Reduction in Perioperative Risk in Patients with Spinal Muscular Atrophy Following the Release of Disease-Modifying Therapies: An Analysis of the National Surgical Quality Improvement Program Database
Abstract
Highlights
- A year following widespread implementation of new disease-modifying therapies, patients with SMA demonstrated a reduced risk for postoperative pulmonary complications and a reduction in length of stay at a population level compared to patients with SMA before the new treatment era.
- Improved motor outcomes and evolving clinical phenotypes with early treatment initiation may account for this trend.
- Multivariable prediction models must consider the dynamic effects of new therapies on the perioperative outcomes of affected populations.
Abstract
1. Introduction
2. Materials and Methods
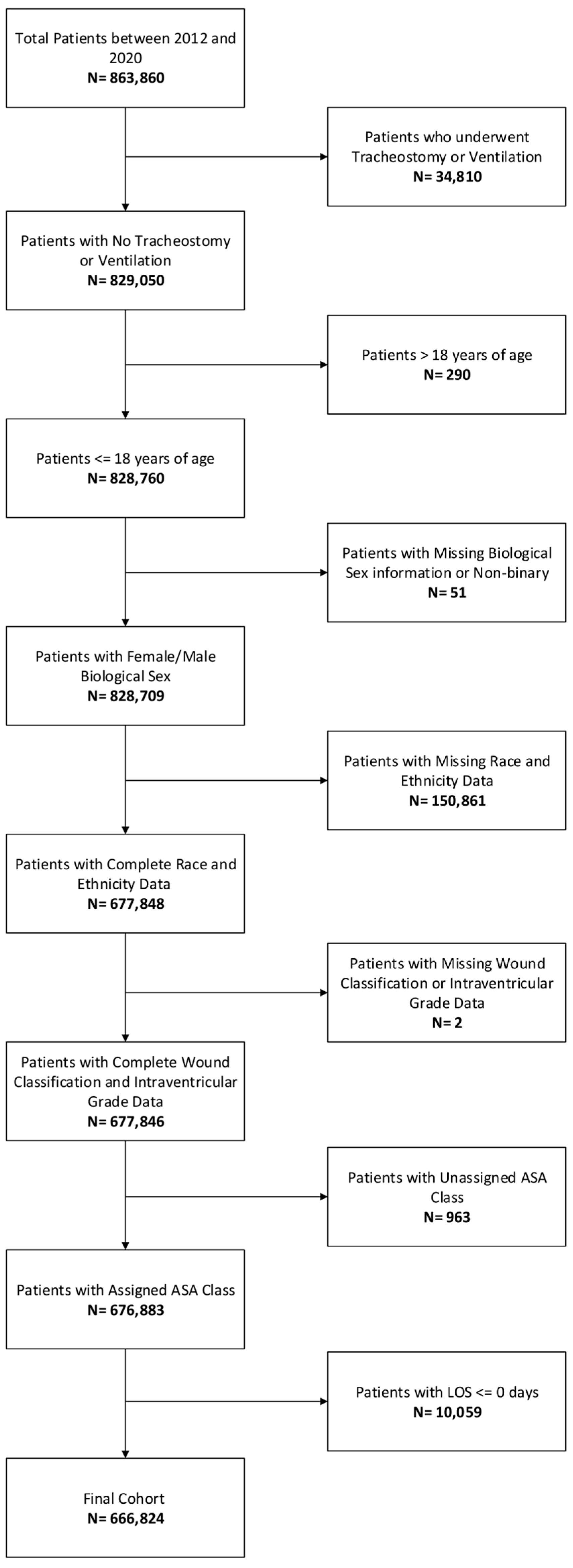
2.1. Patients and Data Collection
2.2. Primary Outcome: Postoperative Pulmonary Complication
2.3. Baseline Characteristics
2.4. Statistical Analysis
3. Results
3.1. Postoperative Pulmonary Complications (PPC): Pre-2018 vs. Post-2018
3.2. Length of Stay (LOS): Pre-2018 vs. Post-2018
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | American College of Surgeons |
| ASA | American Society of Anesthesiologists |
| CI | confidence interval |
| IRB | institutional review board |
| LOS | length of stay |
| NSQIP-P | National Surgical Quality Improvement Program Pediatric |
| OR | operating room |
| POD | postoperative day |
| PPC | postoperative pulmonary complication |
| TRIPOD | Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis |
References
- D′Amico, A.; Mercuri, E.; Tiziano, F.D.; Bertini, E. Spinal muscular atrophy. Orphanet J. Rare Dis. 2011, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Schorling, D.C.; Pechmann, A.; Kirschner, J. Advances in Treatment of Spinal Muscular Atrophy—New Phenotypes, New Challenges, New Implications for Care. J. Neuromuscul. Dis. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Graham, R.J.; Athiraman, U.; Laubach, A.E.; Sethna, N.F. Anesthesia and perioperative medical management of children with spinal muscular atrophy. Pediatr. Anesth. 2009, 19, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, F.; Athiraman, U.; Clendenin, D.J.; Hoagland, M.; Sethna, N.F.; Veyckemans, F. Anesthetic management of 877 pediatric patients undergoing muscle biopsy for neuromuscular disorders: A 20—Year review. Pediatr. Anesth. 2016, 26, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Hache, M.; Swoboda, K.J.; Sethna, N.; Farrow-Gillespie, A.; Khandji, A.; Xia, S.; Bishop, K.M. Intrathecal Injections in Children With Spinal Muscular Atrophy: Nusinersen Clinical Trial Experience. J. Child. Neurol. 2016, 31, 899–906. [Google Scholar] [CrossRef]
- Albrechtsen, S.S.; Born, A.P.; Boesen, M.S. Nusinersen treatment of spinal muscular atrophy—A systematic review. Dan. Med. J. 2020, 67, A02200100. [Google Scholar]
- Mercuri, E.; Muntoni, F.; Baranello, G.; Masson, R.; Boespflug-Tanguy, O.; Bruno, C.; Corti, S.; Daron, A.; Deconinck, N.; Servais, L.; et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): An open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021, 20, 832–841. [Google Scholar] [CrossRef]
- Day, J.W.; Howell, K.; Place, A.; Long, K.; Rossello, J.; Kertesz, N.; Nomikos, G. Advances and limitations for the treatment of spinal muscular atrophy. BMC Pediatr. 2022, 22, 632. [Google Scholar] [CrossRef]
- De Vivo, D.C.; Darryl, C.; Bertini, E.; Swoboda, K.J.; Hwu, W.L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef]
- Bielsky, A.R.; Fuhr, P.G.; Parsons, J.A.; Yaster, M. A retrospective cohort study of children with spinal muscular atrophy type 2 receiving anesthesia for intrathecal administration of nusinersen. Pediatr. Anesth. 2018, 28, 1105–1108. [Google Scholar] [CrossRef]
- Brollier, L.D.; Matuszczak, M.; Marri, T.; Carbajal, J.G.; Moorman, A.T.; Sorial, E.M.; Jain, R. Anesthetic management of pediatric patients undergoing intrathecal nusinersen administration for treatment of spinal muscular atrophy: A single—Center experience. Pediatr. Anesth. 2021, 31, 160–166. [Google Scholar] [CrossRef]
- Sever, F.; Özmert, S.; Öz, N.A. Procedural sedation for paediatric patients with spinal muscular atrophy undergoing intrathecal treatment. Br. J. Clin. Pharmacol. 2023, 89, 2465–2471. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): The TRIPOD Statement. Ann. Intern. Med. 2015, 162, 55–63. [Google Scholar] [CrossRef]
- American College of Surgeons National Surgical Quality Improvement Program—Pediatri. User Guide for the 2013 ACS NSQIP Pediatric Participant Use Data File (PUF); American College of Surgeons: Chicago, IL, USA, 2014. [Google Scholar]
- Futier, E.; Constantin, J.-M.; Paugam-Burtz, C.; Pascal, J.; Eurin, M.; Neuschwander, A.; Marret, E.; Beaussier, M.; Gutton, C.; Lefrant, J.-Y.; et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N. Engl. J. Med. 2013, 369, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Davenport, D.L.; Henderson, W.G.; Khuri, S.F.; Mentzer, J.R.M. Preoperative risk factors and surgical complexity are more predictive of costs than postoperative complications: A case study using the National Surgical Quality Improvement Program (NSQIP) database. Ann. Surg. 2005, 242, 463–468; discussion 468–471. [Google Scholar] [CrossRef]
- Kay, D.M.; Stevens, C.F.; Parker, A.; Saavedra-Matiz, C.A.; Sack, V.; Chung, W.K.; Chiriboga, C.A.; Engelstad, K.; Laureta, E.; Farooq, O.; et al. Implementation of population-based newborn screening reveals low incidence of spinal muscular atrophy. Genet. Med. 2020, 22, 1296–1302. [Google Scholar] [CrossRef]
- Coratti, G.; Pane, M.; Lucibello, S.; Pera, M.C.; Pasternak, A.; Montes, J.; Sansone, V.A.; Duong, T.; Young, S.D.; Messina, S.; et al. Age related treatment effect in type II Spinal Muscular Atrophy pediatric patients treated with nusinersen. Neuromuscul. Disord. 2021, 31, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Halanski, M.; Steinfeldt, A.; Hetzel, S.; Schroth, M.; Muldowney, B. Peri-operative management of children with spinal muscular atrophy. Indian J. Anaesth. 2020, 64, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Gu, T.; Chen, E.; Punekar, R.; Shieh, P.B. Healthcare Utilization, Costs of Care, and Mortality Among Psatients With Spinal Muscular Atrophy. J. Health Econ. Outcomes Res. 2019, 6, 185–195. [Google Scholar] [CrossRef]
- Holm, A.; Hansen, S.N.; Klitgaard, H.; Kauppinen, S. Clinical advances of RNA therapeutics for treatment of neurological and neuromuscular diseases. RNA Biol. 2022, 19, 594–608. [Google Scholar] [CrossRef]
- Liu, J.; Barrett, J.S.; Leonardi, E.T.; Lee, L.; Roychoudhury, S.; Chen, Y.; Trifillis, P. Natural History and Real—World Data in Rare Diseases: Applications, Limitations, and Future Perspectives. J. Clin. Pharmacol. 2022, 62 (Suppl. 2), S38–S55. [Google Scholar] [CrossRef]
- Clift, A.K.; Coupland, C.A.C.; Keogh, R.H.; Diaz-Ordaz, K.; Williamson, E.; Harrison, E.M.; Hayward, A.; Hemingway, H.; Horby, P.; Mehta, N.; et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: National derivation and validation cohort study. BMJ 2020, 371, m3731. [Google Scholar] [CrossRef]
- Hao, B.; Hu, Y.; Sotudian, S.; Zad, Z.; Adams, W.G.; Assoumou, S.A.; Hsu, H.; Mishuris, R.G.; Paschalidis, I.C. Development and validation of predictive models for COVID-19 outcomes in a safety-net hospital population. J. Am. Med. Inform. Assoc. 2022, 29, 1253–1262. [Google Scholar] [CrossRef]
| Pre-2018 | Post-2018 | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | No PPC (N = 387,719) | Yes PPC (N = 1536) | Odds Ratio (95% CI) | p Value | No PPC (N = 375,762) | Yes PPC (N = 1142) | Odds Ratio (95% CI) | p Value |
| Age (years) | 7.63 (5.69) | 5.61 (5.83) | 0.94 [0.93;0.94] | <0.001 | 8.25 (5.83) | 6.08 (5.95) | 0.94 [0.92;0.95] | <0.001 |
| SMA | ||||||||
| No | 387,425 (99.9%) | 1531 (99.7%) | Ref. | Ref. | 276,424 (99.9%) | 846 (99.6%) | Ref. | Ref. |
| Yes | 294 (0.08%) | 5 (0.33%) | 4.44 [1.56;9.65] | 0.007 | 296 (0.11%) | 3 (0.35%) | 3.48 [0.84;9.12] | 0.079 |
| Sex | ||||||||
| Female | 168,253 (43.4%) | 679 (44.2%) | Ref. | Ref. | 122,699 (44.3%) | 393 (46.3%) | Ref. | Ref. |
| Male | 219,466 (56.6%) | 857 (55.8%) | 0.97 [0.87;1.07] | 0.540 | 154,021 (55.7%) | 456 (53.7%) | 0.92 [0.81;1.06] | 0.254 |
| ASA Classification | ||||||||
| I | 123,846 (31.9%) | 68 (4.43%) | Ref. | Ref. | 77,381 (28.0%) | 28 (3.30%) | Ref. | Ref. |
| II | 174,639 (45.0%) | 345 (22.5%) | 3.59 [2.79;4.70] | <0.001 | 129,270 (46.7%) | 202 (23.8%) | 4.30 [2.95;6.52] | <0.001 |
| III | 83,476 (21.5%) | 935 (60.9%) | 20.4 [16.0;26.3] | <0.001 | 65,905 (23.8%) | 525 (61.8%) | 21.9 [15.3;32.8] | <0.001 |
| IV/V | 5758 (1.49%) | 188 (12.2% | 59.4 [45.2;79.0] | <0.001 | 4164 (1.50%) | 94 (11.1%) | 62.1 [41.2;96.7] | <0.001 |
| Wound Class | ||||||||
| Clean | 186,606 (48.1%) | 665 (43.3%) | Ref. | Ref. | 121,595 (43.9%) | 395 (46.5%) | Ref. | Ref. |
| Clean/Contaminated | 144,282 (37.2%) | 685 (44.6%) | 1.33 [1.20;1.48] | <0.001 | 107,789 (39.0%) | 362 (42.6%) | 1.03 [0.90;1.19] | 0.648 |
| Contaminated | 34,275 (8.84%) | 88 (5.73%) | 0.72 [0.57;0.90] | 0.003 | 31,080 (11.2%) | 45 (5.30%) | 0.45 [0.32;0.60] | <0.001 |
| Dirty/Infected | 22,556 (5.82%) | 98 (6.38%) | 1.22 [0.98;1.50] | 0.073 | 16,256 (5.87%) | 47 (5.54%) | 0.89 [0.65;1.20] | 0.457 |
| Septic Shock | ||||||||
| No | 387,582 (100.0%) | 1528 (99.5%) | Ref. | Ref. | 276,611 (100.0%) | 846 (99.6%) | Ref. | Ref. |
| Yes | 137 (0.04%) | 8 (0.52%) | 15.1 [6.75;28.9] | <0.001 | 109 (0.04%) | 3 (0.35%) | 9.46 [2.25;25.1] | 0.005 |
| SIRS | ||||||||
| No | 370,231 (95.5%) | 1469 (95.6%) | Ref. | Ref. | 259,662 (93.8%) | 806 (94.9%) | Ref. | Ref. |
| Yes | 17,488 (4.51%) | 67 (4.36%) | 0.97 [0.75;1.23] | 0.793 | 17,058 (6.16%) | 43 (5.06%) | 0.82 [0.59;1.09] | 0.179 |
| Esophageal/GI Disease | ||||||||
| No | 326,550 (84.2%) | 809 (52.7%) | Ref. | Ref. | 235,212 (85.0%) | 491 (57.8%) | Ref. | Ref. |
| Yes | 61,169 (15.8%) | 727 (47.3%) | 4.80 [4.34;5.30] | <0.001 | 41,508 (15.0%) | 358 (42.2%) | 4.13 [3.60;4.74] | <0.001 |
| Case Type | ||||||||
| Elective | 286,296 (73.8%) | 1116 (72.7%) | Ref. | Ref. | 199,721 (72.2%) | 652 (76.8%) | Ref. | Ref. |
| Emergent | 59,527 (15.4%) | 250 (16.3%) | 1.08 [0.94;1.23] | 0.288 | 38,795 (14.0%) | 103 (12.1%) | 0.81 [0.66;1.00] | 0.048 |
| Urgent | 41,896 (10.8%) | 170 (11.1%) | 1.04 [0.88;1.22] | 0.621 | 38,204 (13.8%) | 94 (11.1%) | 0.75 [0.60;0.93] | 0.048 |
| Pulmonary Disease | ||||||||
| No | 339,457 (87.6%) | 960 (62.5%) | Ref. | Ref. | 241,533 (87.3%) | 511 (60.2%) | Ref. | Ref. |
| Yes | 48,262 (12.4%) | 576 (37.5%) | 3.33 [3.01;3.68] | <0.001 | 35,187 (12.7%) | 338 (39.8%) | 4.54 [3.95;5.21] | <0.001 |
| Neurological Disorder | ||||||||
| No | 297,824 (76.8%) | 766 (49.9%) | Ref. | Ref. | 208,818 (75.5%) | 353 (41.6%) | Ref. | Ref. |
| Yes | 89,895 (23.2%) | 770 (50.1%) | 3.33 [3.01;3.68] | <0.001 | 67,902 (24.5%) | 496 (58.4%) | 4.32 [3.77;4.96] | <0.001 |
| RVU (per unit increase) | 12.4 [9.45;21.5] | 25.6 [14.1;46.3] | 1.02 [1.02;1.02] | <0.001 | 13.5 [9.45;23.1] | 22.2 [12.5;38.5] | 1.02 [1.01;1.02] | <0.001 |
| Pre-2018 | Post-2018 | ||||
|---|---|---|---|---|---|
| Variables | Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | |
| Age | 0.967 (0.958–0.976) | <0.001 | 0.968 (0.955–0.980) | <0.001 | |
| SMA | 1.958 (0.799–4.800) | 0.142 | 1.031 (0.327–3.257) | 0.958 | |
| ASA Classification | |||||
| II vs. I | 2.162 (1.663–2.811) | <0.001 | 2.805 (1.884–4.175) | <0.001 | |
| III vs. I | 6.964 (5.378–9.020) | <0.001 | 7.703 (5.185–11.443) | <0.001 | |
| IV/V vs. I | 14.428 (10.724–19.413) | <0.001 | 16.408 (10.513–25.608) | <0.001 | |
| Outpatient Procedure | 0.149 (0.119–0.188) | <0.001 | 0.203 (0.157–0.263) | <0.001 | |
| Cardiac Risk Factor | 1.405 (1.242–1.590) | <0.001 | 1.228 (1.043–1.446) | 0.014 | |
| Cog Imp/Dev Delay | 1.376 (1.228–1.542) | <0.001 | 1.808 (1.560–2.096) | <0.001 | |
| Malignancy | 1.011 (0.822–1.243) | 0.917 | 1.214 (0.931–1.583) | 0.151 | |
| Structural Pulmonary Abnormality 1 | 1.746 (1.522–2.003) | <0.001 | 2.048 (1.730–2.424) | <0.001 | |
| RVU | 1.012 (1.011–1.014) | <0.001 | 1.008 (1.006–1.011) | <0.001 | |
| Pre-2018 | Post-2018 | ||||
|---|---|---|---|---|---|
| Variables | Coefficient (95% CI) | p Value | Coefficient (95% CI) | p Value | |
| Age | −0.004 (−0.006–−0.003) | <0.001 | −0.041 (−0.043–−0.039) | <0.001 | |
| SMA | 0.666 (0.321–1.010) | <0.001 | 0.194 (−0.160–0.548) | 0.283 | |
| Sex | |||||
| Male vs. Female | −0.448 (−0.468–−0.429) | <0.001 | −0.461 (−0.458–−0.438) | <0.001 | |
| ASA Classification | |||||
| II vs. I | 1.312 (1.289–1.335) | <0.001 | 1.261 (1.232–1.290) | <0.001 | |
| III vs. I | 2.593 (2.561–2.625) | <0.001 | 2.505 (2.466–2.543) | <0.001 | |
| IV/V vs. I | 3.550 (3.468–3.632) | <0.001 | 3.570 (3.471–3.670) | <0.001 | |
| Wound Class | |||||
| Clean/Contaminated vs. Clean | 0.247 (0.225–0.269) | <0.001 | −0.227 (−0.254–−0.200) | <0.001 | |
| Contaminated vs. Clean | 0.327 (0.287–0.367) | <0.001 | −0.255 (−0.301–−0.210) | <0.001 | |
| Dirty/Infected vs. Clean | 1.662 (1.617–1.707) | <0.001 | 1.572 (1.518–1.627) | <0.001 | |
| Septic Shock | −0.514 (−1.010–−0.018) | 0.042 | −0.357 (−0.935–0.222) | 0.227 | |
| SIRS | 0.161 (0.110–0.212) | <0.001 | 0.037 (−0.018–0.091 | 0.185 | |
| Esophageal/GI Disease | 1.115 (1.086–1.143) | <0.001 | 0.966 (0.958–1.028) | <0.001 | |
| Case Type | |||||
| Emergent vs. Elective | 1.087 (1.048–1.125) | <0.001 | 0.404 (0.357–0.450) | <0.001 | |
| Urgent vs. Elective | 1.432 (1.393–1.470) | <0.001 | 0.668 (0.624–0.713) | <0.001 | |
| Pulmonary Disease | −0.129 (−0.159–−0.098) | <0.001 | −0.016 (−0.053–0.021) | 0.500 | |
| Neurological Disease | 0.638 (0.612–0.664) | <0.001 | 0.363 (0.332–0.395) | <0.001 | |
| RVU | 0.067 (0.067–0.068) | <0.001 | 0.054 (0.053–0.055) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toaz, E.; Pinto, N.; Kilner, K.; Cheon, E. Reduction in Perioperative Risk in Patients with Spinal Muscular Atrophy Following the Release of Disease-Modifying Therapies: An Analysis of the National Surgical Quality Improvement Program Database. Children 2025, 12, 1255. https://doi.org/10.3390/children12091255
Toaz E, Pinto N, Kilner K, Cheon E. Reduction in Perioperative Risk in Patients with Spinal Muscular Atrophy Following the Release of Disease-Modifying Therapies: An Analysis of the National Surgical Quality Improvement Program Database. Children. 2025; 12(9):1255. https://doi.org/10.3390/children12091255
Chicago/Turabian StyleToaz, Erin, Nisha Pinto, Keith Kilner, and Eric Cheon. 2025. "Reduction in Perioperative Risk in Patients with Spinal Muscular Atrophy Following the Release of Disease-Modifying Therapies: An Analysis of the National Surgical Quality Improvement Program Database" Children 12, no. 9: 1255. https://doi.org/10.3390/children12091255
APA StyleToaz, E., Pinto, N., Kilner, K., & Cheon, E. (2025). Reduction in Perioperative Risk in Patients with Spinal Muscular Atrophy Following the Release of Disease-Modifying Therapies: An Analysis of the National Surgical Quality Improvement Program Database. Children, 12(9), 1255. https://doi.org/10.3390/children12091255





