Neurodevelopmental Outcomes in Tetralogy of Fallot: A Systematic Review
Abstract
:1. Introduction
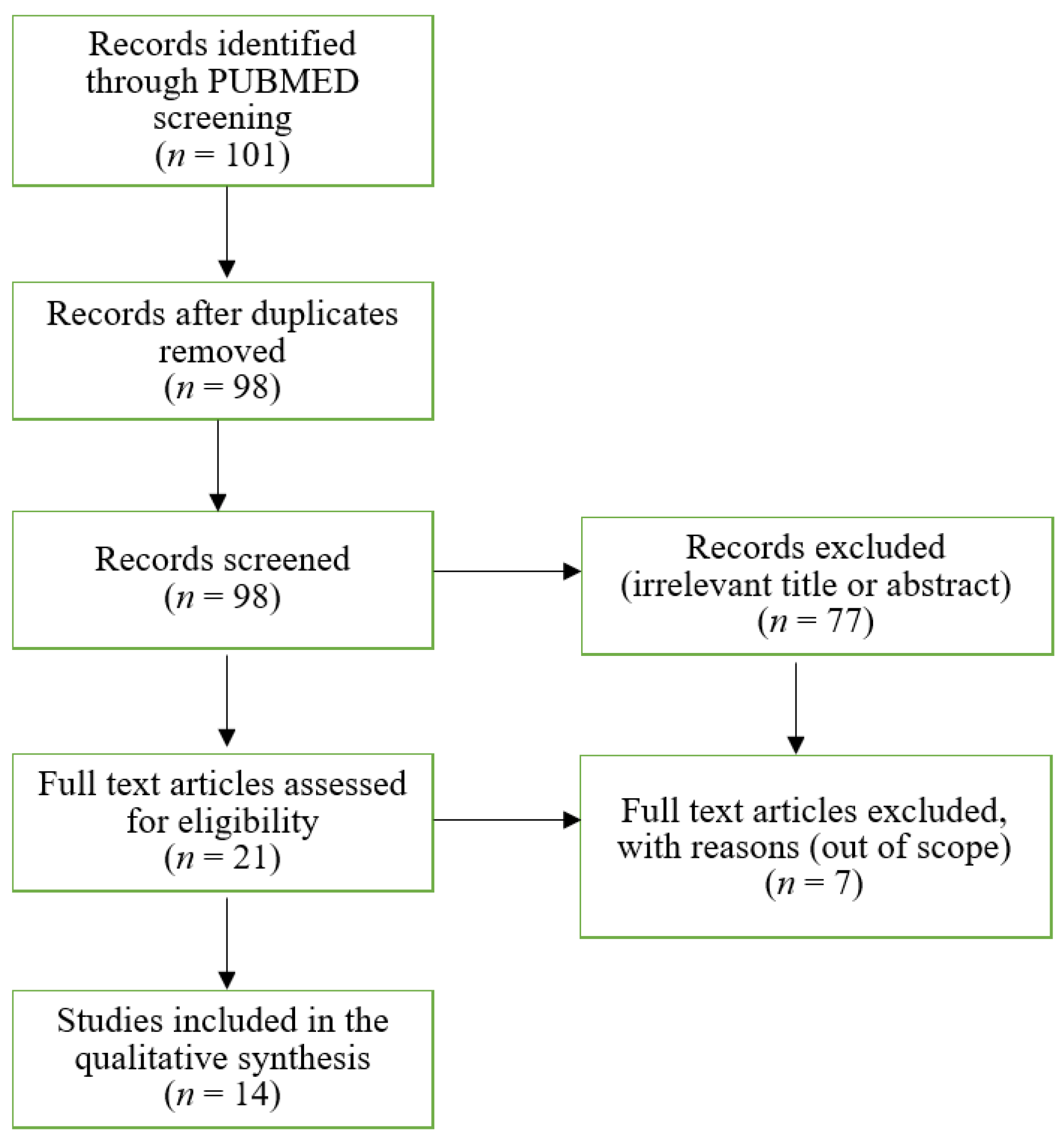
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
3. Results
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
Appendix A
| Study | Incident Cases | Age | Populations’ Characteristics | Definition/Features of TOF Patients | Definition/Assessment Method of Neurodevelopment | Imaging Evaluation Methods | Outcome |
|---|---|---|---|---|---|---|---|
| Miatton (2006) | 18 | 8.3 years | TOF patients operated on at Ghent University Hospital between 1995 and 1999 | Inclusion criteria: birth weight of greater than 2000 g. Exclusion criteria: perinatal problems, noncardiac malformations, genetic abnormalities (Down syndrome, velocar-diofacial syndrome, and Di George syndrome) | Wechsler Intelligence Scale for Children, 3rd edition, Dutch version, neuropsychological assessment battery NEPSY, Child Behavior Checklist (CBCL) | - | -Lower scores on the Full Scale IQ (p < 0.05), on the NEPSY domains Language (p < 0.01), Sensorimotor Functioning (p < 0.01) and on the subtests Tower (p < 0.05), Memory for Names (p < 0.05), Narrative Memory (p < 0.05), and Design Copy (p < 0.05) -Significantly higher scores on attention problems (p < 0.05) and the total problem scale (p < 0.05) by Parental reports -Significantly lower school performances (p < 0.01) |
| Hοvels-Gurich (2006) | 20 | 7.4 years | Patients with regular form of TOF (without Pulmonary Atresia) | Eligibility criteria: good general and cardiac health condition and normal chromosomal status (46, XY and XX, respectively), primary corrective cardiac surgery at a mean age of 0.7 ± 0.3 years, following standardized DHCA and combined low-flow CPB. Exclusion criteria: microdeletion 22q11.2, familial accumulation with respect to TOF, additional somatic malformation, phenotypic suspicion of syndrome, cardiac-related medication | Kiphard and Schilling Body Coordination Test, Kaufman Assessment Battery for Children (K-ABC), Bruce walking treadmill protocol | - | Compared with the normal population, motor function, formal intelligence, academic achievement, and expressive and receptive language were significantly reduced (p < 0.01 to p < 0.001) in the TOF group. |
| Hοvels-Gurich (2007) | 20 | 7.4 years | Patients with regular form of TOF (without Pulmonary Atresia) | Eligibility criteria: good general and cardiac health condition and normal chromosomal status (46, XY and XX, respectively), primary corrective cardiac surgery at a mean age of 0.7 ± 0.3 years, following standardized DHCA and combined low-flow CPB. Exclusion criteria: microdeletion 22q11.2, familial accumulation with respect to TOF, additional somatic malformation, phenotypic suspicion of syndrome, cardiac-related medication | Attention Network Test, performance measures of three networks of attention: alerting, orienting, and executive attention functions (conflict). Child Behavior Checklist. | - | Conflict performance was significantly reduced in the TOF group (p = 0.005), alerting and orienting were found normal. |
| Hοvels-Gurich (2007) | 20 | 7.4 years | Patients with regular form of TOF (without Pulmonary Atresia) | Eligibility criteria: good general and cardiac health condition and normal chromosomal status (46, XY and XX, respectively), primary corrective cardiac surgery at a mean age of 0.7 ± 0.3 years, following standardized DHCA and combined low-flow CPB. Exclusion criteria: microdeletion 22q11.2, familial accumulation with respect to TOF, additional somatic malformation, phenotypic suspicion of syndrome, cardiac-related medication | Child Behavior Checklist (CBCL), KINDL | - | -Elevated Internalizing and externalizing problems -Reduced school performance and total competence -Normal self- and parent-reported quality of life |
| Hοvels-Gurich (2008) | 19 | 7.4 years | Patients with regular form of TOF (without Pulmonary Atresia) | Eligibility criteria: good general and cardiac health condition and normal chromosomal status (46, XY and XX, respectively), primary corrective cardiac surgery at a mean age of 0.7 ± 0.3 years, following standardized DHCA and combined low-flow CPB. Exclusion criteria: microdeletion 22q11.2, familial accumulation with respect to TOF, additional somatic malformation, phenotypic suspicion of syndrome, cardiac-related medication | Oral and Speech Motor Control Protocol, Mayo Test of Speech and Oral Apraxia (Children’s Battery), Auditory Closure (AC) subtest of the Illinois Test of Psycholinguistic Abilities (ITPA), Test of Auditory Analysis Skills (TAAS) | - | -Total scores on oral and speech motor control functions (TFS) as well as on oral and speech apraxia (Mayo Test) were significantly reduced (p < 0.02 to <0.05), and scores on anatomical oral structures tended to be lower (p ≤ 0.09) in the TOF group. -No differences were found for auditory word recognition and phonological awareness |
| Zeltser (2008) | 60 | 1 year | Infants with TOF | Inclusion criteria: TOF/pulmonary stenosis or TOF/pulmonary atresia undergoing complete biventricular repair by the age of 6 months. Exclusion criteria: TOF/absent pulmonary valve and TOF/pulmonary atresia with multiple aortopulmonary collaterals, major recognizable congenital defects, recognizable genetic or phenotypic syndrome other than chromosome 22q11 microdeletions, and language other than English spoken | Bayley Scales of Infant Development-II | - | The mean MDI was 89 ± 13, and the mean PDI was 81 ± 17. Scores for the MDI (76 ± 13 vs. 92 ± 11) and PDI (63 ± 13 vs. 85 ± 15) were significantly lower for patients with genetic syndromes |
| Gaynor (2010) | 44 | 4 years | Preschool patients after cardiac surgery in infancy | Eligibility criteria: Patients 6 months of age or younger undergoing surgery for CHD using CPB with or without DHCA. Exclusion criteria: Multiple congenital anomalies recognizable genetic or phenotypic syndrome, language other than English | Wechsler Preschool and Primary Scale of Intelligence 3rd Edition (WPPSI-III), Preschool Language Scale-4 Total Language Score (PLS-4TLS), Neuro-Psychology Statue Test, Visual Motor Integration, Goldman Fristoe Test of Articulation 2 (GFTA), Woodcock Johnson 3, Wide Range Assessment of Visual Motor Abilities pegboard | - | -The mean scores for the neurodevelopmental outcomes domains tested were in the normal range for preschool children -Significant impairments in at least 1 domain were identified in 20% (8/41) with TOF |
| Bellinger (2014) | 91 | 13–16 years | TOF patients | Admission criteria: TOF with or without pulmonary atresia, and an interval of at least 3 months between the last cardiac surgery and neurodevelopmental testing. Exclusion criteria: patients with disorders that would prevent completion of the study assessments – metal implants – trisomy 21, or lack of reading fluency by the primary caregiver. | Wechsler Intelligence, Scale for Children-4rd Edition, Wechsler Individual Achievement Test-Second Edition, General Memory Index of the Children’s Memory Scale, Delis–Kaplan Executive Function System, Behavior Rating Inventory of Executive Function, General Executive Composite score, Test of Visual-Perceptual Skills (non-motor) (Upper Level)-Revised, Rey-Osterrieth Complex Figure, Sense of Directions Scale, Attention Deficit Hyperactivity Disorder Index score, Eyes Test-Revised, Autism Spectrum Quotient, Toronto Alexithymia Scale | Brain MRI | TOF patients both with or without genetic abnormalities or syndrome performed significantly worse than the referent group or population norms in all of the neuro-psychological domains assessed. Patients with genetic syndrome had markedly greater neuropsychological morbidities than did patients without a syndrome. |
| Cassidy (2015) | 68 | 13–16 years | TOF patients | Eligibility criteria: TOF patients with or without pulmonary atresia, who underwent surgical repair at least 6 months before assessment. Exclusion criteria: identified genetic/syndromic conditions, diagnosis of trisomy 21 and/or presence of a disorder/device contraindicated for MRI. | Delis-Kaplan Executive Function System (D-KEFS), questionnaire data from the Behavior Rating Inventory of Executive Function (BRIEF) | - | -Visuo-spatially mediated executive function abilities were impaired in TOF (p < 0.001) |
| Jaworski (2017) | 68 | 13–16 years | TOF patients | Eligibility criteria: TOF patients with or without pulmonary atresia, who underwent surgical repair at least 6 months before assessment, Exclusion criteria: identified genetic/syndromic conditions (e.g., 22q11.2 deletion syndrome) | Wechsler Individual Achievement Test (WIAT), Rey-Osterrieth Complex Figure | - | -Greater odds of receiving lower scores in all trials of Structural Accuracy and in the Copy trial of Incidental Accuracy (p ≤ 0.5) |
| Cassidy (2017) | 68 | 13–16 years | TOF patients | Eligibility criteria: TOF patients with or without pulmonary atresia, who underwent surgical repair at least 6 months before assessment, Exclusion criteria: identified genetic/syndromic conditions (e.g., 22q11.2 deletion syndrome) | Schedule for Affective Disorders and Schizophrenia for School-Aged Children—Present and Lifetime Version (K-SADS-PL), Children’s Memory Scale | - | -Same level as the normal population (standard mean [SM] 5 100, SD 5 15) on the measures of intelligence and academic attainments -Below expected population means on most CMS variables, except for the Sequences and Stories: Immediate subtests -Immediate and delayed visual- spatial memory deficits |
| Holland (2017) | 91 | 13–16 years | TOF patients | Eligibility criteria: TOF patients with or without pulmonary atresia, Exclusion criteria: patients with disorders that would prevent completion of the study assessments—metal implants—trisomy 21, or lack of reading fluency by the primary caregiver | Schedule for Affective Disorders and Schizophrenia for School-Aged Children—Present and Lifetime Version (K-SADS-PL), Children’s Global Assessment Scale (CGAS) and the Brief Psychiatric Rating Scale for Children (BPRS-C), Revised Children’s Manifest Anxiety Scale (RCMAS), Child Stress Disorders Checklist, Conners ADHD Rating Scales, Conduct Disorder Scale, Children’s Depression Inventory | Brain MRI | -TOF with a genetic diagnosis showed an increased lifetime prevalence of anxiety disorder (43%) and lower global psychosocial functioning compared with TOF without genetic diagnosis (p = 0.04 and <0.001, respectively) and referents (p = 0.001 and <0.001, respectively). -TOF with and without a genetic diagnosis had a higher lifetime prevalence of attention deficit- hyperactivity disorder (ADHD) than referents (p = 0.04 and 0.002, respectively) and worse outcomes on parent-/self-report ratings of anxiety and disruptive behavior compared with referents |
| Holst (2020) | 52 | 13.3 years | TOF patients | Inclusion criteria: surgically corrected TOF in Denmark, 10–16 years, Danish- speaking parents. Exclusion criteria: presence of syndromes, genetic, or neurological abnormalities or other chronic diseases | Attention-Deficit/Hyperactivity Disorder-Rating Scale (parent and school teacher), Pediatric Quality of Life (PedsQLTM) version 4.0 | - | -The rate of scores of TOF children in the clinical range of inattention was almost 6 times more frequent than in the controls -High hyperactivity/impulsivity scores were absent -Significantly higher total attention-deficit/hyperactivity disorder mean score than the control group (p = 0.004) |
| Favilla (2021) | 49 | 1 year | Cardiac Kids Developmental Follow-up Program (CKDP) between 2012 and 2018 | Inclusion criteria: TOF repair between 0 and 12 months of age and neurodevelopment evaluation performed between 0 and 4 years of age at the CKDP. Patients with genetic syndromes were included | Bayley Infant Neurodevelopmental Screener (BINS), Peabody Developmental Motor Scale (PDMS), and Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) | - | -Deficits in at least one domain on the BINS in 43% of patients -Gross motor deficits were the most common (n = 16), followed by deficits in receptive language, cognitive, and fine motor skills, which were seen in one-third of patients -The median composite scores on the PDMS and Bayley- III tests were lower than the normative population’s expected scores |
| Study | Sex (Male) | Gestational Age (Weeks) | APGAR Score (1st min) | Syndromic Patients | Pulmonary Atresia | Age at First Operation | Duration of CPB (min) | Use of DHCA | Total Cardiac Surgeries | Seizures |
|---|---|---|---|---|---|---|---|---|---|---|
| Miatton (2006) | 10 | N/M | <7:33% 1 min | 0% | N/M | 173 days | 89.2 ± 31.9 | 0% | N/M | N/M |
| Hοvels-Gurich (2006, 2007, 2008) | N/M | <37w: 10% | <7: 5% 5 min | 0% | 0% | 0.7 ± 0.3 months | 63.0 ± 20.4 | 100% | N/M | 0 |
| Zeltser (2008) | 31 | 38.0 ± 3.0 | 8 ± 1.44 1 min 8.79 ± 0.55 5 min | 18% | 5% | 2.66 ± 1.4 months | 56.3 ± 19.7 | 37% | Single: 98% | N/M |
| Gaynor (2010) | 21 | 35.5 ± 1.9 | N/M | 0% | N/M | 90.7 ± 50.8 days | 59.5 ± 21.1 | 23% | Additional No.: 0.11 ± 0.4 | N/M |
| Bellinger (2014) | 55 | 39.2 ± 2.3 | N/M | 25% | 29% | 112 days | N/M | N/M | ⩾2: >50% | 20 |
| Cassidy (2015, 2017) | 38 | 39.17 ± 2.49 | 7.68 ± 1.73 1 min 8.83 ± 0.65 5 min | 0% | Included % N/M | 185.56 days | N/M | N/M | 1–7 | 7 |
| Jaworski (2017) | 38 | 39.2 ± 2.5 | N/M | 0% | N/M | 113 days | N/M | N/M | 1–7 | N/M |
| Holland (2017) | 38 12 (G/D) | 39.2 ± 2.5 39.1 ± 1.8 (G/D) | N/M | 25% | 28% 7% (G/D) | N/M | N/M | 100% | Single 33% 11% (G/D) | 7 4 (G/D) |
| Holst (2020) | 33 | 38.1 ± 3.0 | N/M | 0% | N/M | N/M | N/M | N/M | N/M | N/M |
| Favilla (2021) | 33 | 38 | N/M | 41% | 20% | 90 days | 67 min | N/M | N/M | 1 |
References
- Reller, M.D.; Strickland, M.J.; Riehle-Colarusso, T.; Mahle, W.T.; Correa, A. Prevalence of Congenital Heart Defects in Metropolitan Atlanta, 1998–2005. J. Pediatrics 2008, 153, 807–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.S.; Lee, J.R.; Lim, H.G.; Kim, W.H.; Kim, Y.J. The long-term result of total repair for tetralogy of Fallot. Eur. J. Cardio-Thorac. Surg. 2010, 38, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Limperopoulos, C.; Majnemer, A.; Shevell, M.I.; Rosenblatt, B.; Rohlicek, C.; Tchervenkov, C. Neurologic status of newborns with congenital heart defects before open heart surgery. J. Pediatr. 1999, 103, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mahle, W.T.; Wernovsky, G. Cardiovascular disease in the neonate long-tem developmental outcome of children with complex congenital heart disease. Clin. Perinatol. 2001, 28, 235–247. [Google Scholar] [CrossRef]
- Newburger, J.W.; Jonas, R.A.; Wernovsky, G.; Wypij, D.; Hickey, P.R.; Kuban, K.; Farrell, D.M.; Holmes, G.L.; Helmers, S.L.; Constantinou, J.; et al. A Comparison of the Perioperative Neurologic Effects of Hypothermic Circulatory Arrest versus Low-Flow Cardiopulmonary Bypass in Infant Heart Surgery. N. Engl. J. Med. 1993, 329, 1057–1064. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Wypij, D.; du Plessis, A.J.; Rappaport, L.A.; Riviello, J.; Jonas, R.A.; Newburger, J.W. Developmental and neurologic effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J. Thorac. Cardiovasc. Surg. 2001, 121, 374–383. [Google Scholar] [CrossRef] [Green Version]
- Bellinger, D.C.; Wypij, D.; Kuban, K.; Rappaport, L.A.; Hickey, P.R.; Wernovsky, G.; Jonas, R.A.; Newburger, J.W. Developmental and Neurological Status of Children at 4 Years of Age After Heart Surgery with Hypothermic Circulatory Arrest or Low-Flow Cardiopulmonary Bypass. J. Circ. 1999, 100, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C.; Wypij, D.; Duplessis, A.J.; Rappaport, L.A.; Jonas, R.A.; Wernovsky, G.; Newburger, J.W. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. J. Thorac. Cardiovasc. Surg. 2003, 126, 1385–1396. [Google Scholar] [CrossRef] [Green Version]
- Michielon, G.; Marino, B.; Formigari, R.; Gargiulo, G.; Picchio, F.; Digilio, M.C.; Anaclerio, S.; Oricchio, G.; Sanders, S.; Di Donato, R.M. Genetic Syndromes and Outcome After Surgical Correction of Tetralogy of Fallot. Ann. Thorac. Surg. 2006, 81, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Goldmuntz, E.; Clark, B.J.; Mitchell, L.E.; Jawad, A.F.; Cuneo, B.F.; Reed, L.; McDonald-McGinn, D.; Chien, P.; Feuer, J.; Zackai, E.H.; et al. Frequency of 22q11 deletions in patients with conotruncal defects. J. Am. Coll. Cardiol. 1998, 32, 492–498. [Google Scholar] [CrossRef] [Green Version]
- McElhinney, D.B.; Krantz, I.D.; Bason, L.; Piccoli, D.A.; Emerick, K.M.; Spinner, N.B.; Goldmuntz, E. Analysis of Cardiovascular Phenotype and Genotype-Phenotype Correlation in Individuals with a JAG1 Mutation and/or Alagille Syndrome. J. Circ. 2002, 106, 2567–2574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldmuntz, E. THE EPIDEMIOLOGY AND GENETICS OF CONGENITAL HEART DISEASE. Clin. Perinatol. 2001, 28, 1–10. [Google Scholar] [CrossRef]
- Marino, B.S.; Lipkin, P.; Newburger, J.W.; Peacock, G.; Gerdes, M.; Gaynor, J.W.; Mussatto, K.A.; Uzark, K.; Goldberg, C.S.; Johnsonjr, W.H.; et al. Neurodevelopmental Outcomes in Children with Congenital Heart Disease: Evaluation and Management: A scientific statement from the american heart association. J. Circ. 2012, 126, 1143–1172. [Google Scholar] [CrossRef] [Green Version]
- Laskowitz, D.T.; Horsburgh, T.; Roses, A.D. Apolipoprotein E and the eNS Response to Injury. J. Cereb. Blood Flow Metab. 1998, 18, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiefermeier, M.; Kollegger, H.; Madl, C.; Schwarz, C.; Holzer, M.; Kofler, J.; Sterz, F. Apolipoprotein E Polymorphism Survival and Neurological Outcome After Cardiopulmonary Resusci-tation. Stroke 2000, 31, 2068–2073. [Google Scholar] [CrossRef]
- Favilla, E.; Faerber, J.A.; Hampton, L.E.; Tam, V.; DeCost, G.; Ravishankar, C.; Gaynor, J.W.; Burnham, A.; Licht, D.J.; Mercer-Rosa, L. Early Evaluation and the Effect of Socioeconomic Factors on Neurodevelopment in Infants with Tetralogy of Fallot. Pediatr. Cardiol. 2021, 42, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Zeltser, I.; Jarvik, G.P.; Bernbaum, J.; Wernovsky, G.; Nord, A.S.; Gerdes, M.; Zackai, E.; Clancy, R.; Nicolson, S.C.; Spray, T.L.; et al. Genetic factors are important determinants of neurodevelopmental outcome after repair of tetralogy of Fallot. J. Thorac. Cardiovasc. Surg. 2008, 135, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaynor, J.W.; Gerdes, M.; Nord, A.; Bernbaum, J.; Zackai, E.; Wernovsky, G.; Clancy, R.R.; Heagerty, P.J.; Solot, C.B.; McDonald-McGinn, D.; et al. Is cardiac diagnosis a predictor of neurodevelopmental outcome after cardiac surgery in infancy? J. Thorac. Cardiovasc. Surg. 2010, 140, 1230–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hövels-Gürich, H.H.; Bauer, S.B.; Schnitker, R.; Hinckeldey, K.W.-V.; Messmer, B.J.; Seghaye, M.-C.; Huber, W. Long-term outcome of speech and language in children after corrective surgery for cyanotic or acyanotic cardiac defects in infancy. Eur. J. Paediatr. Neurol. 2008, 12, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hövels-Gürich, H.H.; Konrad, K.; Skorzenski, D.; Herpertz-Dahlmann, B.; Messmer, B.J.; Seghaye, M.-C. Attentional Dysfunction in Children After Corrective Cardiac Surgery in Infancy. Ann. Thorac. Surg. 2007, 83, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Hövels-Gürich, H.H.; Konrad, K.; Skorzenski, D.; Nacken, C.; Minkenberg, R.; Messmer, B.J.; Seghaye, M.-C. Long-Term Neurodevelopmental Outcome and Exercise Capacity After Corrective Surgery for Tetralogy of Fallot or Ventricular Septal Defect in Infancy. Ann. Thorac. Surg. 2006, 81, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Hövels-Gürich, H.H.; Konrad, K.; Skorzenski, D.; Minkenberg, R.; Herpertz-Dahlmann, B.; Messmer, B.J.; Seghaye, M.-C. Long-Term Behavior and Quality of Life After Corrective Cardiac Surgery in Infancy for Tetralogy of Fallot or Ventricular Septal Defect. Pediatr. Cardiol. 2007, 28, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Miatton, M.; De Wolf, D.; François, K.; Thiery, E.; Vingerhoets, G. Intellectual, neuropsychological, and behavioral functioning in children with tetralogy of Fallot. J. Thorac. Cardiovasc. Surg. 2007, 133, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Bellinger, D.C.; Rivkin, M.J.; DeMaso, D.; Robertson, R.L.; Stopp, C.; Dunbar-Masterson, C.; Wypij, D.; Newburger, J.W. Adolescents with tetralogy of Fallot: Neuropsychological assessment and structural brain imaging. Cardiol. Young 2015, 25, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.R.; Newburger, J.W.; Bellinger, D.C. Learning and Memory in Adolescents with Critical Biventricular Congenital Heart Disease. J. Int. Neuropsychol. Soc. 2017, 23, 627–639. [Google Scholar] [CrossRef]
- Cassidy, A.R.; White, M.T.; DeMaso, D.R.; Newburger, J.W.; Bellinger, D.C. Executive Function in Children and Adolescents with Critical Cyanotic Congenital Heart Disease. J. Int. Neuropsychol. Soc. 2015, 21, 34–49. [Google Scholar] [CrossRef] [Green Version]
- Holland, J.E.; Cassidy, A.R.; Stopp, C.; White, M.T.; Bellinger, D.C.; Rivkin, M.J.; Newburger, J.W.; DeMaso, D.R. Psychiatric Disorders and Function in Adolescents with Tetralogy of Fallot. J. Pediatr. 2017, 187, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Bean Jaworski, J.L.; White, M.T.; DeMaso, D.R.; Newburger, J.W.; Bellinger, D.C.; Cassidy, A.R. Visuospatial processing in adolescents with critical congenital heart disease: Organization, integration, and implications for academic achievement. Child Neuropsychol. 2017, 24, 451–468. [Google Scholar] [CrossRef]
- Holst, L.M.; Kronborg, J.B.; Jepsen, J.R.M.; Christensen, J.Ø.; Vejlstrup, N.G.; Juul, K.; Bjerre, J.V.; Bilenberg, N.; Ravn, H.B. Attention-deficit/hyperactivity disorder symptoms in children with surgically corrected Ventricular Septal Defect, Transposition of the Great Arteries, and Tetralogy of Fallot. Cardiol. Young 2020, 30, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Carotti, A.; Albanese, S.; Filippelli, S.; Ravà, L.; Guccione, P.; Pongiglione, G.; Di Donato, R.M. Determinants of outcome after surgical treatment of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. J. Thorac. Cardiovasc. Surg. 2010, 140, 1092–1103. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, S.P.; Hanley, F.L. Surgical Management of Pulmonary Atresia with Ventricular Septal Defect and Major Aortopulmonary Collaterals: A Protocol-Based Approach. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2009, 12, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kordopati-Zilou, K.; Sergentanis, T.; Pervanidou, P.; Sofianou-Petraki, D.; Panoulis, K.; Vlahos, N.; Eleftheriades, M. Neurodevelopmental Outcomes in Tetralogy of Fallot: A Systematic Review. Children 2022, 9, 264. https://doi.org/10.3390/children9020264
Kordopati-Zilou K, Sergentanis T, Pervanidou P, Sofianou-Petraki D, Panoulis K, Vlahos N, Eleftheriades M. Neurodevelopmental Outcomes in Tetralogy of Fallot: A Systematic Review. Children. 2022; 9(2):264. https://doi.org/10.3390/children9020264
Chicago/Turabian StyleKordopati-Zilou, Kalliopi, Theodoros Sergentanis, Panagiota Pervanidou, Danai Sofianou-Petraki, Konstantinos Panoulis, Nikolaos Vlahos, and Makarios Eleftheriades. 2022. "Neurodevelopmental Outcomes in Tetralogy of Fallot: A Systematic Review" Children 9, no. 2: 264. https://doi.org/10.3390/children9020264
APA StyleKordopati-Zilou, K., Sergentanis, T., Pervanidou, P., Sofianou-Petraki, D., Panoulis, K., Vlahos, N., & Eleftheriades, M. (2022). Neurodevelopmental Outcomes in Tetralogy of Fallot: A Systematic Review. Children, 9(2), 264. https://doi.org/10.3390/children9020264








