Auditory Stimulation Improves Gait and Posture in Cerebral Palsy: A Systematic Review with Between- and Within-Group Meta-Analysis
Abstract
1. Introduction
- To evaluate the effect of AStim on spatiotemporal gait parameters from both between- and within-group analyses.
- To evaluate the effect of AStim on postural stability from both between- and within-group analyses.
- To evaluate the effect of AStim on gait kinematic parameters from both between- and within-group analyses.
- To perform subgroup analyses between studies according to their training (i.e., no-training vs. training) and randomization (i.e., randomized controlled trials vs. controlled clinical trials) status.
2. Materials and Methods
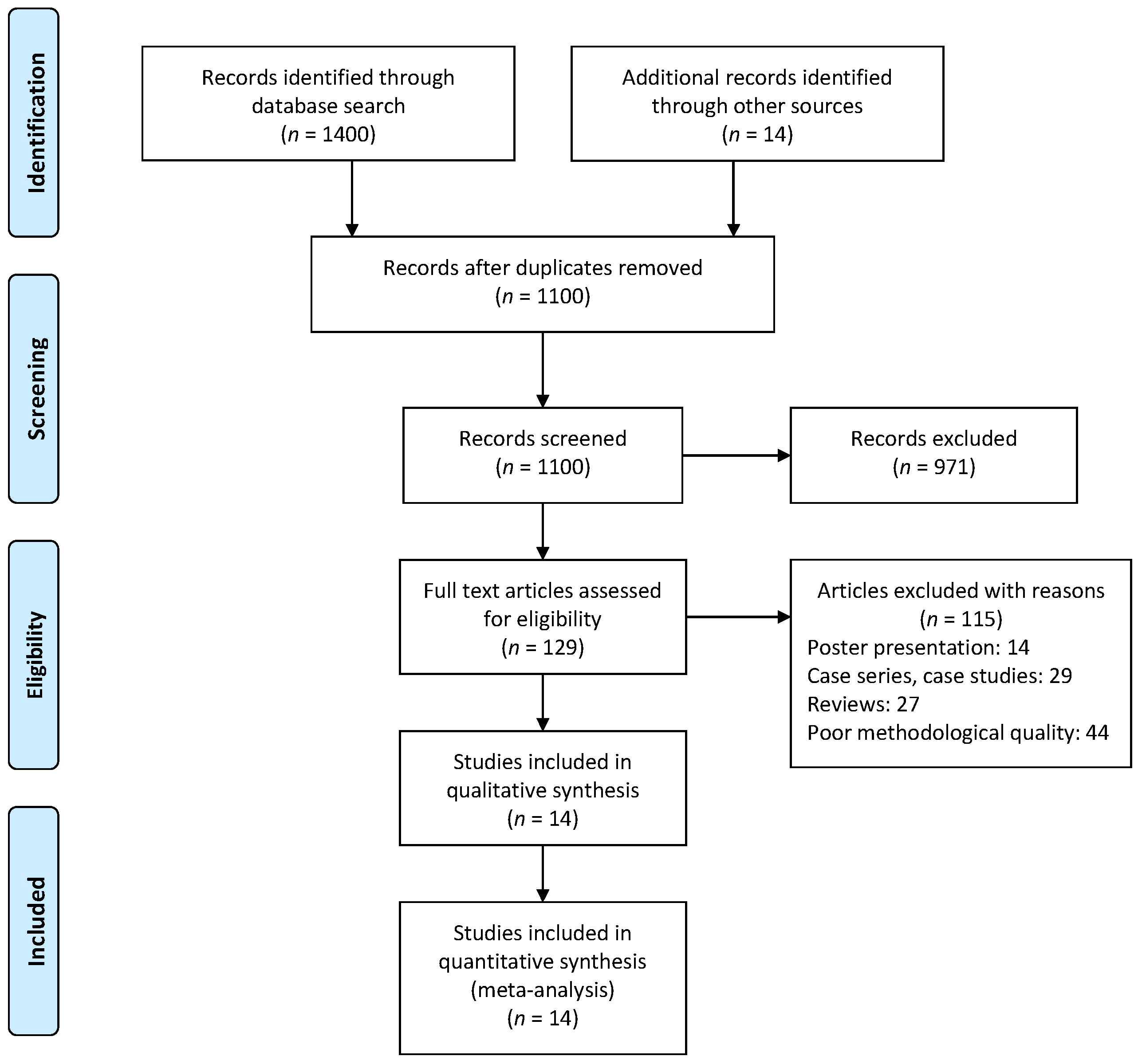
2.1. Sources of Data and Search Strategy
2.2. Assessment of the Risk of Bias
2.3. Data Analysis
3. Results
3.1. Characteristics of Included Studies
| Authors and Country of Research | Sample Size (N) Gender Distribution (F, M) (Age in Years as Mean ± SD/Range) | Gross Motor Function Classification System Level | Outcomes | Training Schedule | Training Groups with Characteristics | Results |
|---|---|---|---|---|---|---|
| Gerek and Moghadamİ [12] Turkey | N = 12 ?F, ?M (13 ± 3.5) | I | Cadence Gait speed Double support time Single support time Limp index Step width Step length Opposite foot lift | - | AStim at 2/4, 4/4 and 6/8 rhythm | Cadence: Significant ↓ with all AStim (2/4, 4/4, 6/8). Gait speed: Significant ↑ with AStim (6/8) and significant ↓ with AStim (2/4, 4/4). Double support time: Significant ↑ with AStim (4/4, 6/8) and no difference with AStim (2/4). Single support time: Significant ↑ with AStim (2/4, 4/4, 6/8). Limp index: Significant ↑ with all AStim (2/4, 4/4, 6/8). Step width: Significant ↑ with AStim (4/4, 6/8) and no difference with AStim (2/4). Step length: Significant ↑ with AStim (2/4, 4/4) and no difference with AStim (6/8). Opposite foot lift: Significant ↓ with all AStim (2/4, 4/4, 6/8). |
| Kim, Yoo [13] South Korea | SC: N = 6 4F, 2M (20 ± 2.8) CC: N = 6 5F, 2M (19.5 ± 5.0) | I to II | Cadence Gait speed Stride length Gait kinematics (pelvis, hip, knee) Range of motion (pelvis, hip, knee, ankle) | Session duration: 30 min Times per week: 3 Weeks: 4 | SC: Simple chord AStim CC: Complex chord AStim | Cadence: Significant ↑ with AStim (SC, CC). Gait speed: Significant ↑ with AStim (SC, CC). Stride length: Significant ↑ with AStim (SC, CC). Gait kinematics: Significant ↑ in maximal ankle plantar flexion in sagittal plane with AStim (SC, CC). Range of motion: Significant ↑ in ankle ROM with AStim (CC), no difference in ankle range of motion with AStim (SC). |
| Duymaz [14] Turkey | Exp: N = 60 ?F, ?M (7.42 ± 2.4) Ct: N = 60 ?F, ?M (7.6 ± 2.6) | I to III | Gross motor function measure dimension D and E | Session duration: 45 min Times per week: 3 Weeks: 5 | Exp: Classical music AStim with neurodevelopment therapy Ct: Neurodevelopment therapy | Gross motor function measurement D (i.e., standing): Significant ↑ with AStim and neurodevelopment therapy. Gross motor function measurement E (i.e., walking): Significant ↑ with AStim and neurodevelopment therapy. |
| Ben-Pazi, Aran [15] Israel | Exp: N = 9 1F, 8M (7.7 ± 4.4) Ct: N = 9 4F, 5M (7.1 ± 3.9) | II to V | Gross motor function measure dimension D and E | Session duration: 30 min Times per week: 4 Weeks: 4 | Exp: AStim with sound frequencies in gamma range modulated in frequency and/or amplitude embedded in nature sound of preference, children actively listened to tracks and gamma tones faded in the background music/sound over the first two minutes Ct: Nature and music sound according to preference | Gross motor function measurement D (i.e., standing): Significant ↑ with AStim. Gross motor function measurement E (i.e., walking): Significant ↑ with AStim. |
| Efraimidou, Tsimaras [17] Greece | Exp: N = 5 0F, 5M (35.2 ± 13) Ct: N = 5 0F, 5M (38.8 ± 12.2) | I to II | Timed-up-and-go test 10 m walk test (normal and fast speed) Berg balance scale Centre of pressure sway | Session duration: 50 min Times per week: 2 Weeks: 8 | Exp: AStim (70–90 bpm), with 4/4 music meter Ct: Conventional physiotherapy | Timed-up-and-go test: Significant ↑ with AStim. 10 m walk test (normal and fast speed): Significant ↑ with AStim. Berg balance scale: Significant ↑ with AStim. Centre of pressure sway: Significant ↓ with AStim. |
| Shin, Chong [19] South Korea | N = 7 4F, 3M (30.1 ± 4.1) | - | Cadence Gait speed Stride length Stride time Step time Single support time Double support time Stance/swing phase Gait kinematics (pelvis, hip, knee, ankle, foot) Gait deviation index | Session duration: 30 min Times per week: 3 Weeks: 4 | AStim by four-chord progression with metronome beat on keyboard at preferred cadence | Cadence: No difference with AStim. Gait speed: No difference with AStim. Stride length: No difference with AStim. Stride time: No difference with AStim. Step time: No difference with AStim. Single support time: No difference with AStim. Double support time: No difference with AStim. Stance/swing phase: No difference with AStim. Gait kinematics: Significant ↑ in maximal ankle plantar flexion in sagittal plane with AStim. Gait deviation index: No difference with AStim. |
| Jiang [68] USA | N = 9 5F, 4M (5–12) | I to III | Cadence Gait speed Stride length | Session duration: 30 min Times per week: 1 Weeks: 3 | AStim delivered by piano, guitar, bass, percussion, with music in 4/4 beat accentuated by metronome Piano was superimposed on the beat to highlight the rhythm at the participant’s preferred cadence | Cadence: Significant ↑ with AStim. Gait speed: Significant ↑ with AStim. Stride length: No difference with AStim. |
| Wang, Peng [24] Taiwan | Exp: N =18 6F, 12M (9 ± 1.9) Ct: N =18 3F, 15M (8.9 ± 2.6) | I to III | Gait speed Gross motor function measure dimensions D and E | Exp: Session duration: 25.2 min Number of sessions: 17.8 Ct: Session duration: 26.9 min Number of sessions: 17.7 | Exp: AStim (PSE of spatial, temporal and force parameters) Ct: Conventional physiotherapy | Gait speed: No difference with AStim. Gross motor function measurement D (i.e., standing): Significant ↑ with AStim. Gross motor function measurement E (i.e., walking): No difference with AStim. |
| Varsamis, Staikopoulos [70] Greece | N = 18 7F, 11M (18.2 ± 3.8) | - | Duration for gait performance Number of steps Cadence Number of steps (intra individual standard deviation) | Session duration: 6 min Times per week: 1 Weeks: 1 | AStim at preferred cadence | Duration for gait performance: Significant ↑ with AStim. Number of steps: No difference with AStim. Cadence: Significant ↑ with AStim. Number of steps (intra individual standard deviation): Significant ↓ with AStim. |
| Kim, Kwak [18] South Korea | Exp: N = 15 5F, 10M (27.3 ± 2.4) Ct: N = 15 6F, 7M (27.3 ± 2.5) | - | Cadence Gait speed Stride length Step length Stride time Step time Stance phase Swing phase Gait kinematics (pelvis, hip, knee, ankle, foot) | Session duration: 30 min Times per week: 3 Weeks: 3 | Exp: AStim at preferred cadence Ct: Neurodevelopmental/Bobath therapy | Cadence: Significant ↑ with AStim. Gait speed: Significant ↑ with AStim. Stride length: Significant ↑ with AStim. Step length: Significant ↑ with AStim. Stride time: Significant ↓ with AStim. Step time: Significant ↓ with AStim. Swing phase: Significant ↑ with AStim. Gait kinematics: Significant ↑ in pelvic minimal angle of anterior tilt in sagittal plane with AStim. Significant ↓ in pelvic anterior tilt initial contact, max angle of anterior tilt in sagittal plane with AStim. Significant ↓ in hip minimal flexion angle in sagittal plane with AStim. Significant ↓ in hip maximum adduction, abduction angle, and abduction/adduction at initial contact in coronal plane with AStim. Significant ↑ in hip maximum internal rotation in transverse plane with AStim. Significant ↓ in hip maximum external rotation in transverse plane with AStim. Significant ↓ in foot internal/external rotation at initial contact in transverse plane with AStim. |
| Baram and Lenger [67] Israel | Exp: N =10 6F, 4M (11.1 ± 6.5) Ct: N = 10 7F, 3M (13.3 ± 6.2) | - | Gait speed Stride length | - | Exp: AStim at preferred cadence Ct: Visual cueing | Gait speed: Significant ↑ with AStim. Stride length: Significant ↑ with AStim. |
| Kim, Kwak [66] South Korea | N = 14 5F, 9M (25.6 ± 7.3) | I to II | Cadence Gait speed Stride length Step length Stride time Step time Stance phase Swing phase Gait kinematics (pelvis, hip, knee, ankle, foot) Gait deviation index | - | AStim at preferred cadence | Cadence: No difference with AStim. Gait speed: No difference with AStim. Stride length: No difference with AStim. Step length: No difference with AStim. Stride time: No difference with AStim. Step time: No difference with AStim. Stance phase: No difference with AStim. Swing phase: No difference with AStim. Gait kinematics: Significant ↓ in pelvic anterior tilt at initial contact in sagittal plane with AStim. Significant ↓ in pelvic maximal, minimal angle of anterior tilt in sagittal plane with AStim. Significant ↓ in hip maximal, minimal flexion angle in sagittal plane with AStim. Significant ↓ in hip internal/external rotation at initial contact in transverse plane with AStim. Gait deviation index: Significant ↑ with AStim. |
| Hamed and Abd-elwahab [65] Egypt | Exp: N = 15 ?F, ?M (7.03 ± 0.76) Ct: N = 15 ?F, ?M (7.07 ± 0.82) | - | Gait speed Stride length Cadence Gait cycle time | Session duration: 60 min Times per week: 5 Weeks: 12 | Exp: Melodious AStim at preferred cadence Ct: Conventional physiotherapy | Gait speed: Significant ↑ with AStim. Stride length: Significant ↑ with AStim. Cadence: Significant ↓ with AStim. Gait cycle time: Significant ↑ with AStim. |
| Kwak [69] USA | TGT: N = 10 ?F, ?M SGT: N = 10 ?F, ?M Ct: N = 10 ?F, ?M (6–20) | - | Stride length Gait speed Cadence Gait symmetry | Session duration: 30 min Times per week: 5 Weeks: 3 | TGT: Therapist guided AStim training SGT: Self-guided AStim training Ct: Conventional physiotherapy | Gait speed: Significant ↑ with AStim. Stride length: Significant ↑ with AStim. Cadence: No difference with AStim. Gait symmetry: Significant ↑ with AStim. |
3.2. Risk of Bias
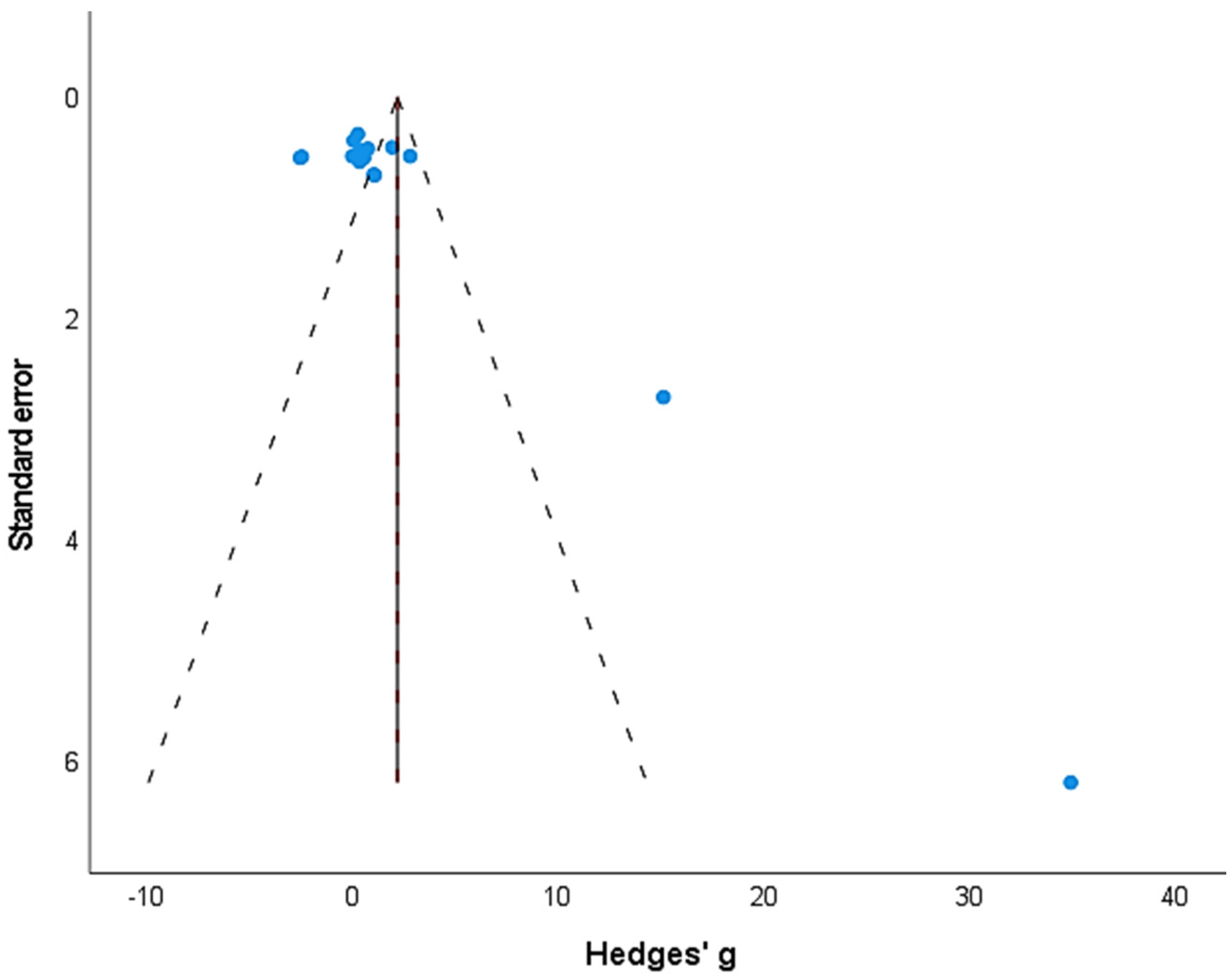
3.3. Publication Bias
3.4. Systematic Review Report
3.4.1. Participants
3.4.2. Gross Motor Function Classification
3.4.3. Outcome
3.4.4. Interventions
3.5. Meta-Analysis Report
| Outcome Number | Outcome Evaluated | Analysis Type | Number of Studies; (References) | Meta-Analysis Outcome Hedge’s G, 95% Confidence Interval, p Value | Heterogeneity Outcome I2 | Figure |
|---|---|---|---|---|---|---|
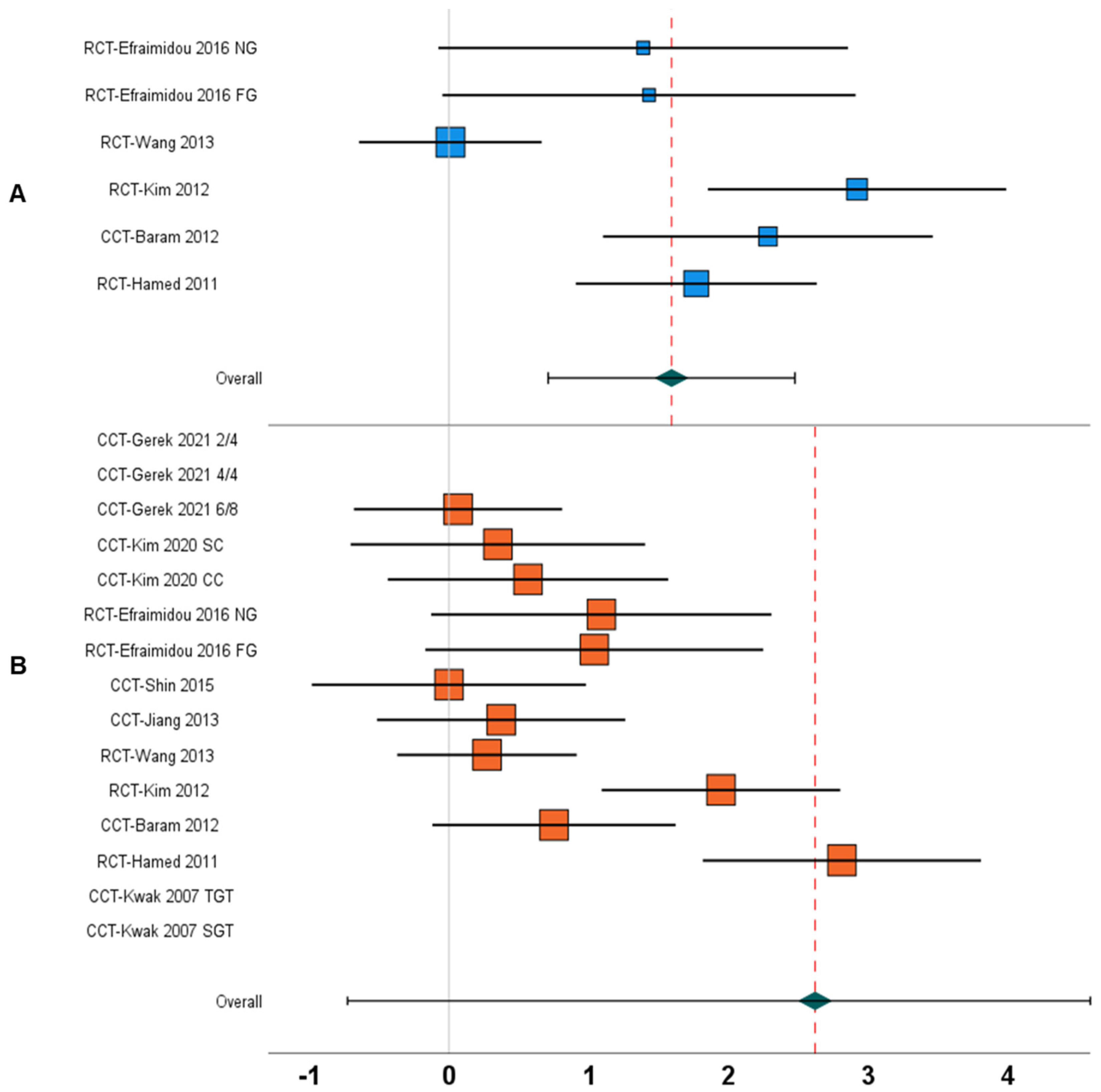
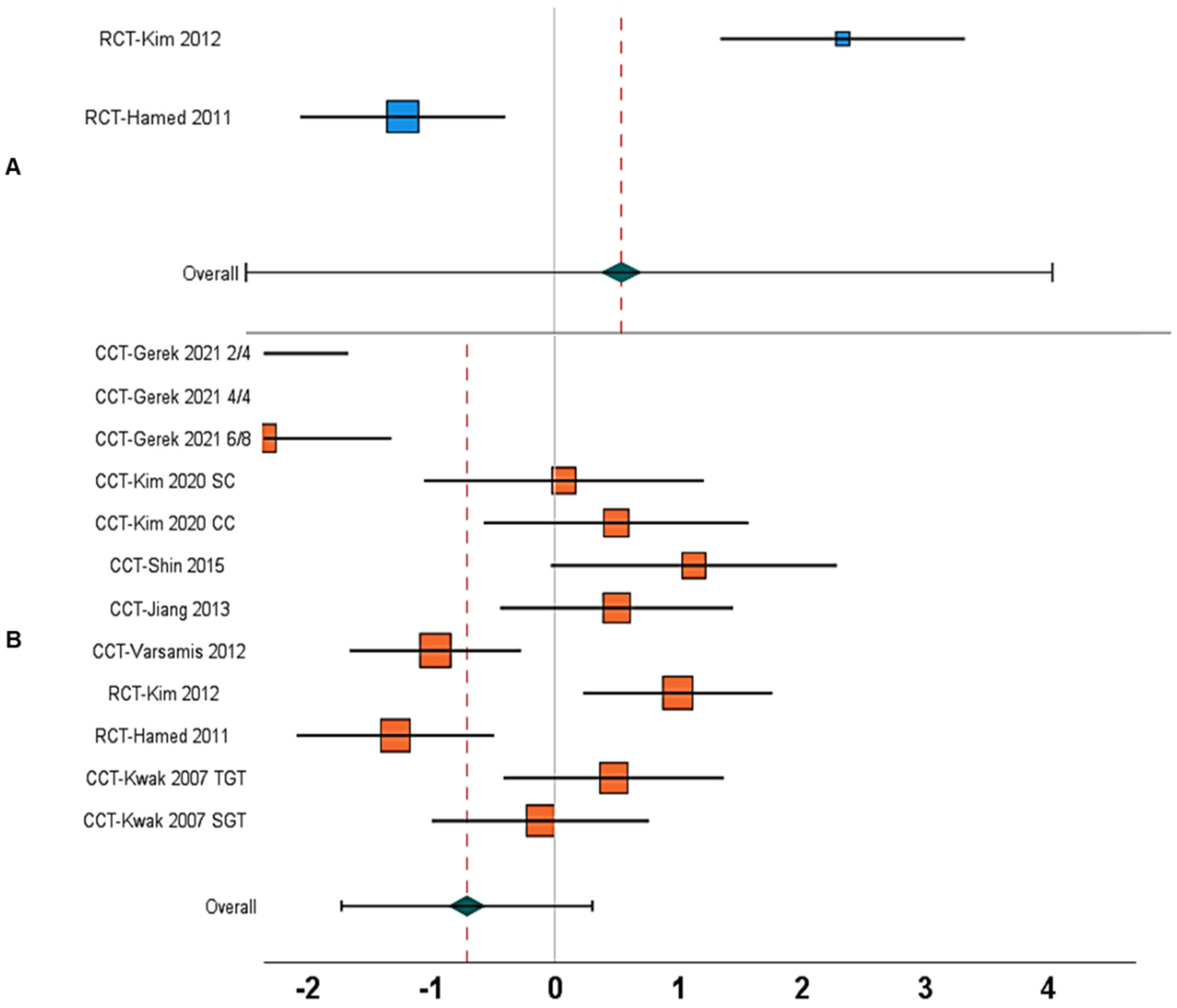
| 1. | Gait speed | Between-group | N = 5; [17,18,24,65,67] | 1.59, 0.71 to 2.47, <0.001 | 77% | Figure 4A |
| 2. | Gait speed (RCT) | Between-group | N = 4; [17,18,24,65] | 1.46, 0.45 to 2.47, 0.004 | 79% | Figure S1 |
| 3. | Gait speed (CCT) | Between-group | N = 1; [67] | - | - | - |
| 4. | Gait speed (with training) | Between-group | Same as outcome number 2 | |||
| 5. | Gait speed (with training and RCT) | Between-group | Same as outcome number 2 | |||
| 6. | Gait speed (with training and CCT) | Between-group | None | - | - | - |
| 7. | Gait speed (no training) | Between-group | N = 1; [67] | - | - | - |
| 8. | Gait speed (no training and RCT) | Between-group | None | - | - | - |
| 9. | Gait speed (no training and CCT) | Between-group | N = 1; [67] | - | - | - |
| 10. | Gait speed | Within-group | N = 10; [12,13,17,18,19,24,65,67,68,69] | 2.61, −0.72 to 5.96, 0.12 | 99% | Figure 4B |
| 11. | Gait speed (RCT) | Within-group | N = 4; [17,18,24,65] | 1.41, 0.49 to 2.34, 0.003 | 76% | Figure S2 |
| 12. | Gait speed (CCT) | Within-group | N = 6; [12,13,19,67,68,69] | 3.64, −2.10 to 9.38, 0.21 | 100% | Figure S3 |
| 13. | Gait speed (with training) | Within-group | N= 8; [13,17,18,19,24,65,68,69] | 4.06, −0.44 to 8.57, 0.07 | 99% | Figure S4 |
| 14. | Gait speed (With training and RCT) | Within-group | Same as outcome number 11 | |||
| 15. | Gait speed (With training and CCT) | Within-group | N = 4; [13,19,68,69] | 7.61, −2.58 to 17.81, 0.14 | 100% | Figure S5 |
| 16. | Gait speed (no training) | Within-group | N = 2; [12,67] | −1.01, −2.68 to 0.64, 0.23 | 92% | Figure S6 |
| 17. | Gait speed (no training and RCT) | Within-group | None | - | - | - |
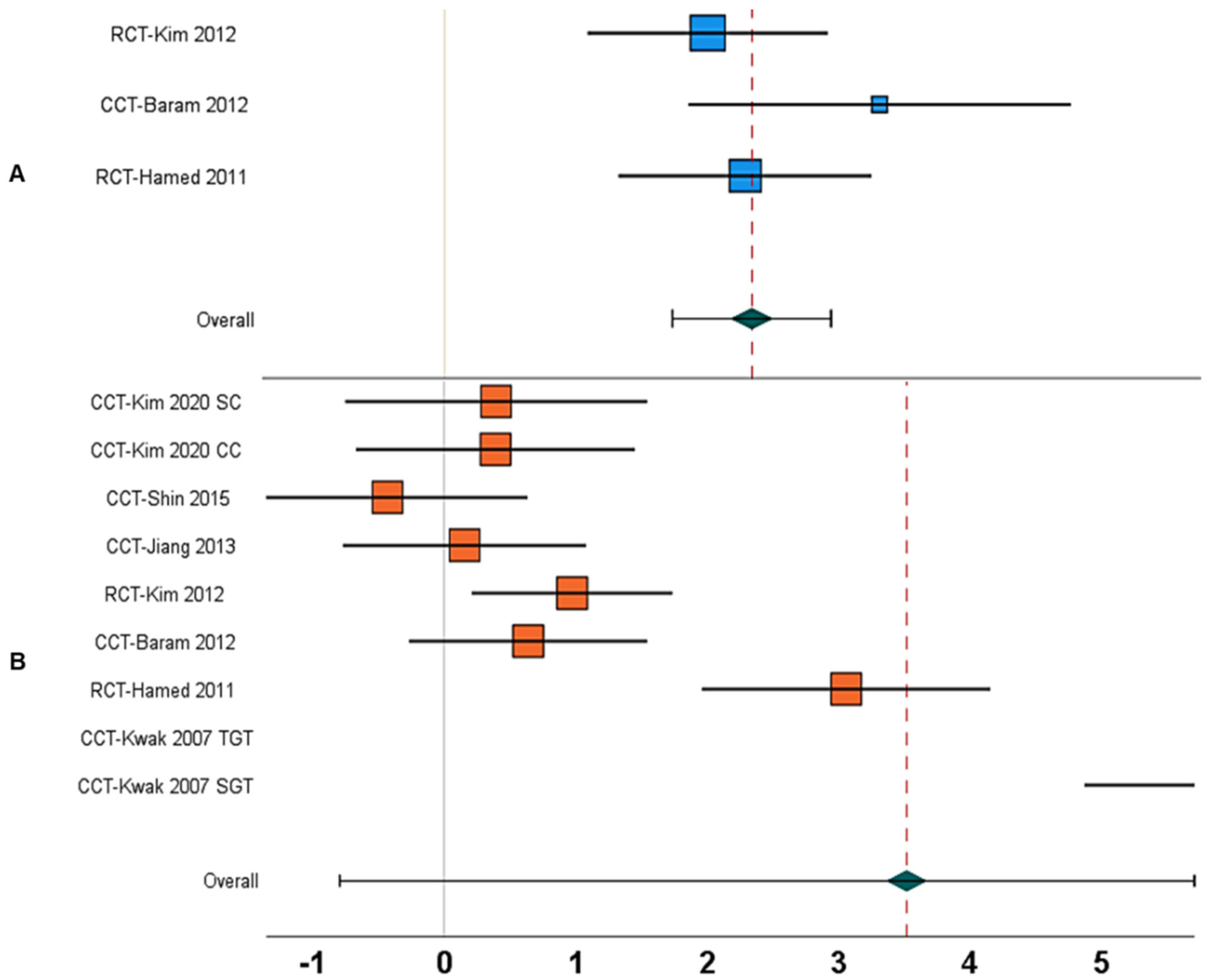
| 18. | Stride length | Between-group | N = 3; [18,65,67] | 2.27, 1.68 to 2.86, <0.001 | 0% | Figure 5A |
| 19. | Stride length (RCT) | Between-group | N = 2; [18,65] | 2.07, 1.43 to 2.72, <0.001 | 0% | Figure S7 |
| 20. | Stride length (CCT) | Between-group | N = 1; [67] | - | - | - |
| 21. | Stride length (with training) | Between-group | Same as outcome number 19 | |||
| 22. | Stride length (with training and RCT) | Between-group | Same as outcome number 19 | |||
| 23. | Stride length (with training and CCT) | Between-group | N = 1; [67] | - | - | - |
| 24. | Stride length | Within-group | N = 7; [13,18,19,65,67,68,69] | 3.51, −0.79 to 7.82, 0.11 | 99% | Figure 5B |
| 25. | Stride length (RCT) | Within-group | N = 2; [18,65] | 1.97, −0.06 to 4.01, 0.05 | 89% | Figure S8 |
| 26. | Stride length (CCT) | Within-group | N = 5; [13,19,67,68,69] | 4.39, −1.98 to 10.77, 0.17 | 99% | Figure S9 |
| 27. | Stride length (with training) | Within-group | N = 6; [13,18,19,65,68,69] | 4.08, −1.09 to 9.26, 0.12 | 99% | Figure S10 |
| 28. | Stride length (with training and RCT) | Within-group | Same as outcome number 25 | |||
| 29. | Stride length (with training and CCT) | Within-group | N = 4; [13,19,68,69] | 5.23, −2.54 to 13.0, 0.18 | 100% | Figure S11 |
| 30. | Stride length (with no training) | Within-group | N = 1; [67] | - | - | - |
| 31. | Stride length (with no training and RCT) | Within-group | None | - | - | - |
| 32. | Stride length (with no training and CCT) | Within-group | N = 1; [67] | - | - | - |
| 33. | Cadence | Between-group | N = 2; [18,65] | 0.51, −2.77 to 3.80, 0.76 | 97% | Figure 6A |
| 34. | Cadence (RCT) | Between-group | Same as outcome number 33 | |||
| 35. | Cadence (CCT) | Between-group | None | - | - | - |
| 36. | Cadence (with training) | Between-group | Same as outcome number 33 | |||
| 37. | Cadence (with training and RCT) | Between-group | Same as outcome number 33 | |||
| 38. | Cadence (with training and CCT) | Between-group | None | - | - | - |
| 39. | Cadence (with no training) | Between-group | None | - | - | - |
| 40. | Cadence | Within-group | N = 8; [12,13,18,19,65,68,69,70] | −0.70, −1.72 to 0.30, 0.17 | 93% | Figure 6B |
| 41. | Cadence (RCT) | Within-group | N = 2; [18,65] | −0.14, −2.38 to 2.09, 0.90 | 94% | Figure S12 |
| 42. | Cadence (CCT) | Within-group | N = 6; [12,13,19,68,69,70] | −0.83, −2.01 to 0.35, 0.16 | 93% | Figure S13 |
| 43. | Cadence (with training) | Within-group | N = 7; [13,18,19,65,68,69,70] | 0.10, -0.46 to 0.67, 0.71 | 72% | Figure S14 |
| 44. | Cadence (with training and RCT) | Within-group | Same as outcome number 41 | |||
| 45. | Cadence (with training and CCT) | Within-group | N = 5; [13,19,68,69,70] | 0.15, −0.37 to 0.69, 0.56 | 55% | Figure S15 |
| 46. | Cadence (with no training) | Within-group | N = 1; [12] | - | - | - |
| 47. | Cadence (with no training and RCT) | Within-group | None | - | - | - |
| 48. | Cadence (with no training and CCT) | Within-group | N = 1; [12] | - | - | - |
| 49. | Gait deviation index | Between-group | None | - | - | - |
| 50. | Gait deviation index | Within-group | N = 4; [13,18,19,66] | 0.61, −0.15 to 1.39, 0.11 | 69% | Figure S16 |
| 51. | Gait deviation index (RCT) | Within-group | N =1; [18] | - | - | - |
| 52. | Gait deviation index (CCT) | Within-group | N = 3; [13,18,19] | 0.26, −0.22 to 0.74, 0.28 | 0% | Figure S17 |
| 53. | Gait deviation index (with training) | Within-group | Same as outcome number 52 | |||
| 54. | Gait deviation index (with training and RCT) | Within-group | N = 1; [18] | - | - | - |
| 55. | Gait deviation index (with training and CCT) | Within-group | Same as outcome number 52 | |||
| 56. | Gait deviation index (with no training) | Within-group | None | - | - | - |
| 57. | GMFM-D | Between-group | N = 3; [14,15,24] | 0.50, 0.20 to 0.81, <0.001 | 0% | Figure S18 |
| 58. | GMFM-D (RCT) | Between-group | Same as outcome number 57 | |||
| 59. | GMFM-D (CCT) | Between-group | None | - | - | - |
| 60. | GMFM-D (with training) | Between-group | Same as outcome number 57 | |||
| 61. | GMFM-D (with training and RCT) | Between-group | Same as outcome number 57 | |||
| 62. | GMFM-D (with training and CCT) | Between-group | None | - | - | - |
| 63. | GMFM-D (with no training) | Between-group | None | - | - | - |
| 64. | GMFM-D | Within-group | N = 3; [14,15,24] | 0.44, 0.14 to 0.74, 0.004 | 0% | Figure S19 |
| 65. | GMFM-D (RCT) | Within-group | Same as outcome number 64 | |||
| 66. | GMFM-D (CCT) | Within-group | None | - | - | - |
| 67. | GMFM-D (with training) | Within-group | Same as outcome number 64 | |||
| 68. | GMFM-D (with training and RCT) | Within-group | Same as outcome number 64 | |||
| 69. | GMFM-D (with training and CCT) | Within-group | None | |||
| 70. | GMFM-D (with no training) | Within-group | None | - | - | - |
| 71. | GMFM-E | Between-group | N = 3; [14,15,24] | 0.24, −0.05 to 0.53, 0.11 | 0% | Figure S20 |
| 72. | GMFM-E (RCT) | Between-group | Same as outcome number 71 | |||
| 73. | GMFM-E (CCT) | Between-group | None | - | - | - |
| 74. | GMFM-E (with training) | Between-group | Same as outcome number 71 | |||
| 75. | GMFM-E (with training and RCT) | Between-group | Same as outcome number 71 | |||
| 76. | GMFM-E (with training and CCT) | Between-group | None | - | - | - |
| 77. | GMFM-E (with no training) | Between-group | None | - | - | - |
| 78. | GMFM-E | Within-group | N = 3; [14,15,24] | 0.78, −0.33 to 1.90, 0.17 | 88% | Figure S21 |
| 79. | GMFM-E (RCT) | Within-group | Same as outcome number 78 | |||
| 80. | GMFM-E (CCT) | Within-group | None | - | - | - |
| 81. | GMFM-E (with training) | Within-group | Same as outcome number 78 | |||
| 82. | GMFM-E (with training and RCT) | Within-group | Same as outcome number 78 | |||
| 83. | GMFM-E (with training and CCT) | Within-group | None | - | - | - |
| 84. | GMFM-E (with no training) | Within-group | None | - | - | - |
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobson, F.; Morris, M.E.; Baker, R.; Graham, H.K. Gait classification in children with cerebral palsy: A systematic review. Gait Posture 2007, 25, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Hsue, B.-J.; Miller, F.; Su, F.-C. The dynamic balance of the children with cerebral palsy and typical developing during gait. Part I: Spatial relationship between COM and COP trajectories. Gait Posture 2009, 29, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.L.; Malouin, F. Cerebral palsy: Definition, assessment and rehabilitation. Handb. Clin. Neurol. 2013, 111, 183–195. [Google Scholar]
- Fluss, J.; Lidzba, K. Cognitive and academic profiles in children with cerebral palsy: A narrative review. Ann. Phys. Rehabil. Med. 2020, 63, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, L. Children with spastic cerebral palsy, their cognitive functioning, and social participation: A review. Child Neuropsychol. 2010, 16, 209–228. [Google Scholar] [CrossRef]
- Parkes, J.; White-Koning, M.; Dickinson, H.O.; Thyen, U.; Arnaud, C.; Beckung, E.; Fauconnier, J.; Marcelli, M.; McManus, V.; Michelsen, S.I. Psychological problems in children with cerebral palsy: A cross-sectional European study. J. Child Psychol. Psychiatry 2008, 49, 405–413. [Google Scholar] [CrossRef]
- Jaspers, E.; Verhaegen, A.; Geens, F.; Van Campenhout, A.; Desloovere, K.; Molenaers, G. Lower limb functioning and its impact on quality of life in ambulatory children with cerebral palsy. Eur. J. Paediatr. Neurol. 2013, 17, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Lundh, S.; Nasic, S.; Riad, J. Fatigue, quality of life and walking ability in adults with cerebral palsy. Gait Posture 2018, 61, 1–6. [Google Scholar] [CrossRef]
- Guttmann, K.; Flibotte, J.; DeMauro, S.B. Parental perspectives on diagnosis and prognosis of neonatal intensive care unit graduates with cerebral palsy. J. Pediatrics 2018, 203, 156–162. [Google Scholar] [CrossRef]
- Mavlyanova, Z.; Burkhanova, G.; Ravshanova, M.; Makhmudov, S.; Kholboyev, S. Improving the tactics of treating children with severe cerebral palsy. Eur. J. Mol. Clin. Med. 2020, 7, 2020. [Google Scholar]
- Vinolo-Gil, M.J.; Casado-Fernández, E.; Perez-Cabezas, V.; Gonzalez-Medina, G.; Martín-Vega, F.J.; Martín-Valero, R. Effects of the Combination of Music Therapy and Physiotherapy in the Improvement of Motor Function in Cerebral Palsy: A Challenge for Research. Children 2021, 8, 868. [Google Scholar] [CrossRef] [PubMed]
- Gerek, Z.; Moghadamİ, A. Biomechanical Investigation of The Effect of Rhythmic Auditory Stimulation on Walking Skills in Children with Cerebral Palsy. Pak. J. Med. Health Sci. 2021, 15, 933–937. [Google Scholar]
- Kim, S.J.; Yoo, G.E.; Shin, Y.K.; Cho, S.R. Gait training for adults with cerebral palsy following harmonic modification in rhythmic auditory stimulation. Ann. N. Y. Acad. Sci. 2020, 1473, 11–19. [Google Scholar] [CrossRef]
- Duymaz, T. The effects of music therapy on gross motor functions, pain and level of functional independence in children with cerebral palsy. Ann. Clin. Anal. Med. 2020, 11, 115–119. [Google Scholar]
- Ben-Pazi, H.; Aran, A.; Pandyan, A.; Gelkop, N.; Ginsberg, G.; Pollak, Y.; Elnatan, D. Auditory stimulation improves motor function and caretaker burden in children with cerebral palsy-a randomized double blind study. PLoS ONE 2018, 13, e0208792. [Google Scholar] [CrossRef]
- Ghai, S.; Ghai, I.; Effenberg, A.O. Effect of rhythmic auditory cueing on gait in cerebral palsy: A systematic review and meta-analysis. Neuropsychiatr. Dis. Treat. 2017, 14, 43–59. [Google Scholar] [CrossRef]
- Efraimidou, V.; Tsimaras, V.; Proios, M.; Christoulas, K.; Giagazoglou, P.; Sidiropoulou, M.; Orologas, A. The effect of a music and movement program on gait, balance and psychological parameters of adults with cerebral palsy. J. Phys. Educ. Sport 2016, 16, 1357. [Google Scholar]
- Kim, S.J.; Kwak, E.E.; Park, E.S.; Cho, S.-R. Differential effects of rhythmic auditory stimulation and neurodevelopmental treatment/Bobath on gait patterns in adults with cerebral palsy: A randomized controlled trial. Clin. Rehabil. 2012, 26, 904–914. [Google Scholar] [CrossRef]
- Shin, Y.-K.; Chong, H.J.; Kim, S.J.; Cho, S.-R. Effect of rhythmic auditory stimulation on hemiplegic gait patterns. Yonsei Med. J. 2015, 56, 1703–1713. [Google Scholar] [CrossRef]
- Bonnyaud, C.; Pradon, D.; Vuillerme, N.; Bensmail, D.; Roche, N. Spatiotemporal and kinematic parameters relating to oriented gait and turn performance in patients with chronic stroke. PLoS ONE 2015, 10, e0129821. [Google Scholar] [CrossRef]
- Thaut, M.H.; Abiru, M. Rhythmic auditory stimulation in rehabilitation of movement disorders: A review of current research. Music Percept. 2010, 27, 263–269. [Google Scholar] [CrossRef]
- Ghisio, S.; Kolykhalova, K.; Volpe, G.; Amadeo, B.; Coletta, P.; Ferrari, N.; Tacchino, C.; Fiscon, S.; Primavera, L.; Moretti, P.; et al. Designing a Platform for Child Rehabilitation Exergames Based on Interactive Sonification of Motor Behavior. In Proceedings of the 4th EAI International Conference on Smart Objects and Technologies for Social Good, Bologna, Italy, 28–30 November 2018; pp. 208–213. [Google Scholar]
- Ghai, S.; Schmitz, G.; Hwang, T.-H.; Effenberg, A.O. Auditory proprioceptive integration: Effects of real-time kinematic auditory feedback on knee proprioception. Front. Neurosci. 2018, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-H.; Peng, Y.-C.; Chen, Y.-L.; Lu, T.-W.; Liao, H.-F.; Tang, P.-F.; Shieh, J.-Y. A home-based program using patterned sensory enhancement improves resistance exercise effects for children with cerebral palsy: A randomized controlled trial. Neurorehabilit. Neural Repair 2013, 27, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Yeo, M.S.; Kim, S.J. Patterned Sensory Enhancement (PSE) music for upper limb function changes in children with spastic cerebral palsy. Korean J. Phys. Mult. Health Disabil. 2019, 62, 257–274. [Google Scholar] [CrossRef]
- Effenberg, A.O. Movement sonification: Effects on perception and action. IEEE MultiMedia 2005, 12, 53–59. [Google Scholar] [CrossRef]
- Sigrist, R.; Rauter, G.; Riener, R.; Wolf, P. Augmented visual, auditory, haptic, and multimodal feedback in motor learning: A review. Psychon. Bull. Rev. 2013, 20, 21–53. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-C.; Lu, T.-W.; Wang, T.-H.; Chen, Y.-L.; Liao, H.-F.; Lin, K.-H.; Tang, P.-F. Immediate effects of therapeutic music on loaded sit-to-stand movement in children with spastic diplegia. Gait Posture 2011, 33, 274–278. [Google Scholar] [CrossRef]
- Spaulding, S.J.; Barber, B.; Colby, M.; Cormack, B.; Mick, T.; Jenkins, M.E. Cueing and gait improvement among people with Parkinson’s disease: A meta-analysis. Arch. Phys. Med. Rehabil. 2013, 94, 562–570. [Google Scholar] [CrossRef]
- Yoo, G.E.; Kim, S.J. Rhythmic auditory cueing in motor rehabilitation for stroke patients: Systematic review and meta-analysis. J. Music Ther. 2016, 53, 149–177. [Google Scholar] [CrossRef]
- Devlin, K.; Alshaikh, J.T.; Pantelyat, A. Music Therapy and Music-Based Interventions for Movement Disorders. Curr. Neurol. Neurosci. Rep. 2019, 19, 83. [Google Scholar] [CrossRef]
- Wittwer, J.E.; Webster, K.E.; Hill, K. Rhythmic auditory cueing to improve walking in patients with neurological conditions other than Parkinson’s disease—what is the evidence? Disabil. Rehabil. 2013, 35, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I.; Effenberg, A.O. Effect of rhythmic auditory cueing on aging gait: A systematic review and meta-analysis. Aging Dis. 2018, 9, 901. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I. Effects of (music-based) rhythmic auditory cueing training on gait and posture post-stroke: A systematic review & dose-response meta-analysis. Sci. Rep. 2019, 9, 2183. [Google Scholar] [PubMed]
- Ghai, S.; Ghai, I. Effects of rhythmic auditory cueing in gait rehabilitation for multiple sclerosis: A mini systematic review and meta-analysis. Front. Neurol. 2018, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Maso, F.D.; Ogourtsova, T.; Porxas, A.-X.; Villeneuve, M.; Penhune, V.; Boudrias, M.-H.; Baillet, S.; Lamontagne, A. Neurophysiological changes induced by music-supported therapy for recovering upper extremity function after stroke: A case series. Brain Sci. 2021, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Crasta, J.E.; Thaut, M.H.; Anderson, C.W.; Davies, P.L.; Gavin, W.J. Auditory priming improves neural synchronization in auditory-motor entrainment. Neuropsychologia 2018, 117, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Altenmüller, E.; Marco-Pallares, J.; Münte, T.F.; Schneider, S. Neural Reorganization Underlies Improvement in Stroke-induced Motor Dysfunction by Music-supported Therapy. Ann. N. Y. Acad. Sci. 2009, 1169, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Jamali, S.; Fujioka, T.; Ross, B. Neuromagnetic beta and gamma oscillations in the somatosensory cortex after music training in healthy older adults and a chronic stroke patient. Clin. Neurophysiol. 2014, 125, 1213–1222. [Google Scholar] [CrossRef]
- Flor, H.; Diers, M. Sensorimotor training and cortical reorganization. NeuroRehabilitation 2009, 25, 19–27. [Google Scholar] [CrossRef]
- Varlet, M.; Nozaradan, S.; Trainor, L.; Keller, P.E. Dynamic modulation of beta band cortico-muscular coupling induced by audio–visual rhythms. Cereb. Cortex Commun. 2020, 1, tgaa043. [Google Scholar] [CrossRef]
- Woelfle, R.; Grahn, J.A. Auditory and visual interhemispheric communication in musicians and non-musicians. PLoS ONE 2013, 8, e84446. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A. The brain that plays music and is changed by it. Ann. N. Y. Acad. Sci. 2001, 930, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I.; Effenberg, A.O. Effects of dual tasks and dual-task training on postural stability: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 557. [Google Scholar] [CrossRef]
- Ronsse, R.; Puttemans, V.; Coxon, J.P.; Goble, D.J.; Wagemans, J.; Wenderoth, N.; Swinnen, S.P. Motor learning with augmented feedback: Modality-dependent behavioral and neural consequences. Cereb. Cortex 2011, 21, 1283–1294. [Google Scholar] [CrossRef]
- Tian, R.; Zhang, B.; Zhu, Y. Rhythmic Auditory Stimulation as an Adjuvant Therapy Improved Post-stroke Motor Functions of the Upper Extremity: A Randomized Controlled Pilot Study. Front. Neurosci. 2020, 14, 649. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, R.S. Auditory rhythmic cueing in movement rehabilitation: Findings and possible mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130402. [Google Scholar] [CrossRef]
- Fritz, T.H.; Halfpaap, J.; Grahl, S.; Kirkland, A.; Villringer, A. Musical feedback during exercise machine workout enhances mood. Front. Psychol. 2013, 4, 921. [Google Scholar] [CrossRef]
- Bella, S.D.; Dotov, D.; Bardy, B.; de Cock, V.C. Individualization of music-based rhythmic auditory cueing in Parkinson’s disease. Ann. N. Y. Acad. Sci. 2018, 1423, 308–317. [Google Scholar] [CrossRef]
- Effenberg, A.O.; Fehse, U.; Schmitz, G.; Krueger, B.; Mechling, H. Movement sonification: Effects on motor learning beyond rhythmic adjustments. Front. Neurosci. 2016, 10, 219. [Google Scholar] [CrossRef]
- Ghai, S.; Ghai, I.; Schmitz, G.; Effenberg, A.O. Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 506. [Google Scholar] [CrossRef]
- Ghai, S. Effects of real-time (sonification) and rhythmic auditory stimuli on recovering arm function post stroke: A systematic review and meta-analysis. Front. Neurol. 2018, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Mohammadi, B.; Hammer, A.; Heldmann, M.; Samii, A.; Münte, T.F.; Effenberg, A.O. Observation of sonified movements engages a basal ganglia frontocortical network. BMC Neurosci. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Yovanoff, M.A.; Chen, H.-E.; Pepley, D.F.; Mirkin, K.A.; Han, D.C.; Moore, J.Z.; Miller, S.R. Investigating the Effect of Simulator Functional Fidelity and Personalized Feedback on Central Venous Catheterization Training. J. Surg. Educ. 2018, 75, 1410–1421. [Google Scholar] [CrossRef] [PubMed]
- Françoise, J.; Bevilacqua, F. Motion-sound mapping through interaction: An approach to user-centered design of auditory feedback using machine learning. ACM Trans. Interact. Intell. Syst. (TiiS) 2018, 8, 1–30. [Google Scholar] [CrossRef]
- Iber, M.; Dumphart, B.; Oliveira, V.-A.d.J.; Ferstl, S.; Reis, J.M.; Slijepčević, D.; Heller, M.; Raberger, A.-M.; Horsak, B. Mind the Steps: Towards Auditory Feedback in Tele-Rehabilitation Based on Automated Gait Classification. In Proceedings of the Audio Mostly 2021, virtual/Trento, Italy, 1–3 September 2021; pp. 139–146. [Google Scholar]
- Thaut, M.H.; McIntosh, G.C.; Hoemberg, V. Neurobiological foundations of neurologic music therapy: Rhythmic entrainment and the motor system. Front. Psychol. 2015, 5, 1185. [Google Scholar] [CrossRef]
- Milligan, G.W. Factors that affect Type I and Type II error rates in the analysis of multidimensional contingency tables. Psychol. Bull. 1980, 87, 238. [Google Scholar] [CrossRef]
- Valentine, J.C.; Thompson, S.G. Issues relating to confounding and meta-analysis when including non-randomized studies in systematic reviews on the effects of interventions. Res. Synth. Methods 2013, 4, 26–35. [Google Scholar] [CrossRef]
- Rasmussen, H.M.; Nielsen, D.B.; Pedersen, N.W.; Overgaard, S.; Holsgaard-Larsen, A. Gait Deviation Index, Gait Profile Score and Gait Variable Score in children with spastic cerebral palsy: Intra-rater reliability and agreement across two repeated sessions. Gait Posture 2015, 42, 133–137. [Google Scholar] [CrossRef]
- Jantakat, C.; Ramrit, S.; Emasithi, A.; Siritaratiwat, W. Capacity of adolescents with cerebral palsy on paediatric balance scale and Berg balance scale. Res. Dev. Disabil. 2015, 36, 72–77. [Google Scholar] [CrossRef]
- Carey, H.; Martin, K.; Combs-Miller, S.; Heathcock, J.C. Reliability and responsiveness of the timed up and go test in children with cerebral palsy. Pediatric Phys. Ther. 2016, 28, 401–408. [Google Scholar] [CrossRef]
- Russell, D.J.; Avery, L.M.; Rosenbaum, P.L.; Raina, P.S.; Walter, S.D.; Palisano, R.J. Improved scaling of the gross motor function measure for children with cerebral palsy: Evidence of reliability and validity. Phys. Ther. 2000, 80, 873–885. [Google Scholar] [PubMed]
- Moseley, A.M.; Rahman, P.; Wells, G.A.; Zadro, J.R.; Sherrington, C.; Toupin-April, K.; Brosseau, L. Agreement between the Cochrane risk of bias tool and Physiotherapy Evidence Database (PEDro) scale: A meta-epidemiological study of randomized controlled trials of physical therapy interventions. PLoS ONE 2019, 14, e0222770. [Google Scholar] [CrossRef] [PubMed]
- Hamed, N.S.; Abd-elwahab, M.S. Pedometer-based gait training in children with spastic hemiparetic cerebral palsy: A randomized controlled study. Clin. Rehabil. 2011, 25, 157–165. [Google Scholar] [CrossRef]
- Kim, S.J.; Kwak, E.E.; Park, E.S.; Lee, D.S.; Kim, K.J.; Song, J.E.; Cho, S.-R. Changes in gait patterns with rhythmic auditory stimulation in adults with cerebral palsy. NeuroRehabilitation 2011, 29, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Baram, Y.; Lenger, R. Gait improvement in patients with cerebral palsy by visual and auditory feedback. Neuromodulation: Technol. Neural Interface 2012, 15, 48–52. [Google Scholar]
- Jiang, A. The Effect of Rhythmic Auditory Stimulation on Gait in Young Children with Spastic Cerebral Palsy. Doctoral dissertation, University of Miami, Coral Gables, FL, USA, 2013. [Google Scholar]
- Kwak, E.E. Effect of Rhythmic Auditory Stimulation on Gait Performance in Children with Spastic Cerebral Palsy. J. Music Ther. 2007, 44, 198–216. [Google Scholar] [CrossRef]
- Varsamis, P.; Staikopoulos, K.; Kartasidou, L. Effect of Rhythmic Auditory Stimulation on Controlling Stepping Cadence of Individuals with Mental Retardation and Cerebral Palsy. Int. J. Spec. Educ. 2012, 27, 68–75. [Google Scholar]
- Espy, D.D.; Yang, F.; Bhatt, T.; Pai, Y.C. Independent influence of gait speed and step length on stability and fall risk. Gait Posture 2010, 32, 378–382. [Google Scholar] [CrossRef]
- Nascimento, L.R.; de Oliveira, C.Q.; Ada, L.; Michaelsen, S.M.; Teixeira-Salmela, L.F. Walking training with cueing of cadence improves walking speed and stride length after stroke more than walking training alone: A systematic review. J. Physiother. 2015, 61, 10–15. [Google Scholar]
- Flather, M.D.; Farkouh, M.E.; Pogue, J.M.; Yusuf, S. Strengths and limitations of meta-analysis: Larger studies may be more reliable. Control. Clin. Trials 1997, 18, 568–579. [Google Scholar]
- Pavão, S.L.; Rocha, N.A.C.F. Sensory processing disorders in children with cerebral palsy. Infant Behav. Dev. 2017, 46, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.J.; Becker, K.M.; Heinrichs-Graham, E.; Wilson, T.W. Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev. Med. Child Neurol. 2014, 56, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.S.; Dracup, K.; Erickson, V.; McCarthy, W.J.; Hamilton, M.A.; Fonarow, G.C. Validity of pedometers for measuring exercise adherence in heart failure patients. J. Card. Fail. 2005, 11, 366–371. [Google Scholar] [CrossRef] [PubMed]
- An, W.W.; Ting, K.-H.; Au, I.P.H.; Zhang, J.H.; Chan, Z.Y.S.; Davis, I.S.; So, W.K.Y.; Chan, R.H.M.; Cheung, R.T.H. Neurophysiological correlates of gait retraining with real-time visual and auditory feedback. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1341–1349. [Google Scholar] [CrossRef]
- Kotchoubey, B.; Pavlov, Y.G.; Kleber, B. Music in research and rehabilitation of disorders of consciousness: Psychological and neurophysiological foundations. Front. Psychol. 2015, 6, 1763. [Google Scholar] [CrossRef] [PubMed]
- Thaut, M.H. The discovery of human auditory–motor entrainment and its role in the development of neurologic music therapy. Prog. Brain Res. 2015, 217, 253–266. [Google Scholar]
- Lamontagne, A.; Fung, J. Faster is better: Implications for speed-intensive gait training after stroke. Stroke 2004, 35, 2543–2548. [Google Scholar] [CrossRef]
- Oudenhoven, L.M.; van Vulpen, L.F.; Dallmeijer, A.J.; de Groot, S.; Buizer, A.I.; van der Krogt, M.M. Effects of functional power training on gait kinematics in children with cerebral palsy. Gait Posture 2019, 73, 168–172. [Google Scholar] [CrossRef]
- Lorentzen, J.; Frisk, R.; Willerslev-Olsen, M.; Bouyer, L.; Farmer, S.F.; Nielsen, J.B. Gait training facilitates push-off and improves gait symmetry in children with cerebral palsy. Hum. Mov. Sci. 2020, 69, 102565. [Google Scholar] [CrossRef]
- Bangert, M.; Altenmüller, E.O. Mapping perception to action in piano practice: A longitudinal DC-EEG study. BMC Neurosci. 2003, 4, 26. [Google Scholar] [CrossRef]
- Brydges, C.R. Effect Size Guidelines, Sample Size Calculations, and Statistical Power in Gerontology. Innov Aging 2019, 3, igz036. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Schmitz, G.; Hwang, T.H.; Effenberg, A.O. Training proprioception with sound: Effects of real-time auditory feedback on intermodal learning. Ann. N. Y. Acad. Sci. 2019, 1438, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Danna, J.; Velay, J.-L. On the auditory-proprioception substitution hypothesis: Movement sonification in two deafferented subjects learning to write new characters. Front. Neurosci. 2017, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Effenberg, A.O.; Schmitz, G. Acceleration and deceleration at constant speed: Systematic modulation of motion perception by kinematic sonification. Ann. N. Y. Acad. Sci. 2018, 1425, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Oppici, L.; Grütters, K.; Garofolini, A.; Rosenkranz, R.; Narciss, S. Deliberate Practice and Motor Learning Principles to Underpin the Design of Training Interventions for Improving Lifting Movement in the Occupational Sector: A Perspective and a Pilot Study on the Role of Augmented Feedback. Front. Sports Act. Living 2021, 3, 746142. [Google Scholar] [CrossRef]
- Oppici, L.; Bobbe, T.; Lüneburg, L.-M.; Nocke, A.; Schwendicke, A.; Winger, H.; Krzywinski, J.; Cherif, C.; Strufe, T.; Narciss, S. Internet of Skills. In Tactile Internet; Elsevier: Amsterdam, The Netherlands, 2021; pp. 75–99. [Google Scholar]
- Trabacca, A.; Vespino, T.; Di Liddo, A.; Russo, L. Multidisciplinary rehabilitation for patients with cerebral palsy: Improving long-term care. J. Multidiscip. Healthc. 2016, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Driller, M.; Ghai, I. Effects of joint stabilizers on proprioception and stability: A systematic review and meta-analysis. Phys. Ther. Sport 2017, 25, 65–75. [Google Scholar] [CrossRef]
- Shamsoddini, A.; Rasti, Z.; Kalantari, M.; Hollisaz, M.T.; Sobhani, V.; Dalvand, H.; Bakhshandeh-Bali, M.K. The impact of Kinesio taping technique on children with cerebral palsy. Iran. J. Neurol. 2016, 15, 219. [Google Scholar]
- Abdel Ghafar, M.A.; Abdelraouf, O.R.; Abdel-aziem, A.A.; Mousa, G.S.; Selim, A.O.; Mohamed, M.E. Combination taping technique versus ankle foot orthosis on improving gait parameters in spastic cerebral palsy: A controlled randomized study. J. Rehabil. Med. 2021, 53, jrm00240. [Google Scholar] [CrossRef]

| PEDro score | Point estimates and variability | Between-group comparison | Intention to treat | Adequate follow up | Blinded assessors | Blinded therapists | Blinded subjects | Baseline comparability | Concealed allocation | Random allocation | Eligibility criteria | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gerek and Moghadamİ [12] | 4 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Kim, Yoo [13] | 5 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Duymaz [14] | 6 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Ben-Pazi, Aran [15] | 8 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Efraimidou, Tsimaras [17] | 5 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Shin, Chong [19] | 5 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Jiang [68] | 5 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Wang, Peng [24] | 8 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Varsamis, Staikopoulos [70] | 5 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Kim, Kwak [18] | 8 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Baram and Lenger [67] | 5 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Kim, Kwak [66] | 5 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Hamed and Abd-elwahab [65] | 8 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Kwak [69] | 5 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghai, S.; Ghai, I.; Narciss, S. Auditory Stimulation Improves Gait and Posture in Cerebral Palsy: A Systematic Review with Between- and Within-Group Meta-Analysis. Children 2022, 9, 1752. https://doi.org/10.3390/children9111752
Ghai S, Ghai I, Narciss S. Auditory Stimulation Improves Gait and Posture in Cerebral Palsy: A Systematic Review with Between- and Within-Group Meta-Analysis. Children. 2022; 9(11):1752. https://doi.org/10.3390/children9111752
Chicago/Turabian StyleGhai, Shashank, Ishan Ghai, and Susanne Narciss. 2022. "Auditory Stimulation Improves Gait and Posture in Cerebral Palsy: A Systematic Review with Between- and Within-Group Meta-Analysis" Children 9, no. 11: 1752. https://doi.org/10.3390/children9111752
APA StyleGhai, S., Ghai, I., & Narciss, S. (2022). Auditory Stimulation Improves Gait and Posture in Cerebral Palsy: A Systematic Review with Between- and Within-Group Meta-Analysis. Children, 9(11), 1752. https://doi.org/10.3390/children9111752








