Neonatal and Postneonatal Pulmonary Hypertension
Abstract
1. Introduction
2. Pulmonary Hypertension in Term Infants
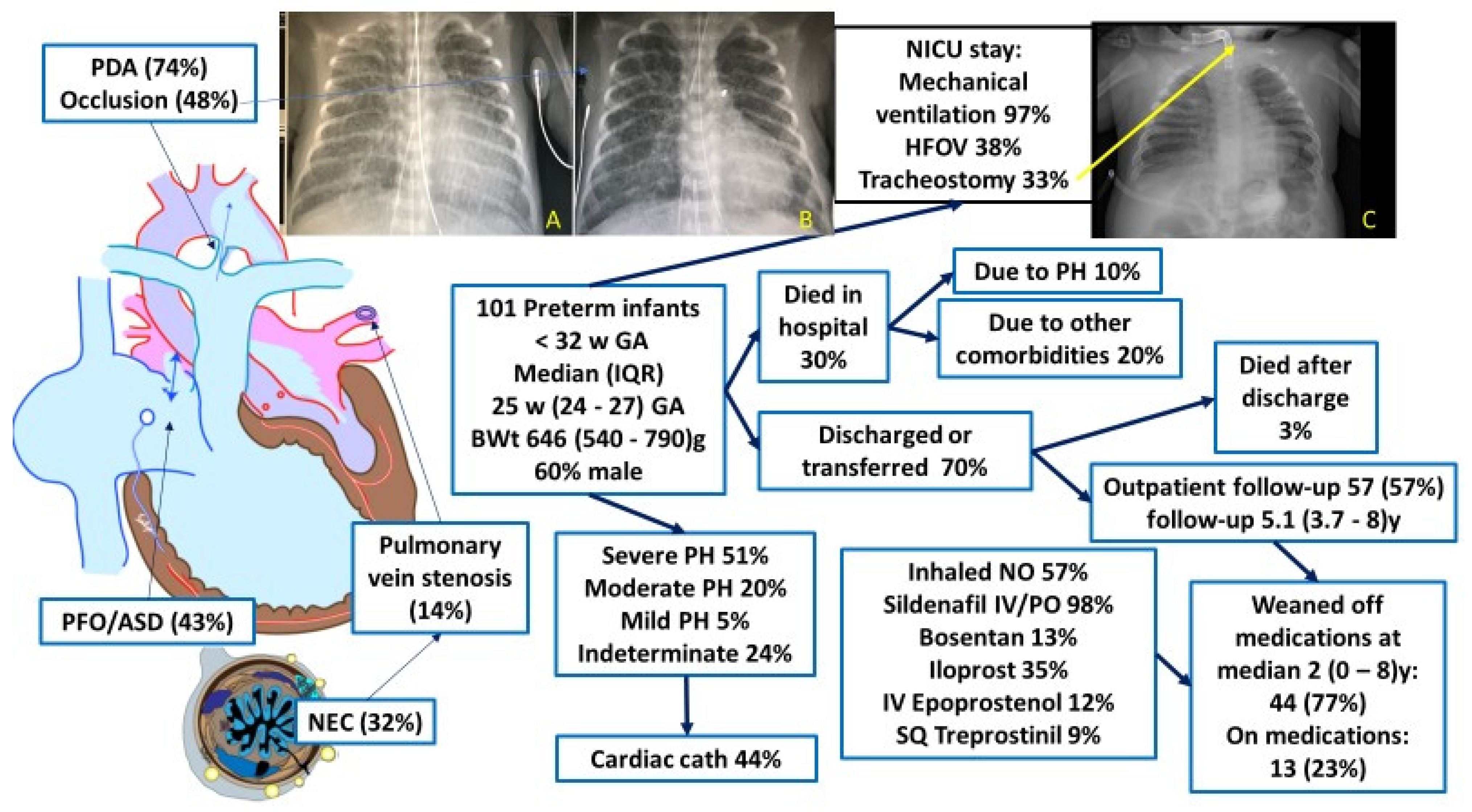
3. Pulmonary Hypertension in Preterm Infants
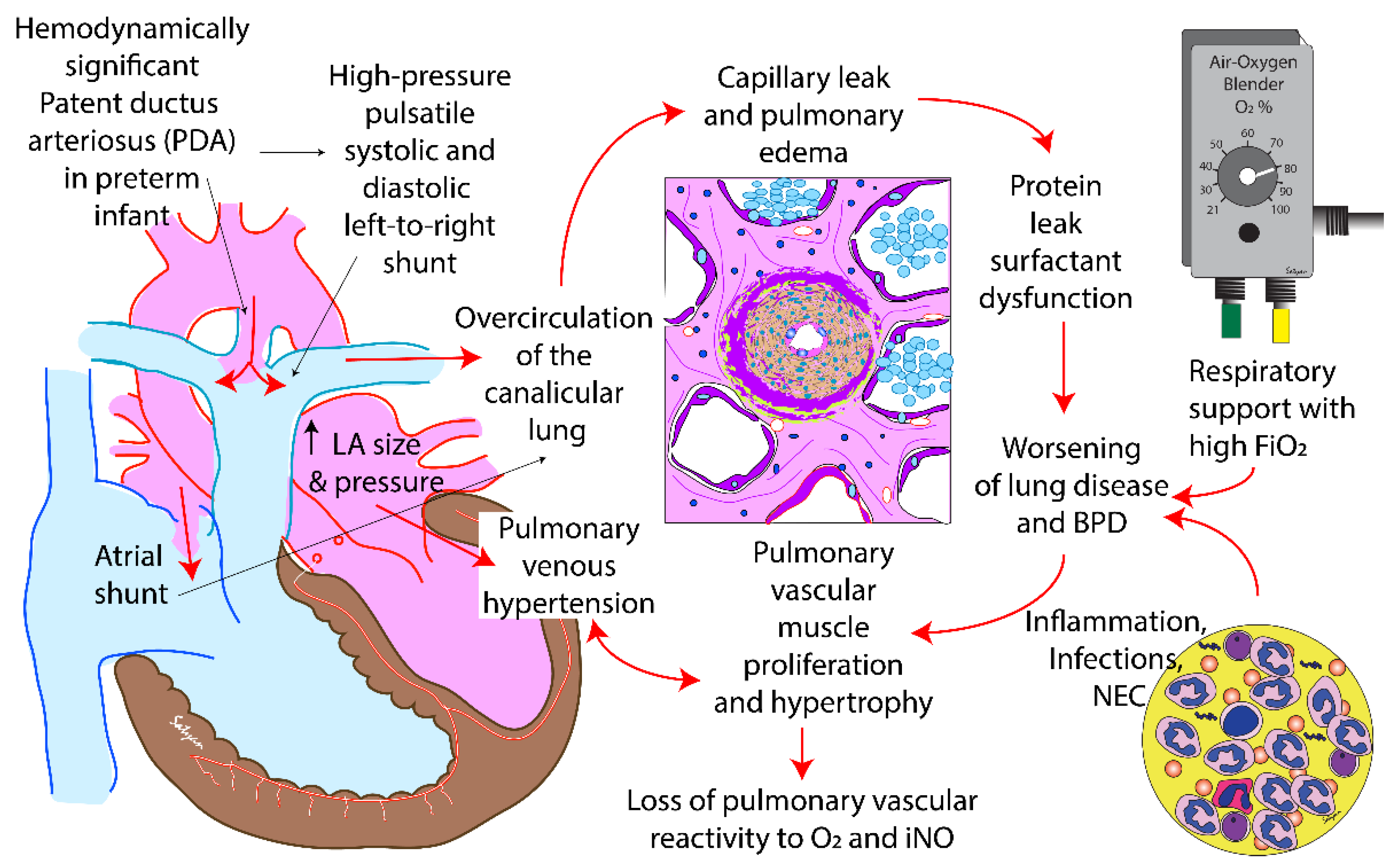
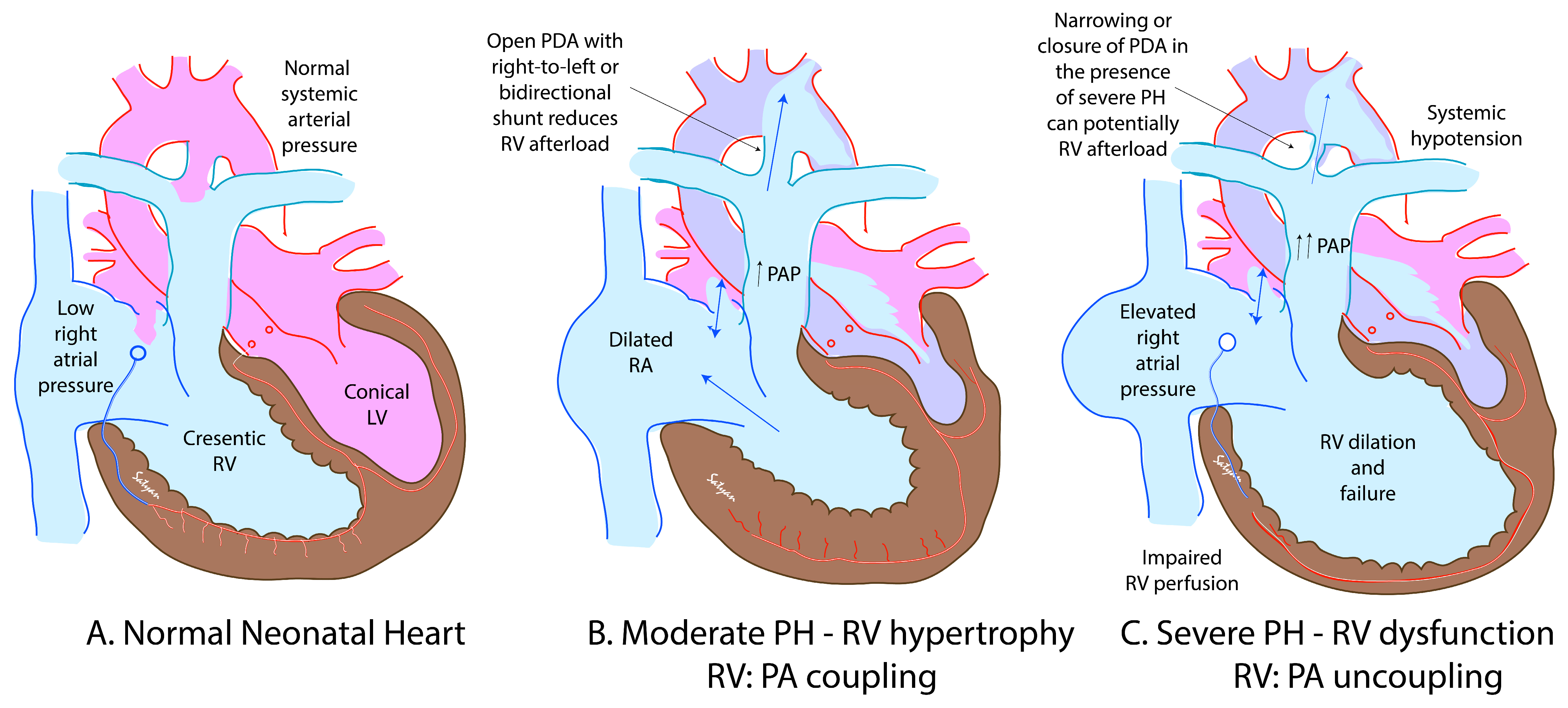
4. Role of the PDA
5. Postneonatal Pulmonary Hypertension and Thiamine Deficiency
6. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vali, P.; Lakshminrusimha, S. The Fetus Can Teach Us: Oxygen and the Pulmonary Vasculature. Children 2017, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.M.; Lakshminrusimha, S.; Ehret, D.E.Y.; Horbar, J.D. NICU Admissions for Meconium Aspiration Syndrome before and after a National Resuscitation Program Suctioning Guideline Change. Children 2019, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Lakshminrusimha, S. Persistent Pulmonary Hypertension in the Newborn. Children 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R. Signaling Pathways Involved in the Development of Bronchopulmonary Dysplasia and Pulmonary Hypertension. Children 2020, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Lesneski, A.; Hardie, M.; Ferrier, W.; Lakshminrusimha, S.; Vali, P. Bidirectional Ductal Shunting and Preductal to Postductal Oxygenation Gradient in Persistent Pulmonary Hypertension of the Newborn. Children 2020, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, P.; Rawat, M.; Lakshminrusimha, S. How Do We Monitor Oxygenation during the Management of PPHN? Alveolar, Arterial, Mixed Venous Oxygen Tension or Peripheral Saturation? Children 2020, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Gien, J.; Kinsella, J.P. Differences in preductal and postductal arterial blood gas measurements in infants with severe congenital diaphragmatic hernia. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F314–F318. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, G.; Koestenberger, M.; Alastalo, T.P.; Apitz, C.; Austin, E.D.; Bonnet, D.; Budts, W.; D’Alto, M.; Gatzoulis, M.A.; Hasan, B.S.; et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2019, 38, 879–901. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, P.; Lakshminrusimha, S.; Abman, S.H. When to say no to inhaled nitric oxide in neonates? Semin. Fetal Neonatal Med. 2021, 101200. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Kinsella, J.P.; Krishnan, U.S.; Van Meurs, K.; Edwards, E.M.; Bhatt, D.R.; Chandrasekharan, P.; Oei, J.L.; Manja, V.; Ramanathan, R.; et al. Just Say No to iNO in Preterms-Really? J. Pediatr. 2020, 218, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Cole, F.S.; Alleyne, C.; Barks, J.D.; Boyle, R.J.; Carroll, J.L.; Dokken, D.; Edwards, W.H.; Georgieff, M.; Gregory, K.; Johnston, M.V.; et al. NIH Consensus Development Conference statement: Inhaled nitric-oxide therapy for premature infants. Pediatrics 2011, 127, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Baczynski, M.; Ginty, S.; Weisz, D.E.; McNamara, P.J.; Kelly, E.; Shah, P.; Jain, A. Short-term and long-term outcomes of preterm neonates with acute severe pulmonary hypertension following rescue treatment with inhaled nitric oxide. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F508–F514. [Google Scholar] [CrossRef] [PubMed]
- Northway, W.H., Jr.; Rosan, R.C.; Porter, D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Mirza, H.; Ziegler, J.; Ford, S.; Padbury, J.; Tucker, R.; Laptook, A. Pulmonary hypertension in preterm infants: Prevalence and association with bronchopulmonary dysplasia. J. Pediatr. 2014, 165, 909–914 e901. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, C.; Hanna, Y.; Ben Fadel, N.; Lemyre, B.; Bijelic, V.; Barrowman, N.; Hoey, L.; Thebaud, B.; Katz, S.L. Target oxygen saturation and development of pulmonary hypertension and increased pulmonary vascular resistance in preterm infants. Pediatr. Pulmonol. 2019, 54, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Vyas-Read, S.; Guglani, L.; Shankar, P.; Travers, C.; Kanaan, U. Atrial Septal Defects Accelerate Pulmonary Hypertension Diagnoses in Premature Infants. Front. Pediatr. 2018, 6, 342. [Google Scholar] [CrossRef] [PubMed]
- Nees, S.N.; Rosenzweig, E.B.; Cohen, J.L.; Valencia Villeda, G.A.; Krishnan, U.S. Targeted Therapy for Pulmonary Hypertension in Premature Infants. Children 2020, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Philip, R.; Lamba, V.; Talati, A.; Sathanandam, S. Pulmonary Hypertension with Prolonged Patency of the Ductus Arteriosus in Preterm Infants. Children (Basel) 2020, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Vali, P.; Lakshminrusimha, S.; Pelech, A.; Underwood, M.; Ing, F. Patent ductus arteriosus in preterm infants: Is early transcatheter closure a paradigm shift? J. Perinatol. 2019, 39, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, N.; Chirla, D.K.; Shetty, R.; Shaikh, F.A.R.; Kumar, P.P.; Madappa, R.; Lingan, A.; Lakshminrusimha, S. Thiamine-Responsive Acute Pulmonary Hypertension of Early Infancy (TRAPHEI)-A Case Series and Clinical Review. Children (Basel) 2020, 7, 199. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakshminrusimha, S. Neonatal and Postneonatal Pulmonary Hypertension. Children 2021, 8, 131. https://doi.org/10.3390/children8020131
Lakshminrusimha S. Neonatal and Postneonatal Pulmonary Hypertension. Children. 2021; 8(2):131. https://doi.org/10.3390/children8020131
Chicago/Turabian StyleLakshminrusimha, Satyan. 2021. "Neonatal and Postneonatal Pulmonary Hypertension" Children 8, no. 2: 131. https://doi.org/10.3390/children8020131
APA StyleLakshminrusimha, S. (2021). Neonatal and Postneonatal Pulmonary Hypertension. Children, 8(2), 131. https://doi.org/10.3390/children8020131




