Update on the Role of High-Flow Nasal Cannula in Infants with Bronchiolitis
Abstract
1. Background
2. High-Flow Nasal Cannula (HFNC) Systems
2.1. The Device
2.2. Mechanisms of Action
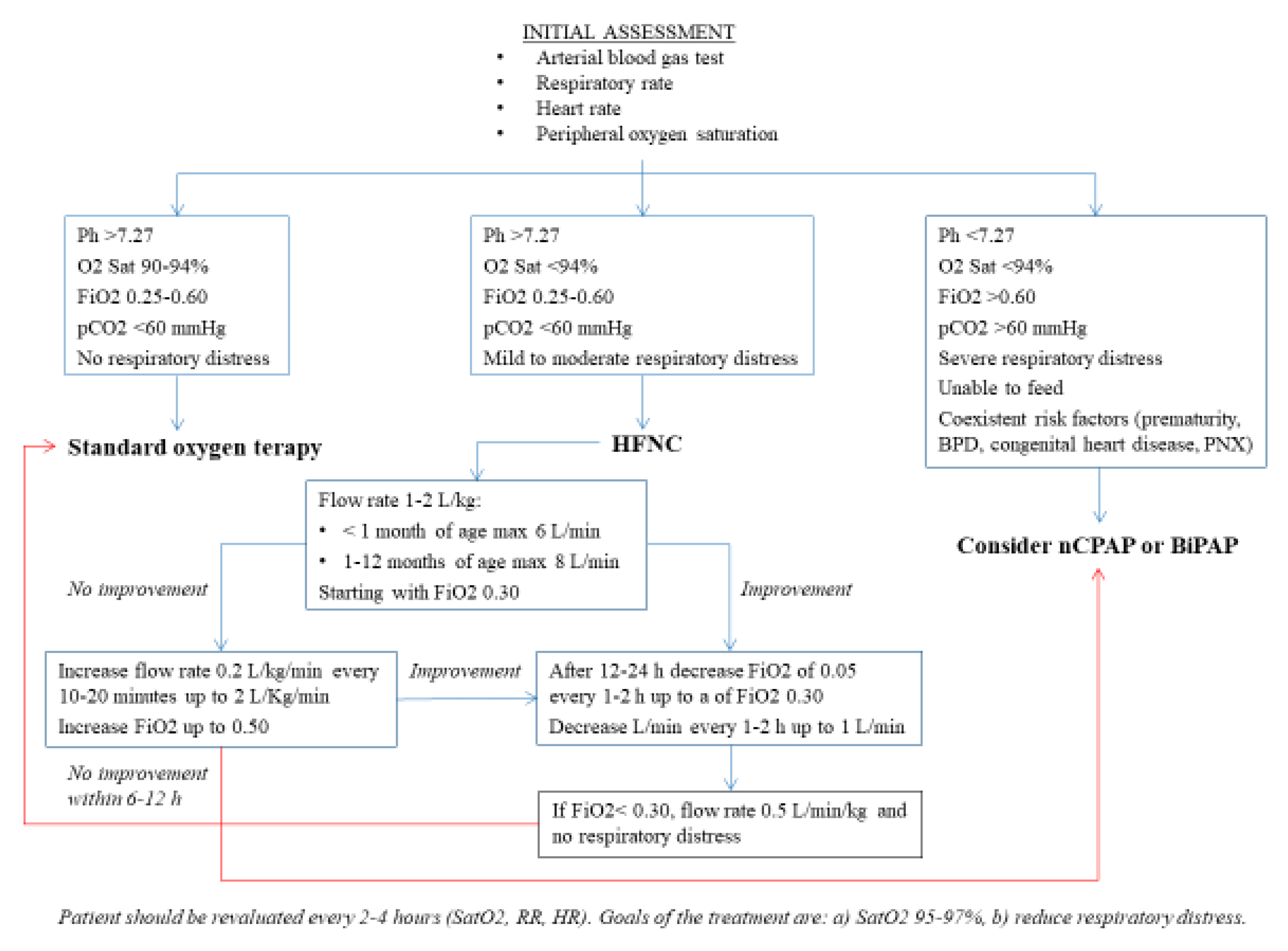
2.3. Use in Children with Bronchiolitis (BR)
3. Efficacy of High-Flow Nasal Cannula (HFNC) Oxygen Administration
3.1. HFNC vs. SOT
3.2. HFNC vs. CPAP and BiPAP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Florin, T.A.; Plint, A.C.; Zorc, J.J. Viral bronchiolitis. Lancet 2017, 389, 211–224. [Google Scholar] [CrossRef]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infec-tions due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Ryu, J.H.; Azadeh, N.; Samhouri, B.; Yi, E. Recent advances in the understanding of bronchiolitis in adults. F1000Research 2020, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.; Hill, V. Incidence of apnea in infants hospitalized with respiratory syncytial virus bronchiolitis: A systematic review. J. Pediatr. 2009, 155, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Damore, D.; Mansbach, J.M.; Clark, S.; Ramundo, M.; Camargo, C.A., Jr. Prospective multicenter bronchiolitis study: Predicting intensive care unit admissions. Acad. Emerg. Med. 2008, 15, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Neumann, R.P.; Von Ungern-Sternberg, B.S.; Ungern-Sternberg, B.S. The neonatal lung—Physiology and ventilation. Pediatr. Anesth. 2013, 24, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Polla, B.; D’Antona, G.; Bottinelli, R.; Reggiani, C. Respiratory muscle fibres: Specialisation and plasticity. Thorax 2004, 59, 808–817. [Google Scholar] [CrossRef]
- Stock, C.; Teyssier, G.; Pichot, V.; Goffaux, P.; Barthélémy, J.-C.; Patural, H. Autonomic dysfunction with early respiratory syncytial virus-related infection. Auton. Neurosci. 2010, 156, 90–95. [Google Scholar] [CrossRef]
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. 2019 Surveillance of Bronchiolitis in Children: Diagnosis and Management (NICE Guideline NG9). Available online: https://www.nice.org.uk/guidance/ng9/resources/2019-surveillance-of-bronchiolitis-in-children-diagnosis-and-management-nice-guideline-ng9-6896788381/chapter/Surveillance-decision?tab=evidence. (accessed on 29 November 2020).
- Baraldi, E.; Lanari, M.; Manzoni, P.; Rossi, G.A.; Vandini, S.; Rimini, A.; Romagnoli, C.; Colonna, P.; Biondi, A.; Biban, P.; et al. Inter-society consensus document on treatment and prevention of bronchiolitis in newborns and infants. Ital. J. Pediatr. 2014, 40, 65. [Google Scholar] [CrossRef]
- Roodsari, G.; Zehtabchi, S. Nebulized hypertonic saline for bronchiolitis. Am. Fam. Physician 2018, 98, 23–24. [Google Scholar] [PubMed]
- Lodeserto, F.J.; Lettich, T.M.; Rezaie, S.R. High-flow nasal cannula: Mechanisms of action and adult and pediatric indications. Cureus 2018, 10, e3639. [Google Scholar] [CrossRef] [PubMed]
- Principi, T.; Fraser, D.D.; Morrison, G.C.; Farsi, S.A.; Carrelas, J.F.; Maurice, E.A.; Kornecki, A. Complications of mechanical venti-lation in the pediatric population. Pediatr. Pulmonol. 2011, 46, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Goligher, E.C.; Slutsky, A.S. Not just oxygen? Mechanisms of benefit from high-flow nasal cannula in hypoxemic respir-atory failure. Am. J. Respir. Crit. Care Med. 2017, 195, 1128–1131. [Google Scholar] [CrossRef]
- Shein, S.L.; Slain, K.N.; Rotta, A.T.; Milési, C.; Cambonie, G. High-flow nasal cannula flow rate in young infants with severe viral bronchiolitis: The question is still open. Intensiv. Care Med. 2018, 45, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M. High-flow nasal cannula oxygen therapy devices. Respir. Care 2019, 64, 735–742. [Google Scholar] [CrossRef]
- US Food and Drug Administration. High Flow/High Velocity Humidified Oxygen Delivery Device. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?id=203. (accessed on 30 November 2020).
- Mauri, T.; Turrini, C.; Eronia, N.; Grasselli, G.; Volta, C.A.; Bellani, G.; Pesenti, A. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am. J. Respir. Crit. Care Med. 2017, 195, 1207–1215. [Google Scholar] [CrossRef]
- Rubin, S.; Ghuman, A.; Deakers, T.; Khemani, R.; Ross, P.; Newth, C.J. Effort of breathing in children receiving high-flow nasal cannula. Pediatr. Crit. Care Med. 2014, 15, 1–6. [Google Scholar] [CrossRef]
- Kwon, J.-W. High-flow nasal cannula oxygen therapy in children: A clinical review. Clin. Exp. Pediatr. 2020, 63, 3–7. [Google Scholar] [CrossRef]
- Cheng, A.Y.; Simon, H.K.; Miller, J.; Wetzel, M.; Zmitrovich, A.; Hebbar, K.B. Survey of current institutional practices in the use of high-flow nasal cannula for pediatric patients. Pediatr. Emerg. Care 2020. [Google Scholar] [CrossRef]
- NSW Government Humidified High Flow Nasal Cannula Oxygen Guideline for Metropolitan Paediatric Wards and EDs, 1st Edition: Guideline. Available online: https://www1.health.nsw.gov.au/pds/ActivePDSDocuments/GL2016_004.pdf. (accessed on 30 November 2020).
- Metge, P.; Grimaldi, C.; Hassid, S.; Thomachot, L.; Loundou, A.; Martin, C.; Michel, F. Comparison of a high-flow humidified nasal cannula to nasal continuous positive airway pressure in children with acute bronchiolitis: Experience in a pediatric intensive care unit. Eur. J. Nucl. Med. Mol. Imaging 2014, 173, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Brink, F.T.; Duke, T.; Evans, J. High-flow nasal prong oxygen therapy or nasopharyngeal continuous positive airway pressure for children with moderate-to-severe respiratory distress?*. Pediatr. Crit. Care Med. 2013, 14, e326–e331. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Iodice, F.; Ricci, Z.; Vitale, V.; De Razza, F.; Haiberger, R.; Iacoella, C.; Conti, G.; Cogo, P. Comparative evaluation of high-flow nasal cannula and conventional oxygen therapy in paediatric cardiac surgical patients: A randomized controlled trial. Interact. Cardiovasc. Thorac. Surg. 2014, 19, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.M.; O’Malley, L.; Mayfield, S.; Martin, S.; Schibler, A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr. Pulmonol. 2015, 50, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Wraight, T.I.; Ganu, S.S. High-flow nasal cannula use in a paediatric intensive care unit over 3 years. Crit. Care Resusc. 2015, 17, 197–201. [Google Scholar] [PubMed]
- Milani, G.P.; Plebani, A.M.; Arturi, E.; Brusa, D.; Esposito, S.; Dell’Era, L.; Laicini, E.A.; Consonni, D.; Agostoni, C.; Fossali, E.F. Using a high-flow nasal cannula provided superior results to low-flow oxygen delivery in moderate to severe bronchiolitis. Acta Paediatr. 2016, 105, e368–e372. [Google Scholar] [CrossRef]
- Franklin, D.; Babl, F.E.; Schlapbach, L.J.; Oakley, E.; Craig, S.; Neutze, J.; Furyk, J.; Fraser, J.F.; Jones, M.; Whitty, J.A.; et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N. Engl. J. Med. 2018, 378, 1121–1131. [Google Scholar] [CrossRef]
- Kepreotes, E.; Whitehead, B.; Attia, J.; Oldmeadow, C.; Collison, A.; Searles, A.; Goddard, B.; Hilton, J.; Lee, M.; Mattes, J. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): An open, phase 4, randomised controlled trial. Lancet 2017, 389, 930–939. [Google Scholar] [CrossRef]
- Hilliard, T.N.; Archer, N.; Laura, H.; Heraghty, J.; Cottis, H.; Mills, K.; Ball, S.; Davis, P. Pilot study of vapotherm oxygen delivery in moderately severe bronchiolitis. Arch. Dis. Child. 2011, 97, 182–183. [Google Scholar] [CrossRef]
- Ergul, A.B.; Calıskan, E.; Samsa, H.; Gokcek, I.; Kaya, A.; Zararsiz, G.; Torun, Y.A. Using a high-flow nasal cannula provides superior results to OxyMask delivery in moderate to severe bronchiolitis: A randomized controlled study. Eur. J. Nucl. Med. Mol. Imaging 2018, 177, 1299–1307. [Google Scholar] [CrossRef]
- Milesi, C.; Pierre, A.-F.; Deho, A.; Pouyau, R.; Liet, J.-M.; Guillot, C.; Guilbert, A.-S.; Rambaud, J.; Millet, A.; Afanetti, M.; et al. A multicenter randomized controlled trial of a 3-L/kg/min versus 2-L/kg/min high-flow nasal cannula flow rate in young infants with severe viral bronchiolitis (TRAMONTANE 2). Intensiv. Care Med. 2018, 44, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, S.; Bogossian, F.; O’Malley, L.; Schibler, A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: Pilot study. J. Paediatr. Child Heal. 2014, 50, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Abboud, P.A.; Roth, P.J.; Skiles, C.L.; Stolfi, A.; Rowin, M.E. Predictors of failure in infants with viral bronchiolitis treated with high-flow, high-humidity nasal cannula therapy*. Pediatr. Crit. Care Med. 2012, 13, e343–e349. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Prodhan, P. Serious air leak syndrome complicating high-flow nasal cannula therapy: A report of 3 cases. Pediatrics 2013, 131, e939–e944. [Google Scholar] [CrossRef] [PubMed]
- Slain, K.N.; Shein, S.L.; Rotta, A.T. The use of high-flow nasal cannula in the pediatric emergency department. J. Pediatr. 2017, 93, 36–45. [Google Scholar] [CrossRef]
- Mikalsen, I.B.; Davis, P.; Øymar, K. High flow nasal cannula in children: A literature review. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 1–12. [Google Scholar] [CrossRef]
- Hutchings, F.A.; Hilliard, T.N.; Davis, P.J. Heated humidified high-flow nasal cannula therapy in children. Arch. Dis. Child. 2014, 100, 571–575. [Google Scholar] [CrossRef]
- Bueno Campaña, M.; Olivares Ortiz, J.; Notario Munoz, C.; Rupérez Lucas, M.; Fernández Rincón, A.; Patiño Hernández, O.; Calvo Rey, C. High flow therapy versus hypertonic saline in bronchiolitis: Randomised controlled trial. Arch. Dis. Child. 2014, 99, 511–515. [Google Scholar] [CrossRef]
- Bressan, S.; Balzani, M.; Krauss, B.; Pettenazzo, A.; Zanconato, S.; Baraldi, E. High-flow nasal cannula oxygen for bronchiolitis in a pediatric ward: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2013, 172, 1649–1656. [Google Scholar] [CrossRef]
- Da Dalt, L.; Bressan, S.; Martinolli, F.; Perilongo, G.; Baraldi, E. Treatment of bronchiolitis: State of the art. Early Hum. Dev. 2013, 89 (Suppl. 1), S31–S36. [Google Scholar] [CrossRef]
- Liu, Z. Clinical observation on the treatment of infant bronchiolitis by warming and humidifying high flow oxygen inha-lation. China Med. Pharm. 2017, 7, 71–73. (In Chinese) [Google Scholar]
- Yan, Z.; Gai, J. Effects of heated, humidified high-flow nasal cannula intreatment of severe bronchiolitis in children: A randomized controlled study of 23 cases. J. Pract. Med. Tech. 2016, 3, 1004–1005. (In Chinese) [Google Scholar]
- Yang, X.; Cui, L.; Mi, Q.; Yan, Z.; Gai, J. Clinical research of humidified high flow nasal cannula for bronchiolitis in children. Chin. Pediatr. Emerg. Med. 2017, 9, 430–433. (In Chinese) [Google Scholar]
- Lin, J.; Zhang, Y.; Xiong, L.; Liu, S.; Gong, C.; Dai, J. High-flow nasal cannula therapy for children with bronchiolitis: A sys-tematic review and meta-analysis. Arch. Dis. Child. 2019, 104, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Moreel, L.; Proesmans, M. High flow nasal cannula as respiratory support in treating infant bronchiolitis: A systematic re-view. Eur. J. Pediatr. 2020, 179, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Sinha, R.; Roychowdhoury, S.; Mukhopadhyay, S.; Ghosh, P.; Dutta, K.; Ghosh, S. Comparative study between nonin-vasive continuous positive airway pressure and hot humidified high-flow nasal cannulae as a mode of respiratory support in infants with acute bronchiolitis in pediatric intensive care unit of a tertiary care hospital. Indian J. Crit. Care Med. 2018, 22, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Vahlkvist, S.; Jürgensen, L.; La Cour, A.; Markoew, S.; Petersen, T.H.; Kofoed, P.-E. High flow nasal cannula and continuous positive airway pressure therapy in treatment of viral bronchiolitis: A randomized clinical trial. Eur. J. Nucl. Med. Mol. Imaging 2019, 179, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Milési, C.; Essouri, S.; Pouyau, R.; Liet, J.-M.; Afanetti, M.; Portefaix, A.; Baleine, J.; Durand, S.; Combes, C.; Douillard, A.; et al. High flow nasal cannula (HFNC) versus nasal continuous positive airway pressure (nCPAP) for the initial respiratory management of acute viral bronchiolitis in young infants: A multicenter randomized controlled trial (TRAMONTANE study). Intensiv. Care Med. 2017, 43, 209–216. [Google Scholar] [CrossRef]
- Clayton, J.A.; McKee, B.; Slain, K.N.; Rotta, A.T.; Shein, S.L. Outcomes of children with bronchiolitis treated with high-flow nasal cannula or noninvasive positive pressure ventilation*. Pediatr. Crit. Care Med. 2019, 20, 128–135. [Google Scholar] [CrossRef]
- Habra, B.; Janahi, I.A.; Dauleh, H.; Chandra, P.; Veten, A. A comparison between high-flow nasal cannula and noninvasive ventilation in the management of infants and young children with acute bronchiolitis in the PICU. Pediatr. Pulmonol. 2020, 55, 455–461. [Google Scholar] [CrossRef]
- Pedersen, M.B.; Vahlkvist, S. Comparison of CPAP and HFNC in management of bronchiolitis in infants and young chil-dren. Children 2017, 4, 28. [Google Scholar] [CrossRef]
- Heikkilä, P.; Sokuri, P.; Mecklin, M.; Nuolivirta, K.; Tapiainen, T.; Peltoniemi, O.; Renko, M.; Korppi, M. Using high-flow nasal cannulas for infants with bronchiolitis admitted to paediatric wards is safe and feasible. Acta Paediatr. 2018, 107, 1971–1976. [Google Scholar] [CrossRef]
- Luo, J.; Duke, T.; Chisti, M.J.; Kepreotes, E.; Kalinowski, V.; Li, J. Efficacy of High-Flow Nasal Cannula vs Standard Oxygen Therapy or Nasal Continuous Positive Airway Pressure in Children with Respiratory Distress: A Meta-Analysis. J. Pediatr. 2019, 215, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Guillot, C.; Le Reun, C.; Behal, H.; Labreuche, J.; Recher, M.; Duhamel, A.; Leteurtre, S. First-line treatment using high-flow nasal cannula for children with severe bronchiolitis: Applicability and risk factors for failure. Arch. Pediatr. 2018, 25, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Suessman, A.; Gray, L.L.; Cavenaugh, S.; Camp, E.A.; Shi, Y.; Meskill, S.D. Clinical factors associated with intubation in the high flow nasal cannula era. Am. J. Emerg. Med. 2020, 38, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.S.; Quinto, D.E.R.; Oliveira, G.C.Z.; Rebello, C.M.; do Prado, C. Nasogastric tube, a warning sign for high-flow nasal cannula failure in infants with bronchiolitis. Sci. Rep. 2020, 10, 15914. [Google Scholar] [CrossRef]
- Kamit, F.; Anil, M.; Anil, A.B.; Berksoy, E.; Gokalp, G. Preemptive high-flow nasal cannula treatment in severe bronchiolitis: Results from a high-volume, resource-limited pediatric emergency department. Pediatr. Int. 2020, 62, 1339–1345. [Google Scholar] [CrossRef]
- Durand, P.; Guiddir, T.; Kyheng, C.; Blanc, F.; Vignaud, O.; Epaud, R.; Dugelay, F.; Breant, I.; Badier, I.; Degas-Bussière, V.; et al. A randomised trial of high-flow nasal cannula in infants with moderate bronchiolitis. Eur. Respir. J. 2020, 56, 1901926. [Google Scholar] [CrossRef]
- Slater, A.; Shann, F.; Pearson, G. PIM2: A revised version of the paediatric index of mortality. Intensiv. Care Med. 2003, 29, 278–285. [Google Scholar] [CrossRef]
| Effects of the High Flow | Effects of the Heated Gas | Effects of Controlled FiO2 |
|---|---|---|
| Physiological dead space washout of waste gases including carbon dioxide (CO2) | Reduction in respiratory workload | No oxygen leak |
| Positive end-expiratory pressure | Reduction of bronchoconstriction | FiO2 up to 1.00 provided to the patient |
| Reduced airway resistance | Increased ciliary clearance | Better monitoring of oxygen saturation |
| Decreased respiratory rate | Better hydration of the mucosa | |
| Increased tidal volume | Better comfort | |
| Increased end-expiratory volume |
| Authors | Year | N. Studies Evaluated | Disease, Type of Patients | Device | Outcome |
|---|---|---|---|---|---|
| Metge et al. [24] | July 2014 | Retrospectively reviewed the medical records of all infants admitted to a pediatric intensive care unit at a tertiary care French hospital during the BR seasons of 2010/11 and 2011/12 | Infants with acute BR | HFNC vs. nCPAP | HFNC is better tolerated, simpler, easier, and associated with less nasal trauma than CPAP. It needs to be confirmed whether HFNC should be used as a first approach in severe BR. |
| Heikkila et al. [55] | November 2018 | Retrospective study | 88 infants under 12 months with BR who received HFNC: 53 on paediatric wards and 35 in paediatric intensive care units | Treatment with HFNC | HFNC treatment was successful in 76 (86%) infants hospitalized in 53 pediatric wards and 23/35 ICU patients. In conclusion, HFNC is safe and often avoids the need for intensive care. |
| Lin et al. [47] | June 2019 | Meta-analysis evaluating 9 RCTs | Infants with BR | Standard therapy with O2 vs. HFNC HFNC vs. nCPAP | This study suggests that HFNC was safe as initial respiratory treatment, but there is insufficient evidence to demonstrate benefits over SOT or nCPAP. |
| Luo et al. [56] | December 2019 | A brief meta-analysis conducted on 8 studies | Infants and children with BR or pneumonia, with mild or severe hypoxemia | Standard therapy with O2 vs. HFNC HFNC vs. nCPAP | Among children <5 years of age with respiratory distress and mild hypoxemia, HFNC reduced the risk of treatment failure compared with SOT. However, nCPAP was associated with a lower risk of treatment failure than HFNC in children aged 1 to 6 months with severe respiratory distress and hypoxemia. No differences in intubation and mortality rates were found between HFNC and SOT or nCPAP. |
| Franklin et al. [30] | 2019 | 9 studies | Children (<2 years) with acute BR | NCPAP vs. HFNC | The use of HFNC therapy reduced intubation rates. |
| Ralston [9] | July 2020 | 6 studies | Children with BR | Standard therapy with O2 vs. HFNC | No overall differences in the length of hospital stay or oxygen therapy between the groups. |
| Authors | Year | Setting | Disease, Type of Patients | Respiratory Support, Device and Flow | Outcome |
|---|---|---|---|---|---|
| Milèsi et al. [34] | 2017 | A multicenter randomized controlled trial performed in 5 pediatric intensive care units | 142 infants up to 6 months old with moderate to severe BR | nCPAP (7 cmH2O) vs. HFNC (2 L/kg/min) | nCPAP was slightly superior to HFNC in the initial respiratory support of these patients. |
| Pedersen et al. [54] | April 2017 | A retrospective study between 2013 and 2015 | 49 children with BR | CPAP vs. HFNC | Evidence of greater effectiveness of CPAP in reducing RR and oxygen need with a higher failure rate for HFNC; no difference emerged, however, for the duration of treatment, the length of hospitalization, or transfer to pediatric intensive care unit. |
| Guillot et al. [57] | April 2018 | Observational prospective study in a pediatric intensive care unit, during two consecutive seasons (2013–2014 without recommendation and 2014–2015 with a study design suggesting HFNC as first-line treatment) | Children with severe BR | HFNC vs. nCPAP HFNC vs. BiPAP | 38% of children on HFNC therapy switched to nCPAP or BiPAP. A high pCO2 value has been correlated with a higher risk of HFNC therapy failure. |
| Clayton et al. [52] | February 2019 | A retrospective study conducted in 92 American pediatric intensive care units | 6496 children with BR | HFNC vs. nCPAP or BiPAP | nCPAP or BiPAP is associated with a greater risk of subsequent need for mechanical ventilation than HFNC. However, confounding factors such as the coexistence of disease or the use of sedatives in children with noninvasive positive pressure ventilation may have influenced the study results. |
| Suessman et al. [58] | December 2019 | A retrospective study from January 2014–January 2018. | 2657 children < 24 months of age with BR. | HFNC in PICU | Lower risk of intubation when heart rate decreased after HFNC application. Increased risk of intubation for infants less than 2 months of age, particularly on days 3 and 4 of RSV infection. |
| Nascimento et al. [59] | September 2020 | A retrospective study from January 2016 to June 2017 | 81 children with BR in pediatric intensive care unit | HFNC in PICU | 21% HFNC failure and need for non-invasive positive pressure ventilation or invasive ventilation. |
| Kamit et al. [60] | May 2020 | A retrospective chart review between 1 January 2015 and 31 December 2016 | 84 patients with severe BR in pediatric intensive care unit | HFNC in PICU | The HFNC failure rate was 27.3%. Risk factors were significant tachycardia, dehydration, and a venous pH <7.30 at hospitalization. |
| Durand et al. [61] | July 2020 | A multicenter RCT performed in 17 hospitals in Paris | 268 infants aged <6 months with moderate BR | HFNC at 3L/kg over standard oxygen therapy | No difference was demonstrated in treatment failure (14% vs. 20%) or in pediatric intensive care unit admission risk (15% vs. 19%). Results were consistent with the review by Moreel et al. which do not support the preventive and routine use of HFNC in patients with moderate BR. |
| Moreel, Proesmans [48] | May 2020 | Systematic literature search was performed in PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) from January 2000 to February 2020: pediatrics department and pediatric intensive care unit | Children (<2 years) with acute BR | 4 studies HFNC vs. oxygen. 3 studies nCPAP vs. HFNC | HFNC seems more appropriate for children who are not adequately supported by oxygen, but there are insufficient data to support the use of HFNC therapy for all children with BR. |
| Vahlkvist et al. [50] | March 2020 | Randomized clinical trial | 50 children with BR | HFNC vs. nCPAP | CPAP and HFNC are comparable in terms of treatment duration, treatment failure, and hospital stay, and have similar effects on respiratory rate, pCO2, and oxygen supply requirement. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fainardi, V.; Abelli, L.; Muscarà, M.; Pisi, G.; Principi, N.; Esposito, S. Update on the Role of High-Flow Nasal Cannula in Infants with Bronchiolitis. Children 2021, 8, 66. https://doi.org/10.3390/children8020066
Fainardi V, Abelli L, Muscarà M, Pisi G, Principi N, Esposito S. Update on the Role of High-Flow Nasal Cannula in Infants with Bronchiolitis. Children. 2021; 8(2):66. https://doi.org/10.3390/children8020066
Chicago/Turabian StyleFainardi, Valentina, Lara Abelli, Maria Muscarà, Giovanna Pisi, Nicola Principi, and Susanna Esposito. 2021. "Update on the Role of High-Flow Nasal Cannula in Infants with Bronchiolitis" Children 8, no. 2: 66. https://doi.org/10.3390/children8020066
APA StyleFainardi, V., Abelli, L., Muscarà, M., Pisi, G., Principi, N., & Esposito, S. (2021). Update on the Role of High-Flow Nasal Cannula in Infants with Bronchiolitis. Children, 8(2), 66. https://doi.org/10.3390/children8020066






