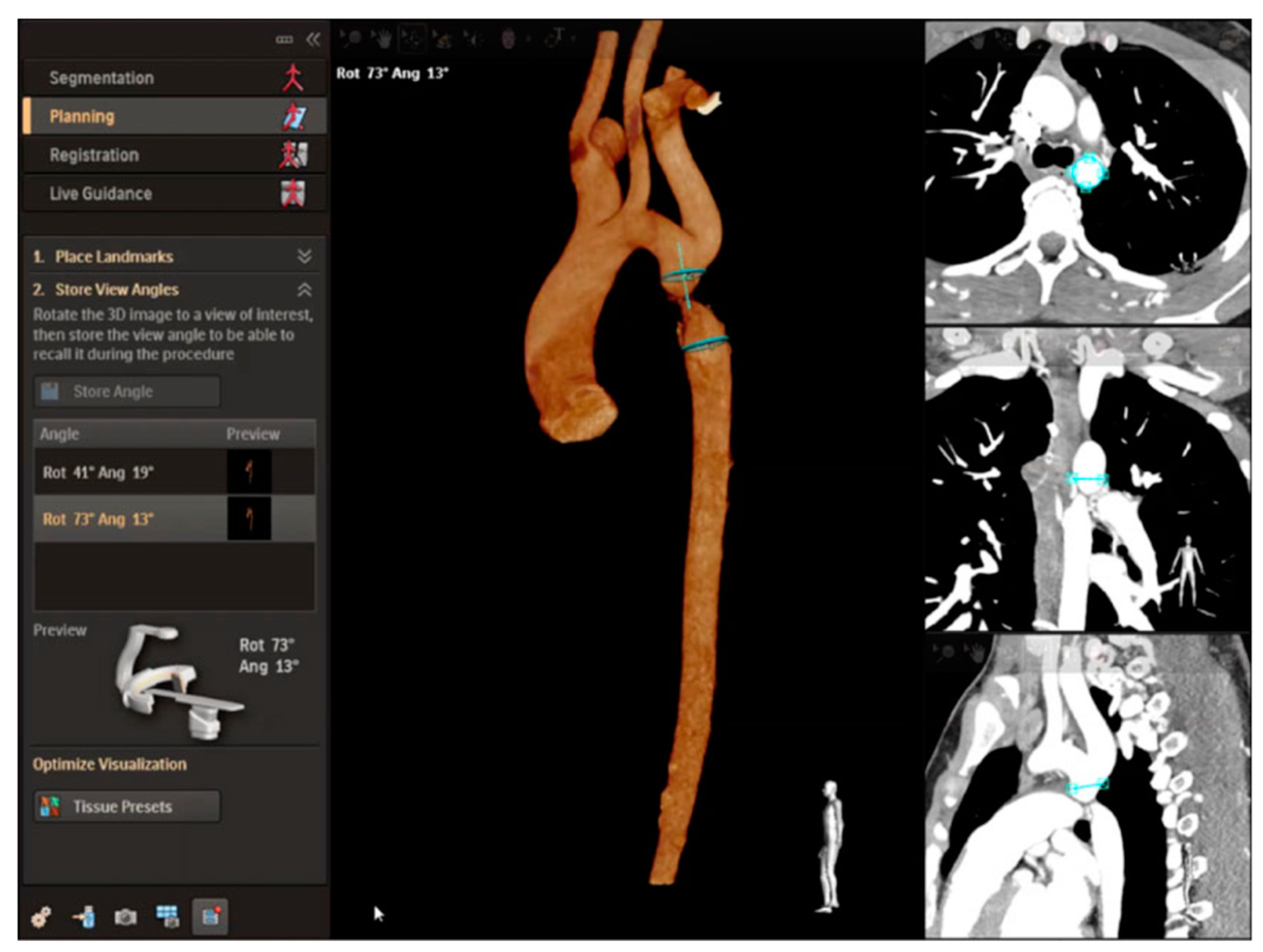
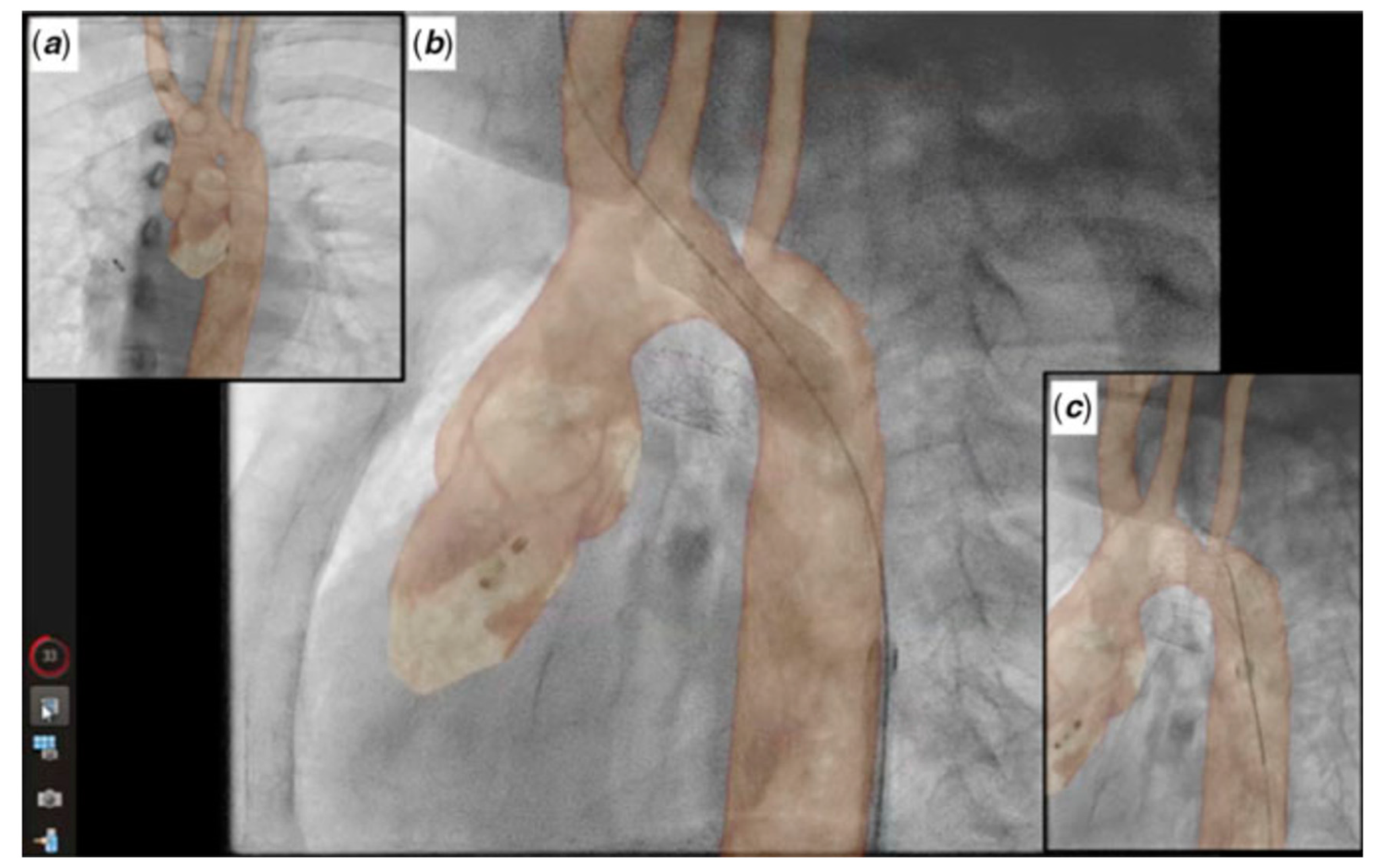
2.4. Cross-Sectional Overlay Fusion in the Catheterization Lab
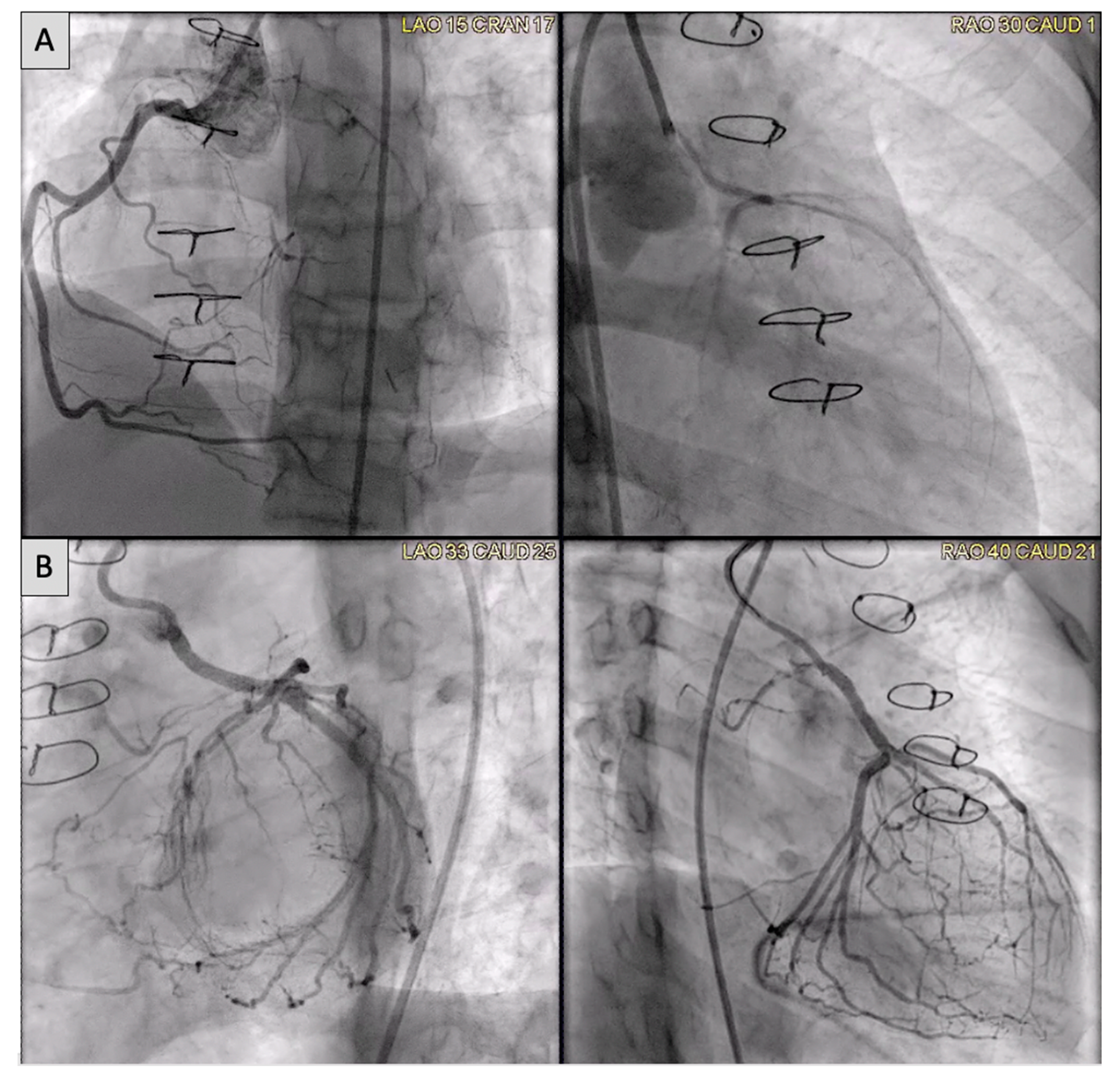
2.5. Coronary Artery Assessment in CHD
3. Future Directions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Congenital heart disease | CHD |
| Computed tomography | CT |
| Cardiac magnetic resonance imaging | CMR |
| Sinus venosus atrial septal defect | SVASD |
| Superior vena cava | SVC |
| Right upper pulmonary vein | RUPV |
| Left atrium | LA |
| Right atrium | RA |
| 3 dimensional | 3D |
| Aorta | Ao |
| Right middle pulmonary vein | RMPV |
| Anomalous pulmonary vein | aPV |
| Inferior vena cava | IVC |
| Patent ductus arteriosus | PDA |
| Drug eluting stent | DES |
| Left carotid artery | LCA |
| Left axillary artery | LAA |
| Left subclavian artery | LSCA |
| Food and drug administration | FDA |
| Landing zone | LZ |
| Pulmonary artery | PA |
| Left pulmonary artery | LPA |
| Coarctation of the aorta | CoA |
| Anomalous left coronary artery from the pulmonary artery | ALCAPA |
| Left main coronary artery | LMCA |
| Invasive CMR | iCMR |
| Left ventricle | LV |
| Right heart catheterization | RHC |
| Left heart catheterization | LHC |
| Fontan Fenestration Test Occlusion | FFTO |
| Fontan fenestration device closure | FFDC |
References
- Naqvi, N.; McCarthy, K.P.; Ho, S.Y. Anatomy of the atrial septum and interatrial communications. J. Thorac. Dis. 2018, 10, S2837–S2847. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Al Zaghal, A.M.; Anderson, R.H. The nature of the superior sinus venosus defect. Clin. Anat. 1998, 11, 349–352. [Google Scholar] [CrossRef]
- Van Praagh, S.; Geva, T.; Lock, J.E.; Nido, P.J.; Vance, M.S.; Van Praagh, R. Biatrial or left atrial drainage of the right superior vena cava: Anatomic, morphogenetic, and surgical considerations—Report of three new cases and literature review. Pediatr. Cardiol. 2003, 24, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.H.; Duong, P.; Jivanji, S.G.; Jones, M.; Kabir, S.; Butera, G.; Qureshi, S.A.; Rosenthal, E. Transcatheter Correction of Superior Sinus Venosus Atrial Septal Defects as an Alternative to Surgical Treatment. J. Am. Coll. Cardiol. 2020, 75, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Burkhardt, B.E.; Batsis, M.; Zellers, T.M.; Forte, M.N.V.; Valverde, I.; McMahan, R.; Guleserian, K.J.; Greil, G.F.; Hussain, T. Sinus Venosus Defects: Anatomic Variants and Transcatheter Closure Feasibility Using Virtual Reality Planning. JACC Cardiovasc. Imaging 2018, 12, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.L.; Rothman, M.T.; Rees, M.R.; Parsons, J.M.; Blackburn, M.E.; Ruiz, C.E. Stenting of the arterial duct: A new approach to palliation for pulmonary atresia. Heart 1992, 67, 240–245. [Google Scholar] [CrossRef]
- Chamberlain, R.C.; Ezekian, J.E.; Sturgeon, G.M.; Barker, P.C.; Hill, K.D.; Fleming, G.A. Preprocedural three-dimensional planning aids in transcatheter ductal stent placement: A single-center experience. Catheter. Cardiovasc. Interv. 2019, 95, 1141–1148. [Google Scholar] [CrossRef]
- Qureshi, A.M.; Goldstein, B.H.; Glatz, A.C.; Agrawal, H.; Aggarwal, V.; Ligon, R.A.; McCracken, C.; McDonnell, A.; Bs, T.M.B.; Whiteside, W.; et al. Classification scheme for ductal morphology in cyanotic patients with ductal dependent pulmonary blood flow and association with outcomes of patent ductus arteriosus stenting. Catheter. Cardiovasc. Interv. 2018, 93, 933–943. [Google Scholar] [CrossRef]
- Arar, Y.; Dimas, V.V.; Nugent, A.W.; Hussain, T.; Kasraie, N.; Reddy, S.R.V.; Zellers, T.M.; Herbert, C. Pre-procedural CT imaging aids neonatal PDA stenting for ductal-dependent pulmonary blood flow with reduction in overall procedural morbidity. Cardiol. Young 2021, 1–6. [Google Scholar] [CrossRef]
- Goreczny, S.; Dryzek, P.; Morgan, G.J.; Lukaszewski, M.; Moll, J.; Moszura, T. Novel Three-Dimensional Image Fusion Software to Facilitate Guidance of Complex Cardiac Catheterization. Pediatr. Cardiol. 2017, 38, 1133–1142. [Google Scholar] [CrossRef]
- Goreczny, S.; Dryzek, P.; Moszura, T.; Kühne, T.; Berger, F.; Schubert, S. 3Dimage fusion for live guidance of stent implantation in aortic coarctation-mag-netic resonance imaging and computed tomography image overlay enhances interventional technique. Postepy Kardiol. Interwencyjnej 2017, 13, 269–272. [Google Scholar]
- Arar, Y.; Reddy, S.R.V.; Kim, H.; Dimas, V.V.; Zellers, T.M.; Zahr, R.A.; Vamsee, R.; Greer, J.S.; Tandon, A.; Pontiki, A.; et al. 3D advanced imaging overlay with rapid registration in CHD to reduce radiation and assist cardiac catheterisation interventions. Cardiol. Young 2020, 30, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Vamsee, R.; Reddy, S.; Dimas, V.; Arar, Y.; Pontiki, A.; Hussain, M. Biplane 3D Overlay Guidance for Congenital Heart Disease to Assist Cardiac Catheterization Interventions—A Pilot Study. J. Cardiovasc. Comput. Tomogr. 2021, 15, S13. [Google Scholar] [CrossRef]
- Duran, S.R.; Huffaker, T.; Dixon, B.; Gooty, V.; Zahr, R.A.; Arar, Y.; Greer, J.S.; Butts, R.J.; Hussain, M.T. Feasibility and safety of quantitative adenosine stress perfusion cardiac magnetic resonance imaging in pediatric heart transplant patients with and without coronary allograft vasculopathy. Pediatr. Radiol. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gooty, V.D.; Dillenbeck, J.; Arar, Y.; Castellanos, D.A.; Greil, G.F.; Hernandez, N.B.; Kirk, R.; Butts, R.; Hussain, T. Coronary CT Angiography in Pediatric and Young Adult Coronary Allograft Vasculopathy: Single-Center Experience. JACC Cardiovasc. Imaging 2020, 14, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Veeram Reddy, S.R.; Arar, Y.; Zahr, R.A.; Gooty, V.; Hernandez, J.; Potersnak, A.; Douglas, P.; Blair, Z.; Greer, J.S.; Roujol, S.; et al. Invasive cardiovascular magnetic resonance (iCMR) for diagnostic right and left heart catheterization using an MR-conditional guidewire and passive visualization in congenital heart disease. J. Cardiovasc. Magn. Reson. 2020, 22, 20. [Google Scholar] [CrossRef]
- Ratnayaka, K.; Faranesh, A.Z.; Guttman, M.A.; Kocaturk, O.; Saikus, C.E.; Lederman, R.J. Interventional cardiovascular magnetic resonance: Still tantalizing. J. Cardiovasc. Magn. Reson. 2008, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Pushparajah, K.; Chubb, H.; Razavi, R. MR-guided Cardiac Interventions. Top. Magn. Reson. Imaging 2018, 27, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Razavi, R.; Hill, D.L.; Keevil, S.F.; Miquel, M.E.; Muthurangu, V.; Hegde, S.; Rhode, K.; Barnett, M.; van Vaals, J.; Hawkes, D.J.; et al. Cardiac catheterization guided by MRI in children and adults with congenital heart disease. Lancet 2003, 362, 1877–1882. [Google Scholar] [CrossRef]
- Campbell-Washburn, A.E.; Rogers, T.; Stine, A.M.; Khan, J.M.; Ramasawmy, R.; Schenke, W.H.; McGuirt, D.R.; Mazal, J.R.; Grant, L.P.; Grant, E.K.; et al. Right heart catheterization using metallic guidewires and low SAR cardiovascular magnetic resonance fluoroscopy at 1.5 Tesla: First in human experience. J. Cardiovasc. Magn. Reson. 2018, 20, 41. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.; Liu, A.; Gurvitz, M.; Guo, L.; Therrien, J.; Laprise, C.; Kaufman, J.S.; Abrahamowicz, M.; Marelli, A.J. Exposure to Low-Dose Ionizing Radiation from Cardiac Procedures and Malignancy Risk in Adults With Congenital Heart Disease. Circulation 2018, 137, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Arar, Y.; Hussain, T.; Greil, G.; Zabala, L.; Das, B. Interventional Cardiovascular Magnetic Resonance Imaging (iCMR) in an Adolescent with Pulmonary Hypertension. Medicina 2020, 56, 636. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.N.V.; Roujol, S.; Ruijsink, B.; Valverde, I.; Duong, P.; Byrne, N.; Krueger, S.; Weiss, S.; Arar, Y.; Reddy, S.R.V.; et al. MRI for Guided Right and Left Heart Cardiac Catheterization: A Prospective Study in Congenital Heart Disease. J. Magn. Reson. Imaging 2020, 53, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Arar, Y.; Hussain, T.; Zahr, R.A.; Gooty, V.; Greer, J.S.; Huang, R.; Hernandez, J.; King, J.; Greil, G.; Reddy, S.R.V. Fick versus flow: A real-time invasive cardiovascular magnetic resonance (iCMR) reproducibility study. J. Cardiovasc. Magn. Reson. 2021, 23, 95. [Google Scholar] [CrossRef]
- Gooty, V.D.; Reddy, S.R.V.; Greer, J.S.; Blair, Z.; Zahr, R.A.; Arar, Y.; Castellanos, D.A.; Pimplawar, S.; Greil, G.F.; Dillenbeck, J.; et al. Lymphatic pathway evaluation in congenital heart disease using 3D whole-heart balanced steady state free precession and T2-weighted cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2021, 23, 16. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arar, Y.; Divekar, A.; Clark, S.; Hussain, T.; Sebastian, R.; Hoda, M.; King, J.; Zellers, T.M.; Reddy, S.R.V. Role of Cross-Sectional Imaging in Pediatric Interventional Cardiac Catheterization. Children 2022, 9, 300. https://doi.org/10.3390/children9030300
Arar Y, Divekar A, Clark S, Hussain T, Sebastian R, Hoda M, King J, Zellers TM, Reddy SRV. Role of Cross-Sectional Imaging in Pediatric Interventional Cardiac Catheterization. Children. 2022; 9(3):300. https://doi.org/10.3390/children9030300
Chicago/Turabian StyleArar, Yousef, Abhay Divekar, Stephen Clark, Tarique Hussain, Roby Sebastian, Mehar Hoda, Jamie King, Thomas M. Zellers, and Surendranath R. Veeram Reddy. 2022. "Role of Cross-Sectional Imaging in Pediatric Interventional Cardiac Catheterization" Children 9, no. 3: 300. https://doi.org/10.3390/children9030300
APA StyleArar, Y., Divekar, A., Clark, S., Hussain, T., Sebastian, R., Hoda, M., King, J., Zellers, T. M., & Reddy, S. R. V. (2022). Role of Cross-Sectional Imaging in Pediatric Interventional Cardiac Catheterization. Children, 9(3), 300. https://doi.org/10.3390/children9030300





