Maternal Singing but Not Speech Enhances Vagal Activity in Preterm Infants during Hospitalization: Preliminary Results
Abstract
:1. Introduction
Early Vocal Contact (EVC) in the NICU
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Intervention
2.4. Main Outcome and Outcome Measures
2.5. Data Analysis
2.5.1. Photoplethysmographic (PPG) Signal Processing
2.5.2. Heart Rate Variability Feature Extraction
- -
- Low Frequency (LF) power and High Frequency (HF) power: the absolute power values;
- -
- LF power nu and HF power nu: the related values normalized to the sum of the LF power and HF power (LF power nu = LF power/(LF power + HF power) and HF power nu = HF power/(LF power + HF power));
- -
- LF/HF: the ratio between LF power and HF power.
2.5.3. Statistical Analysis
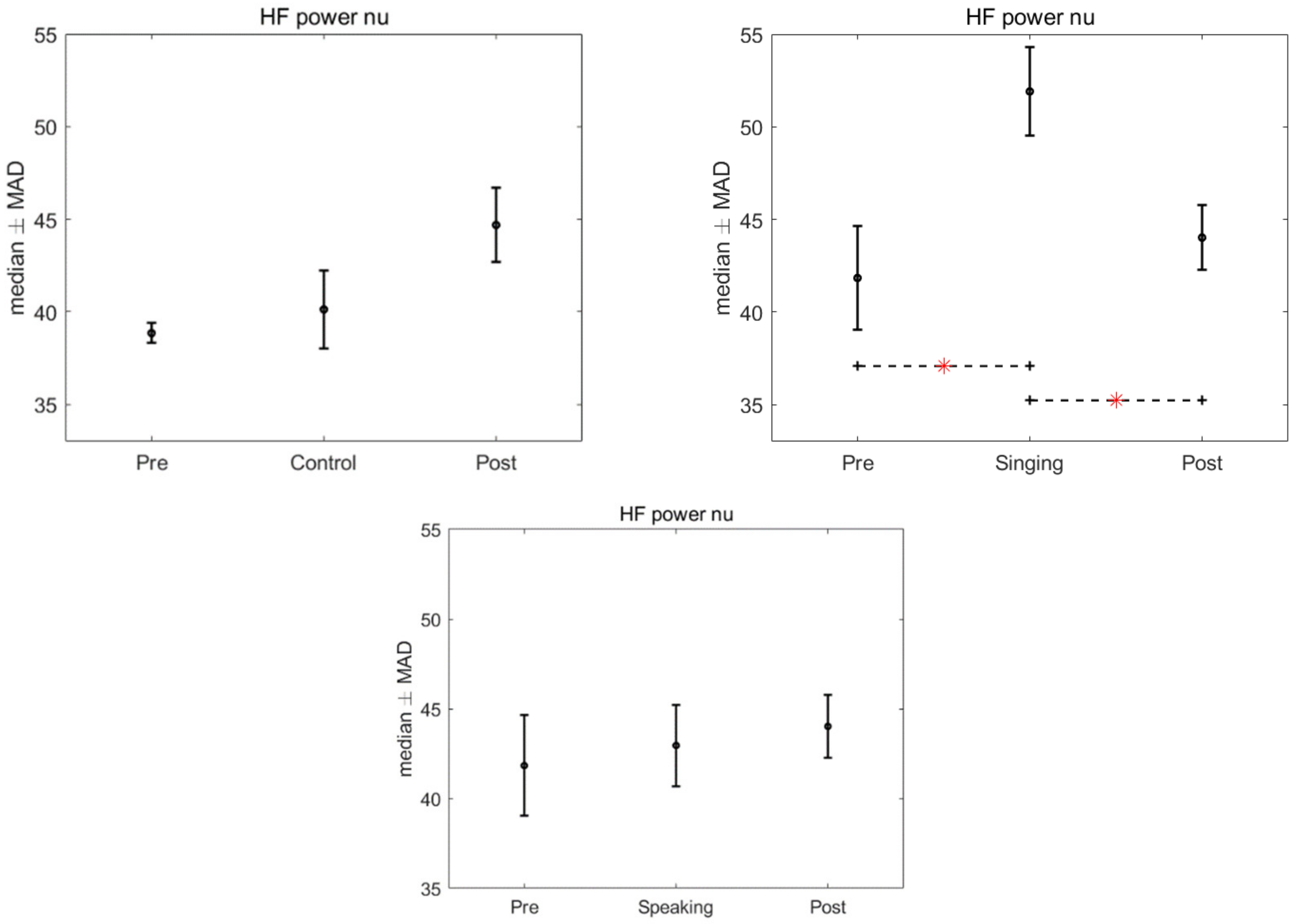
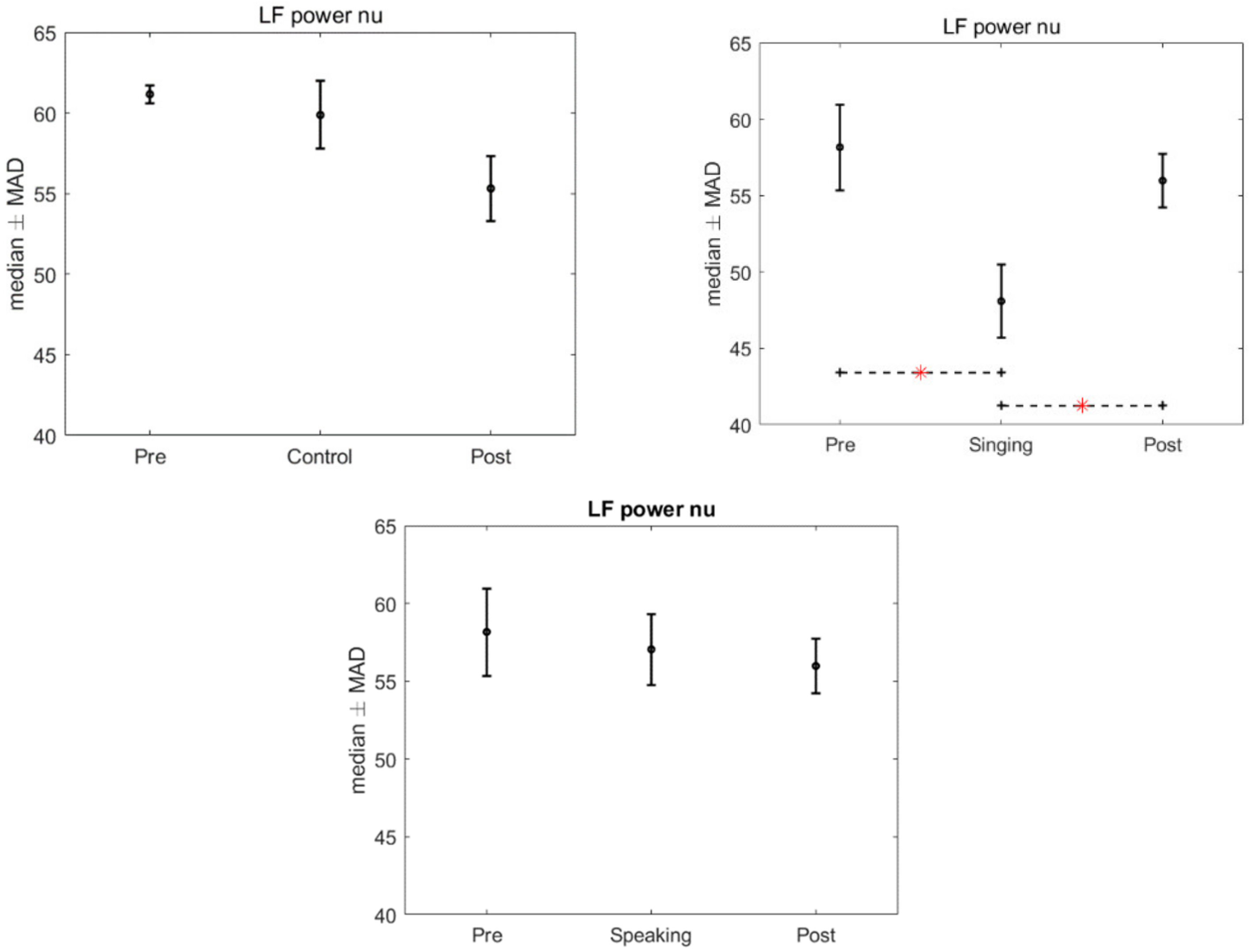
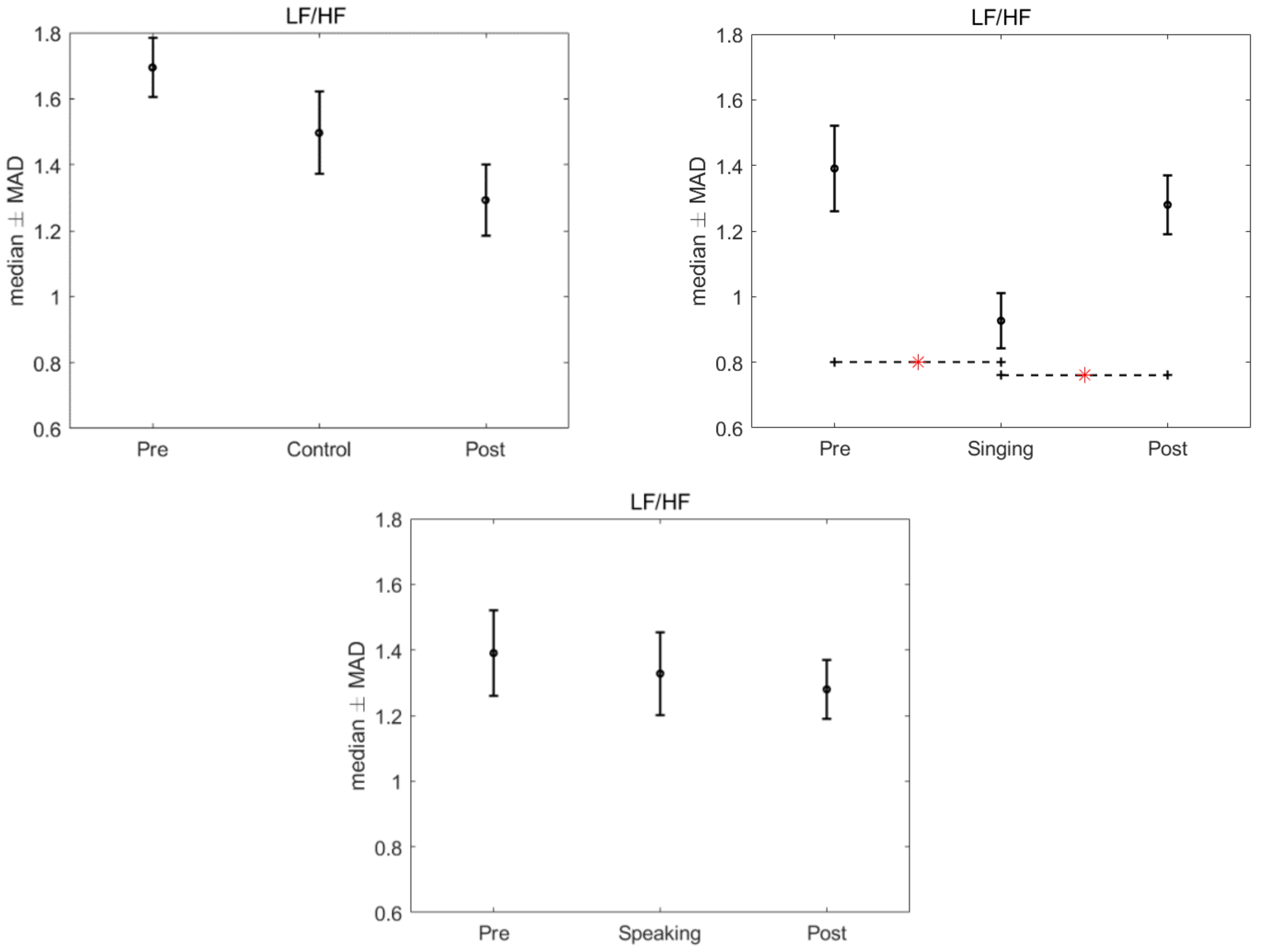
3. Results
4. Discussion
5. Conclusions
6. Limitations and Future Works
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulkey, S.B.; Kota, S.; Swisher, C.B.; Hitchings, L.; Metzler, M.; Wang, Y.; Maxwell, G.L.; Baker, R.; du Plessis, A.J.; Govindan, R. Autonomic nervous system depression at term in neurologically normal premature infants. Early Hum. Dev. 2018, 123, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Yiallourou, S.R.; Witcombe, N.B.; Sands, S.A.; Walker, A.M.; Horne, R.S. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum. Dev. 2013, 89, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sahni, R.; Schulze, K.F.; Kashyap, S.; Ohira-Kist, K.; Fifer, W.P.; Myers, M.M. Maturational changes in heart rate and heart rate variability in low birth weight infants. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 2000, 37, 73–81. [Google Scholar] [CrossRef]
- Porges, S.W. The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, and Self-Regulation (Norton Series on Interpersonal Neurobiology); WW Norton & Company: New York, NY, USA, 2011. [Google Scholar]
- Mulkey, S.B.; du Plessis, A.J. Autonomic nervous system development and its impact on neuropsychiatric outcome. Pediatric Res. 2019, 85, 120–126. [Google Scholar] [CrossRef]
- Chiera, M.; Cerritelli, F.; Casini, A.; Barsotti, N.; Boschiero, D.; Cavigioli, F.; Corti, C.G.; Manzotti, A. Heart Rate Variability in the Perinatal Period: A Critical and Conceptual Review. Front. Neurosci. 2020, 14, 561186. [Google Scholar] [CrossRef]
- DiPietro, J.A.; Costigan, K.A.; Voegtline, K.M. Studies in fetal behavior: Revisited, renewed, and reimagined. Monogr. Soc. Res. Child Dev. 2015, 80, vii-vii94. [Google Scholar]
- Porges, S.W.; Furman, S.A. The early development of the autonomic nervous system provides a neural platform for social behaviour: A polyvagal perspective. Infant Child Dev. 2011, 20, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Mulkey, S.B.; Govindan, R.B.; Hitchings, L.; Al-Shargabi, T.; Herrera, N.; Swisher, C.B.; Eze, A., Jr.; Russo, S.; Schlatterer, S.D.; Jacobs, M.B.; et al. Autonomic nervous system maturation in the premature extrauterine milieu. Pediatric Res. 2021, 89, 863–868. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Porges, S.W. Autonomic regulation and attention. In Attention and Information Processing in Infants and Adults; Campbell, B.A., Hayne, H., Richardson, R., Eds.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1992; pp. 201–223. [Google Scholar]
- Feldman, R.; Eidelman, A.I. Skin-to-skin contact (Kangaroo Care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev. Med. Child Neurol. 2003, 45, 274–281. [Google Scholar] [CrossRef]
- Porges, S.W.; Davila, M.I.; Lewis, G.F.; Kolacz, J.; Okonmah-Obazee, S.; Hane, A.A.; Kwon, K.Y.; Ludwig, R.J.; Myers, M.M.; Welch, M.G. Autonomic regulation of preterm infants is enhanced by Family Nurture Intervention. Dev. Psychobiol. 2019, 61, 942–952. [Google Scholar] [CrossRef]
- Best, K.; Bogossian, F.; New, K. Language exposure of preterm infants in the neonatal unit: A systematic review. Neonatology 2018, 114, 261–276. [Google Scholar] [CrossRef]
- Bieleninik, Ł.; Ghetti, C.; Gold, C. Music therapy for preterm infants and their parents: A meta-analysis. Pediatrics 2016, 138, e20160971. [Google Scholar] [CrossRef] [Green Version]
- Filippa, M.; Panza, C.; Ferrari, F.; Frassoldati, R.; Kuhn, P.; Balduzzi, S.; D’Amico, R. Systematic review of maternal voice interventions demonstrates increased stability in preterm infants. Acta Paediatr. 2017, 106, 1220–1229. [Google Scholar] [CrossRef]
- Provenzi, L.; Broso, S.; Montirosso, R. Do mothers sound good? A systematic review of the effects of maternal voice exposure on preterm infants’ development. Neurosci. Biobehav. Rev. 2018, 88, 42–50. [Google Scholar] [CrossRef]
- Yue, W.; Han, X.; Luo, J.; Zeng, Z.; Yang, M. Effect of music therapy on preterm infants in neonatal intensive care unit: Systematic review and meta-analysis of randomized controlled trials. J. Adv. Nurs. 2021, 77, 635–652. [Google Scholar] [CrossRef]
- Arnon, S.; Diamant, C.; Bauer, S.; Regev, R.; Sirota, G.; Litmanovitz, I. Maternal singing during kangaroo care led to autonomic stability in preterm infants and reduced maternal anxiety. Acta Paediatr. 2014, 103, 1039–1044. [Google Scholar] [CrossRef]
- Filippa, M.; Devouche, E.; Arioni, C.; Imberty, M.; Gratier, M. Live maternal speech and singing have beneficial effects on hospitalized preterm infants. Acta Paediatr. 2013, 102, 1017–1020. [Google Scholar] [CrossRef]
- Filippa, M.; Menin, D.; Panebianco, R.; Monaci, M.G.; Dondi, M.; Grandjean, D. Live maternal speech and singing increase self-touch and eye-opening in preterm newborns: A preliminary study. J. Nonverbal. Behav. 2020, 44, 453–473. [Google Scholar] [CrossRef]
- Filippa, M.; Monaci, M.G.; Spagnuolo, C.; Serravalle, P.; Daniele, R.; Grandjean, D. Maternal speech decreases pain scores and increases oxytocin levels in preterm infants during painful procedures. Sci. Rep. 2021, 11, 17301. [Google Scholar] [CrossRef]
- Filippa, M.; Della Casa, E.; D’amico, R.; Picciolini, O.; Lunardi, C.; Sansavini, A.; Ferrari, F. Effects of Early Vocal Contact in the Neonatal Intensive Care Unit: Study Protocol for a Multi-Centre, Randomised Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 3915. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, J.; Gil, E.; Vergara, J.M.; Laguna, P. Pulse rate variability analysis for discrimination of sleep-apnea-related decreases in the amplitude fluctuations of pulse photoplethysmographic signal in children. IEEE J. Biomed. Health Inform. 2013, 18, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.P.; Ranta-Aho, P.O.; Karjalainen, P.A. An advanced detrending method with application to HRV analysis. IEEE Trans. Biomed. Eng. 2002, 49, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.P.; Georgiadis, S.D.; Ranta-Aho, P.O.; Karjalainen, P.A. Time-varying analysis of heart rate variability signals with a Kalman smoother algorithm. Physiol. Meas. 2006, 27, 225. [Google Scholar] [CrossRef] [Green Version]
- Rosenstock, E.G.; Cassuto, Y.; Zmora, E. Heart rate variability in the neonate and infant: Analytical methods, physiological and clinical observations. Acta Paediatr. 1999, 88, 477–482. [Google Scholar] [CrossRef]
- Hochberg, Y.; Tamhane, A.C. Multiple Comparison Procedures; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1987. [Google Scholar]
- Javorka, K.; Lehotska, Z.; Kozar, M.; Uhrikova, Z.; Kolarovszki, B.; Javorka, M.; Zibolen, M. Heart rate variability in newborns. Physiol. Res. 2017, 66, S203–S214. [Google Scholar] [CrossRef]
- Longin, E.; Gerstner, T.; Schaible, T.; Lenz, T.; König, S. Maturation of the autonomic nervous system: Differences in heart rate variability in premature vs. term infants. J. Perinat. Med. 2006, 34, 303–308. [Google Scholar] [CrossRef]
- Malik, M. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use: Task force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology. Ann. Noninvasive Electrocardiol. 1996, 1, 151–181. [Google Scholar] [CrossRef]
- Acharya, U.R.; Joseph, K.P.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef]
- Filippa, M.; Gratier, M.; Devouche, E.; Grandjean, D. Changes in infant-directed speech and song are related to preterm infant facial expression in the neonatal intensive care unit. Interact. Stud. 2018, 19, 427–444. [Google Scholar] [CrossRef]
- Corbeil, M.; Trehub, S.E.; Peretz, I. Speech vs. singing: Infants choose happier sounds. Front. Psychol. 2013, 4, 372. [Google Scholar] [CrossRef] [Green Version]
- Schön, D.; Boyer, M.; Moreno, S.; Besson, M.; Peretz, I.; Kolinsky, R. Songs as an aid for language acquisition. Cognition 2008, 106, 975–983. [Google Scholar] [CrossRef]
- Cirelli, L.K.; Jurewicz, Z.B.; Trehub, S.E. Effects of maternal singing style on mother–infant arousal and behavior. J. Cogn. Neurosci. 2020, 32, 1213–1220. [Google Scholar] [CrossRef]
- Ferrari, F.; Cioni, G.; Prechtl, H.F.R. Qualitative changes of general movements in preterm infants with brain lesions. Early Hum. Dev. 1990, 23, 193–231. [Google Scholar] [CrossRef]
- Nardelli, M.; Vanello, N.; Galperti, G.; Greco, A.; Scilingo, E.P. Assessing the Quality of Heart Rate Variability Estimated from Wrist and Finger PPG: A Novel Approach Based on Cross-Mapping Method. Sensors 2020, 20, 3156. [Google Scholar] [CrossRef]
- Nardelli, M.; Scilingo, E.P.; Valenza, G. Multichannel Complexity Index (MCI) for a multi-organ physiological complexity assessment. Phys. A Stat. Mech. Its Appl. 2019, 530, 121543. [Google Scholar] [CrossRef]

| Characteristics | Intervention Group (n = 12) Mean (SD) | Control Group (n = 12) Mean (SD) |
|---|---|---|
| Gestational age at birth (weeks) | 29.1 (2.4) | 29.3 (1.5) |
| Birthweight (g) | 1226 (367) | 1204 (235) |
| Apgar score at 5 min | 7.1 (1.3) | 6.4 (2.3) |
| Apgar score at 7 min | 8.1 (1.1) | 7.9 (1.0) |
| Gestational age at test (weeks) | 34.1 (1.3) | 34.2 (1.7) |
| Post-natal age at test (days) | 33.8 (19.2) | 34.7 (15.8) |
| Weight at test (g) | 1767.8 (258) | 1603.8 (308) |
| Maternal citizenship | Italian (80%) | Italian (70%) |
| Primiparous | Yes (72%) | Yes (60%) |
| Mother’s age | 35.6 (3.2) | 37.7 (6.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippa, M.; Nardelli, M.; Della Casa, E.; Berardi, A.; Picciolini, O.; Meloni, S.; Lunardi, C.; Cecchi, A.; Sansavini, A.; Corvaglia, L.; et al. Maternal Singing but Not Speech Enhances Vagal Activity in Preterm Infants during Hospitalization: Preliminary Results. Children 2022, 9, 140. https://doi.org/10.3390/children9020140
Filippa M, Nardelli M, Della Casa E, Berardi A, Picciolini O, Meloni S, Lunardi C, Cecchi A, Sansavini A, Corvaglia L, et al. Maternal Singing but Not Speech Enhances Vagal Activity in Preterm Infants during Hospitalization: Preliminary Results. Children. 2022; 9(2):140. https://doi.org/10.3390/children9020140
Chicago/Turabian StyleFilippa, Manuela, Mimma Nardelli, Elisa Della Casa, Alberto Berardi, Odoardo Picciolini, Sara Meloni, Clara Lunardi, Alessandra Cecchi, Alessandra Sansavini, Luigi Corvaglia, and et al. 2022. "Maternal Singing but Not Speech Enhances Vagal Activity in Preterm Infants during Hospitalization: Preliminary Results" Children 9, no. 2: 140. https://doi.org/10.3390/children9020140
APA StyleFilippa, M., Nardelli, M., Della Casa, E., Berardi, A., Picciolini, O., Meloni, S., Lunardi, C., Cecchi, A., Sansavini, A., Corvaglia, L., Scilingo, E. P., Ferrari, F., & EVC Group. (2022). Maternal Singing but Not Speech Enhances Vagal Activity in Preterm Infants during Hospitalization: Preliminary Results. Children, 9(2), 140. https://doi.org/10.3390/children9020140








