Assessing Discharge Readiness After Propofol-Mediated Deep Sedation in Pediatric Dental Procedures: Revisiting Discharge Practices with the Modified Aldrete Recovery Score
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Power Analysis
2.3. Statistical Analyzes
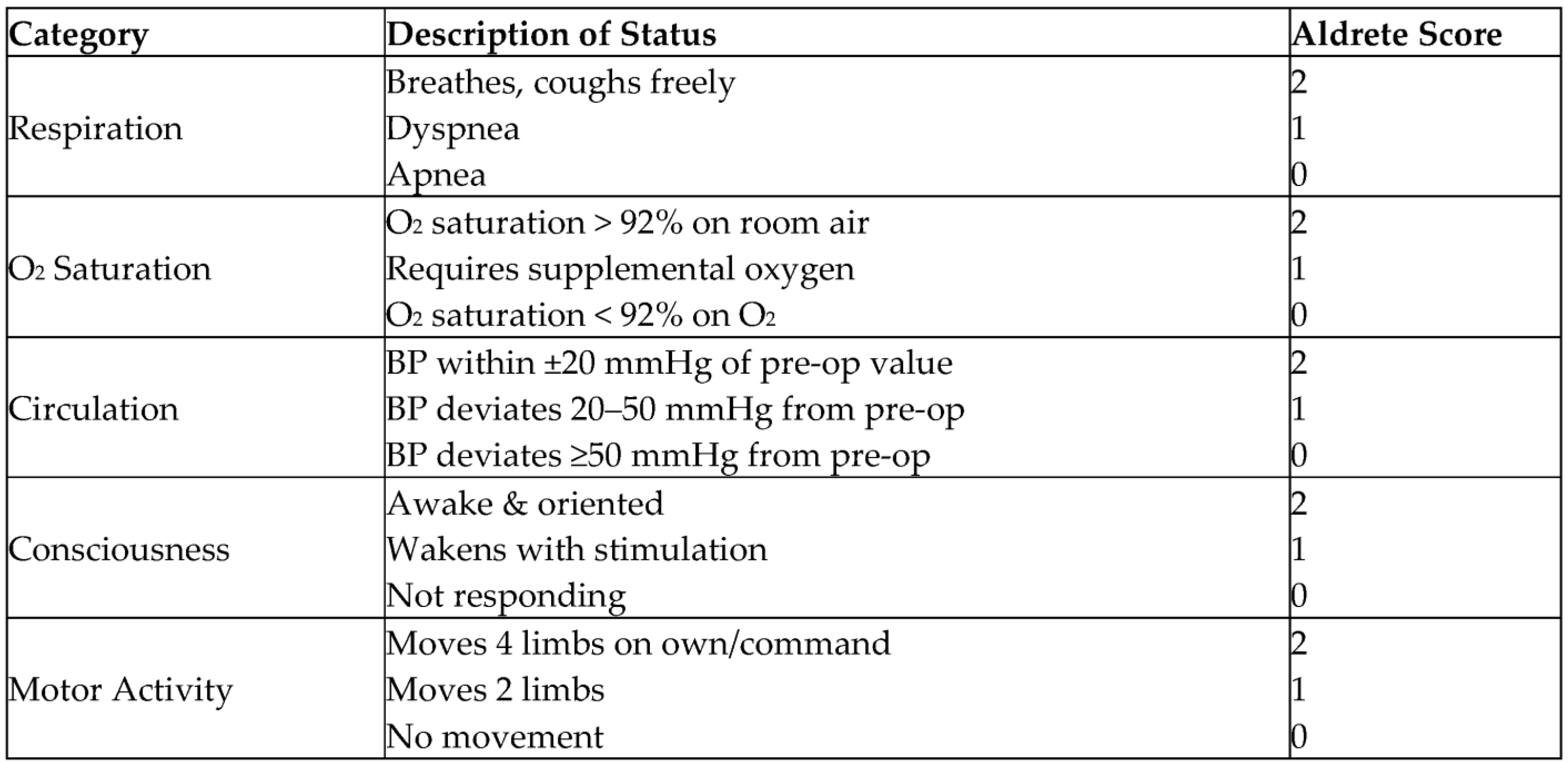
2.4. Anesthesia Procedures
2.5. Evaluation of Discharge Scores
3. Results
- Diabetes mellitus + Asthma
- Down Syndrome + Hypothyroidism
- Aortic stenosis
- Intellectual Disability + Epilepsia + Hypoxic Brain Injury
- Atrial Septal Defect + Thorax deformation
- Atrial Septal Defect + Aortic Regurgitaiton
- Severe F7 Deficiency
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, X.; Song, N.; Chen, L.; Li, Y. Non-pharmacological interventions for reducing dental anxiety in pediatric dentistry: A network meta-analysis. BMC Oral Health 2024, 24, 1151. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Srinivasan, D.; Eagappan, S. Comparative evaluation of three different behavior management techniques among children aged 6–12 years in dental practice: A single-center, double-blind, randomized controlled trial. Dent. Med. Probl. 2024, 61, 641–650. [Google Scholar] [CrossRef]
- Coté, C.J.; Wilson, S.; Riefe, J.; Koteras, R.J.; American Academy of Pediatrics. Guidelines for monitoring and management of pediatric patients before, during, and after sedation for diagnostic and therapeutic procedures. Pediatrics 2019, 143, e20191000. [Google Scholar] [CrossRef]
- Demirel, A.; Önder, N.S.; Kocaoğlu, M.H.; Vural, Ç.; Sarı, Ş. Retrospective evaluation of pediatric dental treatments under deep sedation. J. Clin. Pediatr. Dent. 2024, 48, 124–131. [Google Scholar] [CrossRef]
- Ritwik, P.; Cao, L.T.; Curran, R.; Musselman, R.J. Post-sedation Events in Children Sedated for Dental Care. Anesth. Prog. 2013, 60, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.; Wilson, S. Children Sedated For Dental Care: A Pilot Study of the 24-hour Postsedation Period. Pediatr. Dent. 2005, 28, 260–264. [Google Scholar]
- Merchant, R.; Chartrand, D.; Dain, S.; Dobson, G.; Kurrek, M.M.; Lagacé, A.; Stacey, S.; Thiessen, B. Guidelines to the Practice of Anesthesia—Revised Edition 2016. Can. J. Anesth. 2016, 63, 86–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daud, Y.N.; Carlson, D.W. Pediatric sedation. Clin. North Am. 2014, 61, 703–717. [Google Scholar] [CrossRef]
- Apfelbaum, J.L.; Gross, J.B.; Connis, R.T.; Agarkar, M.; Arnold, D.E.; Coté, C.J.; Dutton, R.; Madias, C.; Nickinovich, D.G. Practice guidelines for moderate procedural sedation and analgesia 2018. Anesthesiology 2018, 128, 437–479. [Google Scholar] [CrossRef]
- Malviya, S.; Voepel-Lewis, T.; Prochaska, G.; Tait, A.R. Prolonged recovery and delayed side effects of sedation for diagnostic imaging studies in children. Pediatrics 2000, 105, e42. [Google Scholar] [CrossRef]
- Ead, H. From Aldrete to PADSS: Reviewing Discharge Criteria After Ambulatory Surgery. J. Perianesthesia Nurs. 2006, 21, 259–267. [Google Scholar] [CrossRef]
- White, P.F.; Song, D. New criteria for fast-tracking after outpatient anesthesia: A comparison with the modified Aldrete’s scoring system. Anesth. Analg. 1999, 88, 1069–1072. [Google Scholar] [CrossRef]
- Aldrete, J.A.; Kroulik, D. A postanesthetic recovery score. Anesth. Analg. 1970, 49, 924–934. [Google Scholar] [CrossRef]
- Aldrete, J.A. The post-anesthesia recovery score revisited. J. Clin. Anesthesia 1995, 7, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Moran, J.L.; Blum, P. Post Anaesthesia Care Unit Discharge: A Clinical Scoring System Versus Traditional Time-based Criteria. Anaesth. Intensive Care 2004, 32, 33–42. [Google Scholar] [CrossRef]
- Armstrong, J.; Forrest, H.; Crawford, M.W. Une étude d’observation prospective, comparant un système de pointage physiologique à des critères de congé fondés sur le temps, chez les patients pédiatriques de chirurgie ambulatoire. Can. J. Anesthesia 2015, 62, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Muralidhar, V.; Aneja, S.; Sharma, A.K. A prospective observational study comparing criteria-based discharge method with traditional time-based discharge method for discharging patients from post-anaesthesia care unit undergoing ambulatory or outpatient minor surgeries under general anaesthesia. Indian J. Anaesth. 2018, 62, 61–65. [Google Scholar] [CrossRef]
- El Aoufy, K.; Forciniti, C.; Longobucco, Y.; Lucchini, A.; Mangli, I.; Magi, C.E.; Bulleri, E.; Fusi, C.; Iovino, P.; Iozzo, P.; et al. A Comparison among Score Systems for Discharging Patients from Recovery Rooms: A Narrative Review. Nurs. Rep. 2024, 14, 2777–2794. [Google Scholar] [CrossRef]
- Awad, I.T.; Chung, F. Factors affecting recovery and discharge following ambulatory surgery. Can. J. Anesth. J. Can. d’anesthésie. 2006, 53, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.; Jellish, W.S.; Kleinman, B.; Fluder, E.; Sawicki, K.; Katsaros, J.; Rahman, R. Use of postanesthesia discharge criteria to reduce discharge delays for inpatients in the postanesthesia care unit. J. Clin. Anesth. 2008, 20, 175–179. [Google Scholar] [CrossRef]
- Micha, G.; Samanta, E.; Damigos, D.; Petridis, A.; Mavreas, V.; Livanios, S. Impact of an anesthesia discharge scoring system on postoperative monitoring after circumcision in children: A randomized trial. Eur. J. Pediatr. Surgery 2009, 19, 293–296. [Google Scholar] [CrossRef]
- Malviya, S.; Voepel-Lewis, T.; Ludomirsky, A.; Marshall, J.; Tait, A.R. Can We Improve the Assessment of Discharge Readiness? Anesthesiology 2004, 100, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Noble, H.; Smith, J. Issues of validity and reliability in qualitative research. Evid. Based Nurs. 2015, 18, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.G.; Waddle, J.P.; Evers, A.S.; Piccirillo, J.F. ECONOMICS AND HEALTH SYSTEMS RESEARCH SECTION EDITOR Postanesthesia Care Unit length of Stay: Quantifying and Assessing Dependent Factors. Anesth. Analg. 1998, 87, 628–633. [Google Scholar] [CrossRef]
- Vural, C.; Kocaoglu, M.H.; Akbarihamed, R.; Demirel, A. A Retrospective Investigation of Patient- and Procedure-Related Factors Associated with Cardiorespiratory Complications in Pediatric Dental Patients Undergoing Deep Sedation. Pediatr. Dent. 2023, 45, 511–519. [Google Scholar]
- Coté, C.J.; Karl, H.W.; Notterman, D.A.; Weinberg, J.A.; McCloskey, C. Adverse Sedation Events in Pediatrics: Analysis of Medications Used for Sedation. Pediatrics 2000, 106, 633–644. [Google Scholar] [CrossRef]
- Scherrer, P.D.; Mallory, M.D.; Cravero, J.P.; Lowrie, L.; Hertzog, J.H.; Berkenbosch, J.W. The impact of obesity on pediatric procedural sedation—related outcomes: Results from the Pediatric Sedation Research Consortium. Pediatr. Anesth. 2015, 25, 689–697. [Google Scholar] [CrossRef]
- Rogerson, C.M.; Abulebda, K.; Hobson, M.J. Association of BMI With Propofol Dosing and Adverse Events in Children With Cancer Undergoing Procedural Sedation. Hosp. Pediatr. 2017, 7, 542–546. [Google Scholar] [CrossRef]
- Chen, S.C.; Chen, C.Y.; Shen, S.J.; Tsai, Y.F.; Ko, Y.C.; Chuang, L.C.; Lin, J.R.; Tsai, H.I. Application of Bispectral Index System (BIS) Monitor to Ambulatory Pediatric Dental Patients under Intravenous Deep Sedation. Diagnostics 2023, 13, 1789. [Google Scholar] [CrossRef]
- Aggarwal, S.; Misquith, J.C.R.; Rao, S.T.; Mahanta, P. Comparison of three scoring criteria to assess recovery from general anesthesia in the postanesthesia care unit in the indian population. Ann. Afr. Med. 2024, 23, 82–86. [Google Scholar] [CrossRef]
- Roelandt, P.; Haesaerts, R.; Demedts, I.; Bisschops, R. Implementation of the Aldrete score reduces recovery time after non-anesthesiologist-administered procedural sedation in gastrointestinal endoscopy. Endosc. Int. Open 2022, 10, E1544–E1547. [Google Scholar] [CrossRef] [PubMed]

| Demographic | n (%) | |
|---|---|---|
| Gender | Male | 55 (55) |
| Female | 45 (45) | |
| BMI-Percentile | Normal | 63 (63) |
| Obese | 3 (3) | |
| Overweight | 11 (11) | |
| Underweight | 23 (23) | |
| Comorbidity | Yes | 29 (29) |
| No | 71 (71) | |
| ASA | I | 71 (71) |
| II | 22 (22) | |
| III | 7 (7) | |
| Variable | X ± S.D. |
|---|---|
| Age (yr) | 5.52 ± 1.75 |
| Anesthesia Duration (min) | 63.99 ± 16.52 |
| Propofol (mg) | 298.23 ± 112.64 |
| Measurements | X ± S.D. | p |
|---|---|---|
| MAS Time (min) | 24.75 ± 7.33 | 0.01 * |
| Institutional Discharge Criteria Time (min) | 36.79 ± 8.59 |
| MAS Time | p | Institutional Discharge Criteria Time | p | Inter-Method Time Difference | p | ||
|---|---|---|---|---|---|---|---|
| (X ± S.D.) | (X ± S.D.) | (X ± S.D.) | |||||
| Gender | Male | 25.87 ± 7.19 | 0.14 | 38.71 ± 8.94 | 0.02 * | 12.84 ± 7.79 | 0.22 |
| Female | 23.38 ± 7.36 | 34.44 ± 7.61 | 11.07 ± 7.24 | ||||
| BMI-Percentile Groups | Normal | 25.59 ± 6.91 | 0.01 * | 37.14 ± 9.12 | 0.01 * | 11.56 ± 7.29 | 0.01 * |
| Obese | 18.33 ± 7.64 | 28.33 ± 10.41 | 10.00 ± 5.01 | ||||
| Overweight | 22.27 ± 8.47 | 37.73 ± 6.07 | 15.45 ± 7.89 | ||||
| Underweight | 24.48 ± 7.66 | 36.48 ± 7.79 | 12.00 ± 8.37 | ||||
| Comorbidity | Yes | 24.52 ± 8.09 | 0.72 | 38.26 ± 10.32 | 0.34 | 13.74 ± 7.39 | 0.14 |
| No | 24.86 ± 7.03 | 36.13 ± 7.69 | 11.28 ± 7.57 | ||||
| ASA | I | 25.21 ± 7.35 | 0.62 | 36.28 ± 7.76 | 0.75 | 11.07 ± 7.56 | 0.13 |
| II | 23.55 ± 7.13 | 37.68 ± 9.73 | 14.14 ± 7.21 | ||||
| III | 23.86 ± 8.41 | 39.14 ± 13.11 | 15.29 ± 7.45 | ||||
| MAS Time (min) | Institutional Discharge Criteria Time (min) | Inter-Method Time Difference (min) | ||
|---|---|---|---|---|
| Age | r | 0.05 | 0.01 | −0.04 |
| p | 0.63 | 0.93 | 0.71 | |
| Weight | r | 0.01 | −0.04 | −0.05 |
| p | 0.94 | 0.71 | 0.62 | |
| Height | r | 0.11 | 0.01 | −0.09 |
| p | 0.29 | 0.90 | 0.38 | |
| BMI | r | −0.15 | −0.11 | 0.03 |
| p | 0.13 | 0.29 | 0.79 | |
| BMI Percentile | r | −0.15 | −0.09 | 0.04 |
| p | 0.14 | 0.36 | 0.70 | |
| Anesthesia Duration (min) | r | −0.07 | 0.05 | 0.13 |
| p | 0.51 | 0.59 | 0.21 | |
| Propofol (mg) | r | 0.01 | 0.09 | 0.09 |
| p | 0.90 | 0.37 | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocaoglu, M.H.; Vural, C. Assessing Discharge Readiness After Propofol-Mediated Deep Sedation in Pediatric Dental Procedures: Revisiting Discharge Practices with the Modified Aldrete Recovery Score. Children 2025, 12, 1155. https://doi.org/10.3390/children12091155
Kocaoglu MH, Vural C. Assessing Discharge Readiness After Propofol-Mediated Deep Sedation in Pediatric Dental Procedures: Revisiting Discharge Practices with the Modified Aldrete Recovery Score. Children. 2025; 12(9):1155. https://doi.org/10.3390/children12091155
Chicago/Turabian StyleKocaoglu, Merve Hayriye, and Cagil Vural. 2025. "Assessing Discharge Readiness After Propofol-Mediated Deep Sedation in Pediatric Dental Procedures: Revisiting Discharge Practices with the Modified Aldrete Recovery Score" Children 12, no. 9: 1155. https://doi.org/10.3390/children12091155
APA StyleKocaoglu, M. H., & Vural, C. (2025). Assessing Discharge Readiness After Propofol-Mediated Deep Sedation in Pediatric Dental Procedures: Revisiting Discharge Practices with the Modified Aldrete Recovery Score. Children, 12(9), 1155. https://doi.org/10.3390/children12091155






