High-Risk Neuroblastoma Treatment Review
Abstract
:1. Introduction
2. High-Risk Neuroblastoma Definition
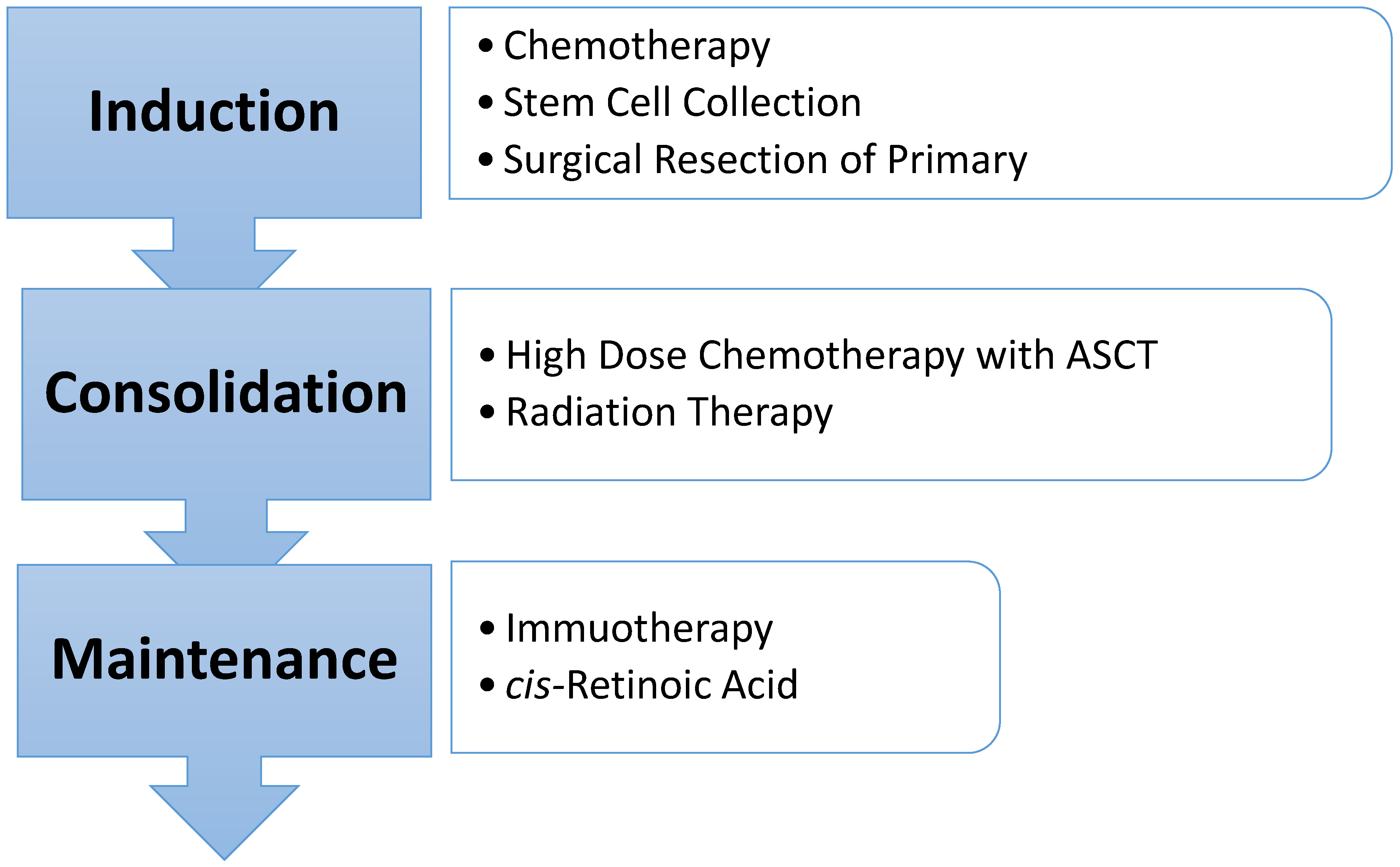
3. Treatment
4. Clinical Trials and Future Directions
5. Conclusions
Funding
Conflicts of Interest
References
- Brodeur, G.; Hogarty, M.; Bagatell, R.; Mosse, Y.; Maris, J. Neuroblastoma. In Principles and Practice of Pediatric Oncology, 7th ed.; Pizzo, P., Poplack, D., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2016; pp. 772–798. ISBN 978-1-4511-9423-4. [Google Scholar]
- Cohn, S.L.; Pearson, A.D.J.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) Classification System: An INRG Task Force Report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monclair, T.; Brodeur, G.M.; Ambros, P.F.; Brisse, H.J.; Cecchetto, G.; Holmes, K.; Kaneko, M.; London, W.B.; Matthay, K.K.; Nuchtern, J.G.; et al. The International Neuroblastoma Risk Group (INRG) Staging System: An INRG Task Force Report. J. Clin. Oncol. 2009, 27, 298–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, N.R.; Applebaum, M.A.; Volchenboum, S.L.; Matthay, K.K.; London, W.B.; Ambros, P.F.; Nakagawara, A.; Berthold, F.; Schleiermacher, G.; Park, J.R.; et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J. Clin. Oncol. 2015, 33, 3008–3017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, C.U.; Shohet, J.M. Neuroblastoma: Molecular Pathogenesis and Therapy. Annu. Rev. Med. 2015, 66, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peinemann, F.; Kahangire, D.A.; van Dalen, E.C.; Berthold, F. Rapid COJEC versus standard induction therapies for high-risk neuroblastoma. Cochrane Database Syst. Rev. 2015, 60, 1135–1151. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Kramer, K.; LaQuaglia, M.P.; Modak, S.; Yataghene, K.; Cheung, N.-K.V. Reduction From Seven to Five Cycles of Intensive Induction Chemotherapy in Children With High-Risk Neuroblastoma. J. Clin. Oncol. 2004, 22, 4888–4892. [Google Scholar] [CrossRef] [PubMed]
- Garaventa, A.; Poetschger, U.; Valteau-Couanet, D.; Castel, V.; Elliott, M.; Ash, S.; Chan, G.C.; Laureys, G.; Beck Popovic, M.; Vettenranta, K.; et al. The Randomised Induction for High-Risk Neuroblastoma Comparing COJEC and the N5-MSKCC Regimen: Early Results from the HR-NBL1.5/SIOPEN Trial. In Proceedings of the Advances in Neuroblastoma Research Association (ANARA), San Francisco, CA, USA, 9–12 May 2018. [Google Scholar]
- Kreissman, S.G.; Seeger, R.C.; Matthay, K.K.; London, W.B.; Sposto, R.; Grupp, S.A.; Haas-Kogan, D.A.; LaQuaglia, M.P.; Alice, L.Y.; Diller, L.; et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 999–1008. [Google Scholar] [CrossRef]
- Adkins, E.S.; Sawin, R.; Gerbing, R.B.; London, W.B.; Matthay, K.K.; Haase, G.M. Efficacy of complete resection for high-risk neuroblastoma: A children’s cancer group study. J. Pediatr. Surg. 2004, 39, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Häberle, B.; Hero, B.; von Schweinitz, D.; Berthold, F. Role of Surgery in the Treatment of Patients With Stage 4 Neuroblastoma Age 18 Months or Older at Diagnosis. J. Clin. Oncol. 2013, 31, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Pohl, A.; Volland, R.; Hero, B.; Dübbers, M.; Cernaianu, G.; Berthold, F.; Schweinitz, D.; Simon, T. Complete surgical resection improves outcome in INRG high-risk patients with localized neuroblastoma older than 18 months. BMC Cancer 2017, 17, 520. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Villablanca, J.G.; Seeger, R.C.; Stram, D.O.; Harris, R.E.; Ramsay, N.K.; Swift, P.; Shimada, H.; Black, C.T.; Brodeur, G.M.; et al. Treatment of High-Risk Neuroblastoma with Intensive Chemotherapy, Radiotherapy, Autologous Bone Marrow Transplantation, and 13-cis-Retinoic Acid. N. Engl. J. Med. 1999, 341, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Reynolds, C.P.; Seeger, R.C.; Shimada, H.; Adkins, E.S.; Haas-Kogan, D.; Gerbing, R.B.; London, W.B.; Villablanca, J.G. Long-Term Results for Children With High-Risk Neuroblastoma Treated on a Randomized Trial of Myeloablative Therapy Followed by 13-cis-Retinoic Acid: A Children’s Oncology Group Study. J. Clin. Oncol. 2009, 27, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Kreissman, S.G.; London, W.B.; Naranjo, A.; Cohn, S.L.; Hogarty, M.D.; Tenney, S.C.; Haas-Kogan, D.; Shaw, P.J.; Geiger, J.D.; et al. A phase III randomized clinical trial (RCT) of tandem myeloablative autologous stem cell transplant (ASCT) using peripheral blood stem cell (PBSC) as consolidation therapy for high-risk neuroblastoma (HR-NB): A Children’s Oncology Group (COG) study. In Proceedings of the American Society of Clinical Oncology (ASCO) Conference, Chicago, IL, USA, 3–7 June 2016. [Google Scholar]
- Qayed, M.; Chiang, K.-Y.; Ricketts, R.; Alazraki, A.; Tahvildari, A.; Haight, A.; George, B.; Esiashvili, N.; Katzenstein, H.M. Tandem stem cell rescue as consolidation therapy for high-risk neuroblastoma. Pediatr. Blood Cancer 2011, 58, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Elborai, Y.; Hafez, H.; Moussa, E.A.; Hammad, M.; Hussein, H.; Lehmann, L.; Elhaddad, A. Comparison of toxicity following different conditioning regimens (busulfan/melphalan and carboplatin/etoposide/melphalan) for advanced stage neuroblastoma: Experience of two transplant centers. Pediatr. Transplant. 2015, 20, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Ladenstein, R.; Pötschger, U.; Pearson, A.D.J.; Brock, P.; Luksch, R.; Castel, V.; Yaniv, I.; Papadakis, V.; Laureys, G.; Malis, J.; et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): An international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 500–514. [Google Scholar] [CrossRef]
- Gaze, M.N.; Boterberg, T.; Dieckmann, K.; Hörmann, M.; Gains, J.E.; Sullivan, K.P.; Ladenstein, R. Results of a Quality Assurance Review of External Beam Radiation Therapy in the International Society of Paediatric Oncology (Europe) Neuroblastoma Group’s High-risk Neuroblastoma Trial: A SIOPEN Study. Radiat. Oncol. Boil. 2013, 85, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, A.; Louis, C.U.; Nuchtern, J.; Kim, E.; Russell, H.; Allen-Rhoades, W.; Krance, R.; Paulino, A.C. Radiation Therapy to the Primary and Postinduction Chemotherapy MIBG-Avid Sites in High-Risk Neuroblastoma. Int. J. Radiat. Oncol. Boil. Phys. 2014, 90, 858–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, D.L.; Kushner, B.H.; Cheung, N.-K.V.; Modak, S.; LaQuaglia, M.P.; Wolden, S.L. Local Control with 21-Gy Radiation Therapy for High-Risk Neuroblastoma. Int. J. Radiat. Oncol. Boil. Phys. 2016, 96, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.M.; Pritchard, J.; Berthold, F.; Carlsen, N.L.; Castel, V.; Castelberry, R.P.; De Bernardi, B.; Evans, A.E.; Favrot, M.; Hedborg, F. Revisions of the international criteria for neuroblastoma diagnosis, staging and response to treatment. Prog. Clin. Biol. Res. 1994, 385, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Hill-Kayser, C.; Tochner, Z.; Both, S.; Lustig, R.; Reilly, A.; Balamuth, N.; Womer, R.; Maris, J.; Grupp, S.; Bagatell, R. Proton versus photon radiation therapy for patients with high-risk neuroblastoma: The need for a customized approach. Pediatr. Blood Cancer. 2013, 60, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Hattangadi, J.A.; Rombi, B.; Yock, T.I.; Broussard, G.; Friedmann, A.M.; Huang, M.; Chen, Y.L.; Lu, H.M.; Kooy, H.; MacDonald, S.M. Proton Radiotherapy for High-Risk Pediatric Neuroblastoma: Early Outcomes and Dose Comparison. Int. J. Radiat. Oncol. Boil. Phys. 2012, 83, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Peinemann, F.; van Dalen, E.C.; Tushabe, D.A.; Berthold, F. Retinoic acid post consolidation therapy for high-risk neuroblastoma patients treated with autologous hematopoietic stem cell transplantation. Cochrane Database Syst. Rev. 2015, 1, CD010685. [Google Scholar] [PubMed]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, N.-K.V.; Cheung, I.Y.; Kushner, B.H.; Ostrovnaya, I.; Chamberlain, E.; Kramer, K.; Modak, S. Murine Anti-GD2 Monoclonal Antibody 3F8 Combined With Granulocyte-Macrophage Colony-Stimulating Factor and 13-Cis-Retinoic Acid in High-Risk Patients With Stage 4 Neuroblastoma in First Remission. J. Clin. Oncol. 2012, 30, 3264–3270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.J.; Doral, M.Y.; DuBois, S.G.; Villablanca, J.G.; Yanik, G.A.; Matthay, K.K. Different outcomes for relapsed vs. refractory neuroblastoma after therapy with 131I-metaiodobenzylguanidine (131I-MIBG). Eur. J. Cancer 2015, 51, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Pugh, T.J.; Morozova, O.; Attiyeh, E.F.; Asgharzadeh, S.; Wei, J.S.; Auclair, D.; Carter, S.L.; Cibulskis, K.; Hanna, M.; Kiezun, A.; et al. The genetic landscape of high-risk neuroblastoma. Nat. Genet. 2013, 45, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peifer, M.; Hertwig, F.; Roels, F.; Dreidax, D.; Gartlgruber, M.; Menon, R.; Krämer, A.; Roncaioli, J.L.; Sand, F.; Heuckmann, J.M. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature 2015, 526, 700–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depuydt, P.; Boeva, V.; Hocking, T.D.; Cannoodt, R.; Ambros, I.M.; Ambros, P.F.; Asgharzadeh, S.; Attiyeh, E.F.; Combaret, V.; Defferrari, R.; et al. Genomic Amplifications and Distal 6q Loss: Novel Markers for Poor Survival in High-risk Neuroblastoma Patients. JNCI J. Natl. Cancer Inst. 2018. [CrossRef] [PubMed]
- Lee, J.W.; Lee, S.; Cho, H.W.; Ma, Y.; Yoo, K.H.; Sung, K.W.; Koo, H.H.; Cho, E.J.; Lee, S.K.; Lim, D.H. Incorporation of high-dose 131I-metaiodobenzylguanidine treatment into tandem high-dose chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma: Results of the SMC NB-2009 study. J. Hematol. Oncol. 2017, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, S.; Rufini, V.; Ruggiero, A.; Di Giannatale, A.; Riccardi, R. Treatment of advanced neuroblastoma in children over 1 year of age: The critical role of 131I-metaiodobenzylguanidine combined with chemotherapy in a rapid induction regimen. Pediatr. Blood Cancer 2011, 56, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Krytska, K.; Ryles, H.T.; Sano, R.; Raman, P.; Infarinato, N.; Hansel, T.D.; Makena, M.R.; Song, M.M.; Reynolds, C.P.; Mossé, Y.P. Crizotinib Synergizes with Chemotherapy in Preclinical Models of Neuroblastoma. Clin. Cancer Res. 2016, 22, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Mossé, Y.P.; Lim, M.S.; Voss, S.D.; Wilner, K.; Ruffner, K.; Laliberte, J.; Rolland, D.; Balis, F.M.; Maris, J.M.; Weigel, B.J.; et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: A Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013, 14, 472–480. [Google Scholar] [CrossRef]
- Bassiri, H.; Benavides, A.; Haber, M.; Gilmour, S.K.; Norris, M.D.; Hogarty, M.D. Translational development of difluoromethylornithine (DFMO) for the treatment of neuroblastoma. Transl. Pediatr. 2015, 4, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Saulnier Sholler, G.L.; Gerner, E.W.; Bergendahl, G.; MacArthur, R.B.; VanderWerff, A.; Ashikaga, T.; Bond, J.P.; Ferguson, W.; Roberts, W.; Wada, R.K.; et al. A Phase I Trial of DFMO Targeting Polyamine Addiction in Patients with Relapsed/Refractory Neuroblastoma. PLoS ONE 2015, 10, e0127246. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Cheung, I.Y.; Modak, S.; Kramer, K.; Ragupathi, G.; Cheung, N.-K.V. Phase I Trial of a Bivalent Gangliosides Vaccine in Combination with β-Glucan for High-Risk Neuroblastoma in Second or Later Remission. Clin. Cancer Res. 2014, 20, 1375–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Stage | Description |
|---|---|
| L1 | Localized tumor not involving vital structures as defined by the list of image-defined risk factors and confined to one body part |
| L2 | Loco-regional tumor with presence of one more image-defined risk factors |
| M | Distant metastatic disease (except stage MS) |
| MS | Metastatic disease in children younger than 18 months with metastases confined to skin, liver, and/or bone marrow |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, V.; Foster, J. High-Risk Neuroblastoma Treatment Review. Children 2018, 5, 114. https://doi.org/10.3390/children5090114
Smith V, Foster J. High-Risk Neuroblastoma Treatment Review. Children. 2018; 5(9):114. https://doi.org/10.3390/children5090114
Chicago/Turabian StyleSmith, Valeria, and Jennifer Foster. 2018. "High-Risk Neuroblastoma Treatment Review" Children 5, no. 9: 114. https://doi.org/10.3390/children5090114
APA StyleSmith, V., & Foster, J. (2018). High-Risk Neuroblastoma Treatment Review. Children, 5(9), 114. https://doi.org/10.3390/children5090114





