A Bayesian Framework to Assess the Usability of Dry Powder Inhalers in a Cohort of Asthma Adolescents in Italy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Virchow, J.C.; Crompton, G.K.; Dal Negro, R.W.; Pedersen, S.; Magnan, A.; Seidemberg, J.; Barnes, P.J. Importance of inhaler devices in the management of airway diseases. Respir. Med. 2008, 102, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, P.; Taylor, A.; Zanen, P.; Chrystyn, H. Can patients use all dry powder inhalers equally well? Int. J. Clin. Pract. Suppl. 2005, 149, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Barrons, R.; Pegram, A.; Borrens, A. Inhaler device selection: Special considerations in elderly patients with chronic obstructive pulmonary disease. Am. J. Health Syst. Pharm. 2011, 68, 1221–1232. [Google Scholar] [CrossRef]
- Newman, S.P.; Busse, W.W. Evolution of dry powder inhaler design, formulation, and performance. Respir. Med. 2002, 96, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Wieshammer, S.; Dreyhaupt, J. Dry Powder Inhalers: Which Factors Determine the Frequency of Handling Errors? Respiration 2008, 75, 18–25. [Google Scholar] [CrossRef]
- Chrystyn, K. Do patients show the same level of adherence with all dry powder inhalers? Int. J. Clin. Pract. 2005, 149, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Melani, A.S. Inhalation therapy training: A priority challenge for the physician. Acta Biomed. 2007, 78, 233–245. [Google Scholar] [PubMed]
- Thomas, M.; Williams, A.E. Are outcomes the same with all dry powder inhalers? Int. J. Clin. Pract. Suppl. 2005, 149, 33–35. [Google Scholar] [CrossRef]
- Barry, P.W.; O’Callaghan, C. The influence of inhaler selection on efficacy of asthma therapies. Adv. Drug Deliv. Rev. 2003, 55, 879–923. [Google Scholar] [CrossRef]
- Lenney, J.; Innes, J.A.; Crompton, G.K. Inappropriate inhaler use: Assessment of use and patient preference of seven inhalation devices. EDICI. Respir. Med. 2000, 94, 496–500. [Google Scholar] [CrossRef] [Green Version]
- Anderson, P. Patient preference for and satisfaction with inhaler devices. Eur. Respir. Rev. 2005, 96, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Schulte, M.; Osseiran, K.; Betz, R.; Wenker, M.; Brand, P.; Meyer, T.; Haidl, P. Handling of and Preferences for Available Dry Powder Inhaler Systems by Patients with Asthma and COPD. J. Aerosol Med. Pulm. Drug Deliv. 2008, 21, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Franks, M.; Briggs, P. Use of a cognitive ergonomics approach to compare usability of a multidose dry powder inhaler and a capsule dry powder inhaler: An open label, randomized, controlled study. Clin. Ther. 2004, 26, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Cross, S. Asthma Inhalation Delivery Systems: The Patient’s Viewpoint. J. Aerosol Med. 2001, 14 (Suppl. S1), 3–7. [Google Scholar] [CrossRef]
- Van Der Palen, J.; Ginko, T.; Kroker, A.; Van Der Valk, P.; Goosens, M.; Padullés, L.; Seoane, B.; Rekeda, L.; Gil, E.G. Preference, satisfaction and errors with two dry powder inhalers in patients with COPD. Expert Opin. Drug Deliv. 2013, 10, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Tordera, M.P.; Viejo, J.L.; Sanchis, J.; Badia, X.; Cobos, N.; Picado, C.; Sobradillo, V.; Del Río, J.M.G.; Duce, F.; Cabrera, L.M. Assessment of patient satisfaction and preferences with inhalers in asthma with the FSI-10 Questionnaire. Arch. Bronconeumol. 2008, 44, 346–352. [Google Scholar] [CrossRef]
- Zervas, E.; Samitas, K.; Gaga, M. Assessment of satisfaction with different dry powder inhalation devices in Greek patients with COPD and asthma: The ANASA study. Int. J. Chron. Obstruct Pulmon. Dis. 2016, 11, 1845–1855. [Google Scholar]
- Kozma, C.M.; Slaton, T.L.; Monz, B.U.; Hodder, R.; Reese, P.R. Development and Validation of a Patient Satisfaction and Preference Questionnaire for Inhalation Devices. Treat. Respir. Med. 2005, 4, 41–52. [Google Scholar] [CrossRef]
- Dal Negro, R.W.; Turco, P.; Povero, M. The Global Usability Score: A Novel Comprehensive Tool for Assessing, Ranking, and Compare Usability of Inhalers in Patients Requiring Airway Treatments. J. Pulm. Respir. Med. 2017, 7, 2. [Google Scholar]
- Povero, M.; Turco, P.; Bonadiman, L.; Dal Negro, R.W. The Global Usability Score Short-Form for the simplified assessment of dry powder inhalers (DPIs) usability. Multidiscip. Respir. Med. 2020, 15, 659. [Google Scholar] [CrossRef]
- Kruger, P.; Ehrlein, B.; Zier, M.; Greguletz, R. Inspiratory flow resistance of marketed dry powder inhalers. Eur. Respir. J. 2014, 44 (Suppl. 58), 4635. [Google Scholar]
- Dal Negro, R.W.; Turco, P.; Povero, M. The contribution of patients’ lung function to the inspiratory airflow rate achievable through a DPIs’ simulator reproducing different intrinsic resistance rates. Multidiscip. Respir. Med. 2021, 16, 752. [Google Scholar] [CrossRef]
- Lunn, D.J.; Thomas, A.; Best, N.; Spiegelhalter, D. A Bayesian modelling framework: Concepts, structure, and extensibility. Stat. Comput. 2000, 10, 325–337. [Google Scholar] [CrossRef]
- Dias, S.; Sutton, A.J.; Ades, A.E.; Welton, N.J. Evidence Synthesis for Decision Making 2: A Generalized Linear Modeling Framework for Pairwise and Network Meta-analysis of Randomized Controlled Trials. Med. Decis. Mak. 2012, 33, 607–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P.A. Graphical Methods and Numerical Summaries for Presenting Results from Multiple-Treatment Meta-Analysis: An Overview and Tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Rajan, S.K.; A Gogtay, J. Ease-of-use, preference, confidence, and satisfaction with Revolizer®, a novel dry powder inhaler, in an Indian population. Lung India 2014, 31, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Dal Negro, R.W. Dry powder inhalers and the right things to remember: A concept review. Multidiscip. Respir. Med. 2015, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Brocklebank, D.; Ram, F.; Wright, J.; Barry, P.; Cates, C.; Davies, L.; Douglas, G.; Muers, M.; Smith, D.; White, J. Comparison of the effectiveness of inhaler devices in asthma and chronic airways disease: A systemic review of the literature. Health Technol. Assess. 2001, 5, 1–149. [Google Scholar] [CrossRef]
- Dal Negro, R.W.; Turco, P.; Povero, M. Assessing the Global Usability of Dry Powder Inhalers: Analysis of Six Devices Widely Used for Asthma. J. Pulm. Med. Respir. Res. 2021, 7, 64. [Google Scholar]
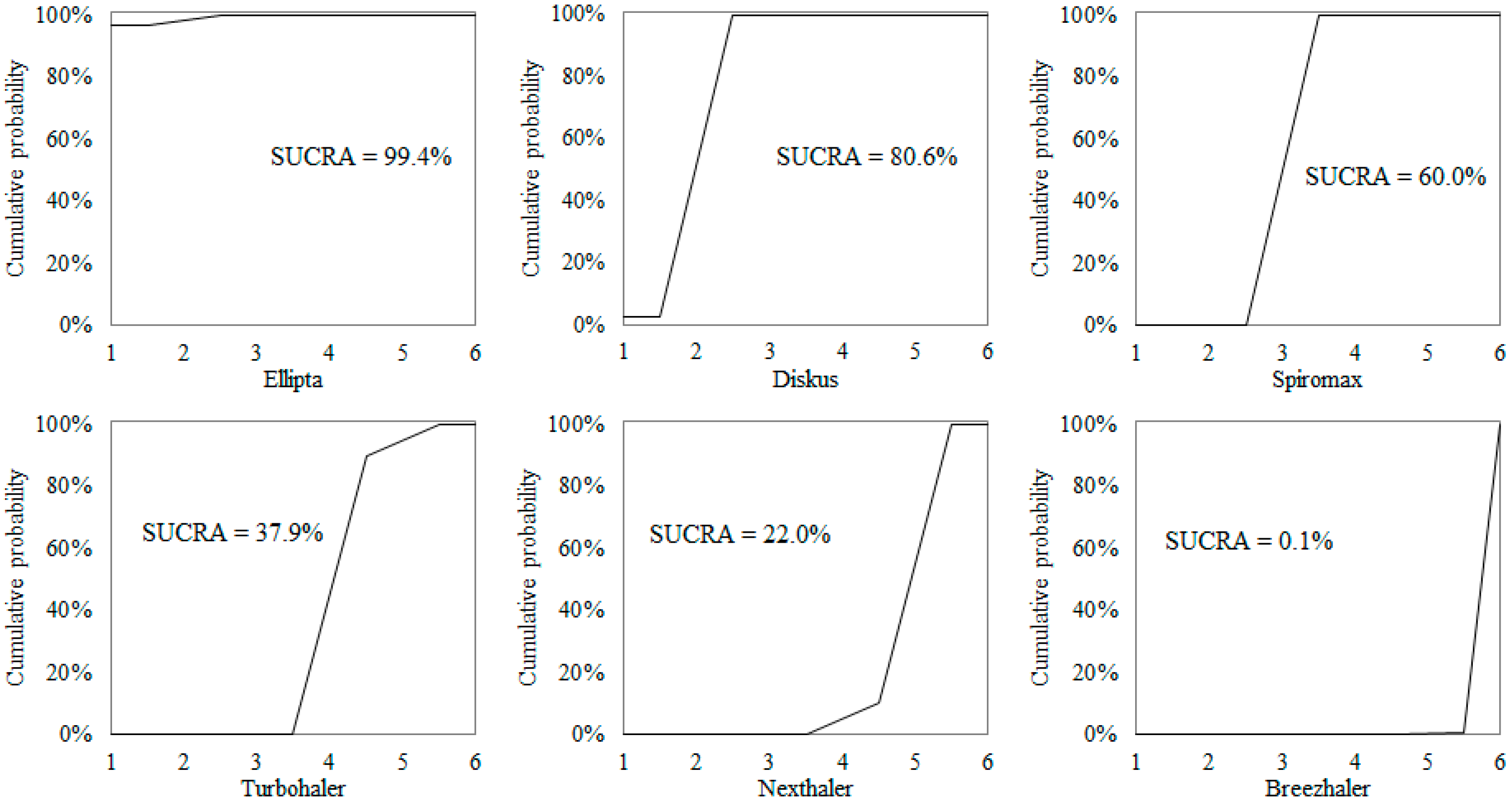

| Total (N = 33) | Group 1 (N = 18) | Group 2 (N = 15) | p-Value | |
|---|---|---|---|---|
| Instructed to DPIs (%) | 17 (51.5%) | 8 (44.4%) | 9 (60.0%) | 0.295 |
| Instructed to MDIs (%) | 11 (33.3%) | 5 (27.8%) | 6 (40.0%) | 0.355 |
| Instructed to SMIs (%) | 2 (6.1%) | 1 (5.6%) | 1 (6.7%) | 0.71 |
| Mean age (SD) | 16.3 (2.47) | 16.4 (2.05) | 16.4 (1.69) | 0.621 |
| Male (%) | 17 (51.5%) | 7 (38.9%) | 10 (66.7%) | 0.107 |
| Country (%) | 0.199 | |||
| North | 25 (75.8%) | 15 (83.3%) | 10 (66.7%) | |
| Center | 3 (9.1%) | 0 (0.0%) | 3 (20.0%) | |
| South and Islands | 5 (15.2%) | 3 (16.7%) | 2 (13.3%) | |
| Education (%) | 0.391 | |||
| Lower secondary | 26 (78.8%) | 15 (83.3%) | 11 (73.3%) | |
| Upper secondary | 7 (21.2%) | 3 (16.7%) | 4 (26.7%) |
| Ellipta S-GUS = 28.5 90% CrI (20.6 to 39.2) | Post p = 0.970 | Post p = 1.000 | Post p = 1.000 | Post p = 1.000 | Post p = 1.000 |
| AMD = 3.44 90% CrI (0.4 to 6.5) | Diskus S-GUS = 25.1 90% CrI (17.2 to 35.8) | Post p = 0.999 | Post p = 1.000 | Post p = 1.000 | Post p = 1.000 |
| AMD = 7.92 90% CrI (5.6 to 10.2) | AMD = 4.48 90% CrI (2.1 to 6.8) | Spiromax S-GUS = 20.6 90% CrI (12.9 to 31.2) | Post p = 1.000 | Post p = 1.000 | Post p = 1.000 |
| AMD = 14.26 90% CrI (11.4 to 17.1) | AMD = 10.82 90% CrI (8.3 to 13.3) | AMD = 6.34 90% CrI (4.2 to 8.5) | Turbohaler S-GUS = 14.3 90% CrI (6.4 to 25.0) | Post p = 0.897 | Post p = 1.000 |
| AMD = 16.32 90% CrI (14.0 to 18.6) | AMD = 12.88 90% CrI (10.0 to 15.7) | AMD = 8.40 90% CrI (6.4 to 10.4) | AMD = 2.06 90% CrI (−0.6 to 4.7) | Nexthaler S-GUS = 12.2 90% CrI (4.4 to 22.9) | Post p = 0.997 |
| AMD = 19.52 90% CrI (17.3 to 21.7) | AMD = 16.08 90% CrI (13.8 to 18.4) | AMD = 11.60 90% CrI (10.0 to 13.2) | AMD = 5.26 90% CrI (3.1 to 7.4) | AMD = 3.20 90% CrI (1.3 to 5.1) | Breezhaler S-GUS = 9.0 90% CrI (1.4 to 19.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Negro, R.W.; Povero, M. A Bayesian Framework to Assess the Usability of Dry Powder Inhalers in a Cohort of Asthma Adolescents in Italy. Children 2022, 9, 28. https://doi.org/10.3390/children9010028
Dal Negro RW, Povero M. A Bayesian Framework to Assess the Usability of Dry Powder Inhalers in a Cohort of Asthma Adolescents in Italy. Children. 2022; 9(1):28. https://doi.org/10.3390/children9010028
Chicago/Turabian StyleDal Negro, Roberto Walter, and Massimiliano Povero. 2022. "A Bayesian Framework to Assess the Usability of Dry Powder Inhalers in a Cohort of Asthma Adolescents in Italy" Children 9, no. 1: 28. https://doi.org/10.3390/children9010028
APA StyleDal Negro, R. W., & Povero, M. (2022). A Bayesian Framework to Assess the Usability of Dry Powder Inhalers in a Cohort of Asthma Adolescents in Italy. Children, 9(1), 28. https://doi.org/10.3390/children9010028






