Tumor–Immune Interactions in Pediatric Oral Rhabdomyosarcoma: A Narrative Review on Immuno-Oncology and Emerging Therapies
Abstract
1. Introduction
1.1. Overview of Pediatric Rhabdomyosarcoma
1.2. Epidemiology and Clinical Significance of Oral Rhabdomyosarcoma
1.3. Importance of the Tumor Immune Microenvironment in Pediatric Rhabdomyosarcoma
1.4. Need for Immuno-Oncology Approaches in Rhabdomyosarcoma
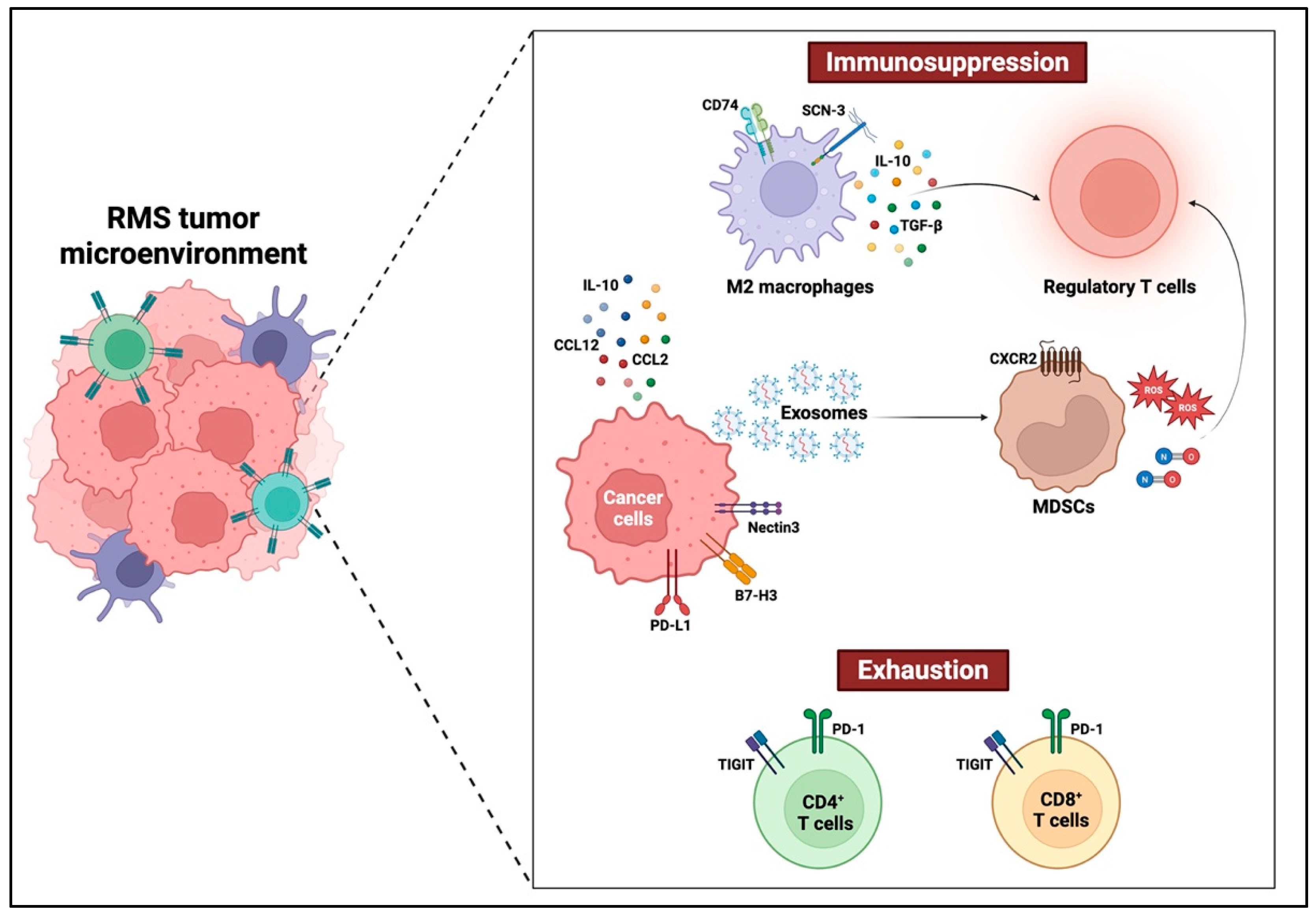
2. Tumor–Immune Microenvironment in Oral Rhabdomyosarcoma
2.1. Tumor-Infiltrating Immune Cells in Oral Rhabdomyosarcoma
2.1.1. Macrophages
2.1.2. Lymphocytes
T Cell Exhaustion and Dysfunction in the Oral Rhabdomyosarcoma Microenvironment
Regulatory T Cell (Treg) Plasticity and Tumor Promotion in Oral Rhabdomyosarcoma
CD4+ CTLs in Oral Rhabdomyosarcoma
2.1.3. Myeloid-Derived Suppressor Cells
Recruitment of MDSCs in the Rhabdomyosarcoma Microenvironment
Activation of MDSCs in Rhabdomyosarcoma
Immunosuppressive Mechanisms of MDSCs in Rhabdomyosarcoma
2.2. Immunosuppressive Cytokines and Chemokines in Oral Rhabdomyosarcoma
2.2.1. IL-10-Driven Immune Suppression in Rhabdomyosarcoma
2.2.2. Contextual “Jekyll and Hyde” of TGF-β Signaling in Rhabdomyosarcoma
2.2.3. Chemokine-Mediated Immune Suppression in Rhabdomyosarcoma
2.3. Immune Evasion Mechanisms in Rhabdomyosarcoma
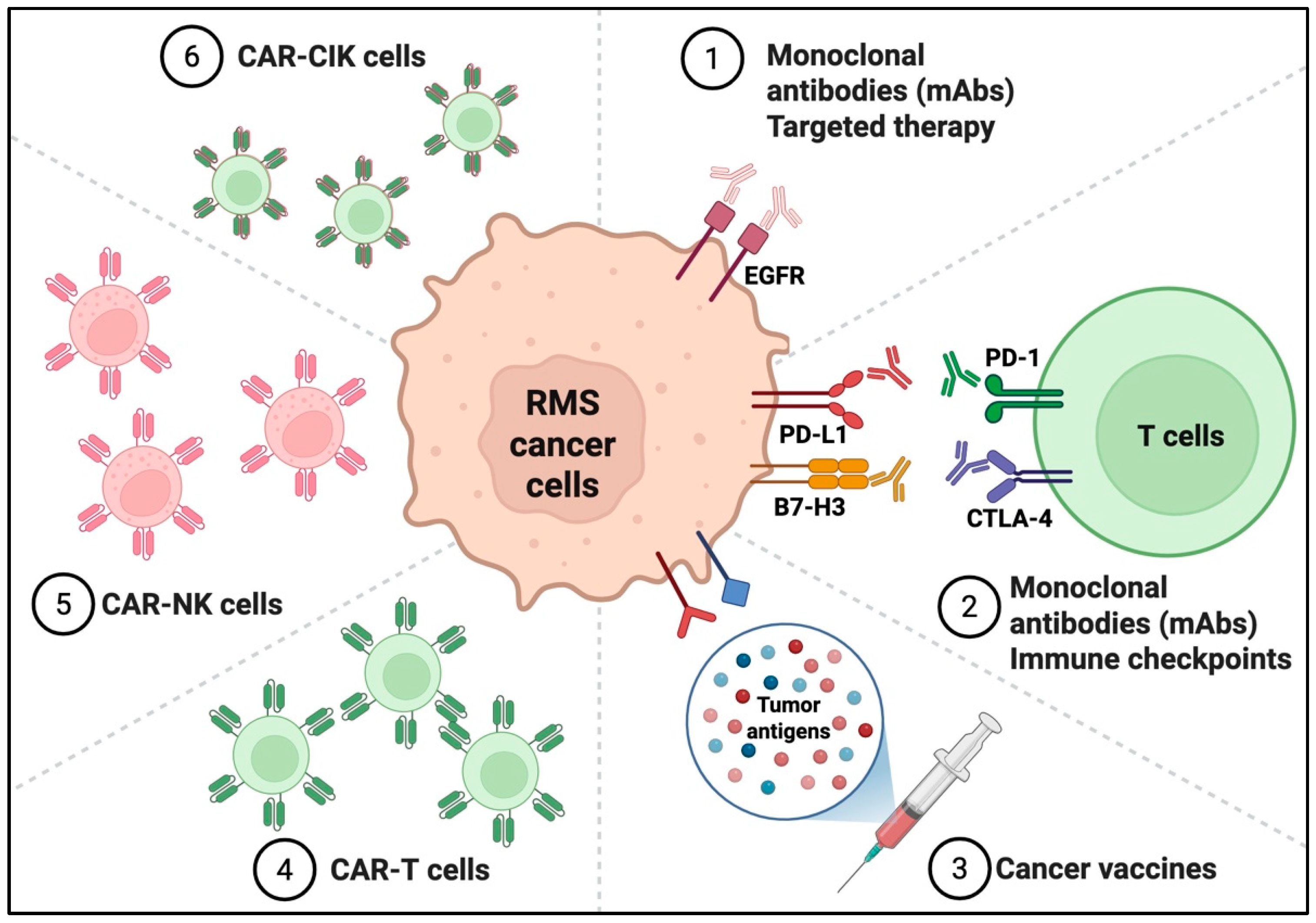
3. Emerging Immunotherapeutic Strategies in Pediatric Rhabdomyosarcoma
3.1. Antibody-Based Therapies
3.2. Immune Checkpoint Inhibitors
3.3. Cancer Vaccines
3.4. Cellular Immunotherapies
3.4.1. CAR-T Cell Therapies
3.4.2. CAR-NK Cells
3.4.3. CAR-Cytokine Induced Killer (CIK) Cells
4. Challenges and Future Directions
4.1. Integrating Immunotherapy with Standard Treatments
4.2. Biological and Developmental Barriers in Pediatric Tumors
4.3. Technical and Ethical Constraints in Pediatric Immunotherapy
4.4. The Biomarker Gap: Stratifying Patients for Response
4.5. Personalizing Immuno-Oncology for Pediatric Rhabdomyosarcoma
4.6. Looking Forward: Translational and Collaborative Imperatives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pathania, A.S. Immune Microenvironment in Childhood Cancers: Characteristics and Therapeutic Challenges. Cancers 2024, 16, 2201. [Google Scholar] [CrossRef]
- Bosse, K.R.; Majzner, R.G.; Mackall, C.L.; Maris, J.M. Immune-Based Approaches for the Treatment of Pediatric Malignancies. Annu. Rev. Cancer Biol. 2020, 4, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Hutzen, B.; Paudel, S.N.; Naeimi Kararoudi, M.; Cassady, K.A.; Lee, D.A.; Cripe, T.P. Immunotherapies for pediatric cancer: Current landscape and future perspectives. Cancer Metastasis Rev. 2019, 38, 573–594. [Google Scholar] [CrossRef] [PubMed]
- Malempati, S.; Hawkins, D.S. Rhabdomyosarcoma: Review of the Children’s Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr. Blood Cancer 2012, 59, 5–10. [Google Scholar] [CrossRef]
- Agaram, N.P. Evolving classification of rhabdomyosarcoma. Histopathology 2022, 80, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Ognjanovic, S.; Linabery, A.M.; Charbonneau, B.; Ross, J.A. Trends in childhood rhabdomyosarcoma incidence and survival in the United States, 1975–2005. Cancer 2009, 115, 4218–4226. [Google Scholar] [CrossRef]
- Rudzinski, E.R.; Anderson, J.R.; Chi, Y.Y.; Gastier-Foster, J.M.; Astbury, C.; Barr, F.G.; Skapek, S.X.; Hawkins, D.S.; Weigel, B.J.; Pappo, A.; et al. Histology, fusion status, and outcome in metastatic rhabdomyosarcoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2017, 64, e26645. [Google Scholar] [CrossRef]
- Chen, C.; Garcia, H.D.; Scheer, M.; Henssen, A.G. Current and Future Treatment Strategies for Rhabdomyosarcoma. Front. Oncol. 2019, 9, 1458. [Google Scholar] [CrossRef]
- Van Tilburg, C.M.; Pfaff, E.; Pajtler, K.W.; Langenberg, K.P.S.; Fiesel, P.; Jones, B.C.; Balasubramanian, G.P.; Stark, S.; Johann, P.D.; Blattner-Johnson, M.; et al. The Pediatric Precision Oncology INFORM Registry: Clinical Outcome and Benefit for Patients with Very High-Evidence Targets. Cancer Discov. 2021, 11, 2764–2779. [Google Scholar] [CrossRef]
- Dasgupta, R.; Fuchs, J.; Rodeberg, D. Rhabdomyosarcoma. Semin. Pediatr. Surg. 2016, 25, 276–283. [Google Scholar] [CrossRef]
- Crist, W.M.; Anderson, J.R.; Meza, J.L.; Fryer, C.; Raney, R.B.; Ruymann, F.B.; Breneman, J.; Qualman, S.J.; Wiener, E.; Wharam, M.; et al. Intergroup rhabdomyosarcoma study-IV: Results for patients with nonmetastatic disease. J. Clin. Oncol. 2001, 19, 3091–3102. [Google Scholar] [CrossRef]
- Dinakar, J.; Gowri, S.; Tryphena, E.T.A. Alveolar type of rhabdomyosarcoma of maxilla-A case report. J. Oral Maxillofac. Pathol. 2023, 27, 406–410. [Google Scholar] [CrossRef]
- Skapek, S.X.; Ferrari, A.; Gupta, A.A.; Lupo, P.J.; Butler, E.; Shipley, J.; Barr, F.G.; Hawkins, D.S. Rhabdomyosarcoma. Nat. Rev. Dis. Primers 2019, 5, 1. [Google Scholar] [CrossRef]
- Rogers, T.N.; Dasgupta, R. Management of Rhabdomyosarcoma in Pediatric Patients. Surg. Oncol. Clin. N. Am. 2021, 30, 339–353. [Google Scholar] [CrossRef]
- Raney, R.B.; Maurer, H.M.; Anderson, J.R.; Andrassy, R.J.; Donaldson, S.S.; Qualman, S.J.; Wharam, M.D.; Wiener, E.S.; Crist, W.M. The Intergroup Rhabdomyosarcoma Study Group (IRSG): Major Lessons From the IRS-I Through IRS-IV Studies as Background for the Current IRS-V Treatment Protocols. Sarcoma 2001, 5, 9–15. [Google Scholar] [CrossRef]
- Crompton, B.D.; Stewart, C.; Taylor-Weiner, A.; Alexe, G.; Kurek, K.C.; Calicchio, M.L.; Kiezun, A.; Carter, S.L.; Shukla, S.A.; Mehta, S.S.; et al. The genomic landscape of pediatric Ewing sarcoma. Cancer Discov. 2014, 4, 1326–1341. [Google Scholar] [CrossRef] [PubMed]
- Shern, J.F.; Chen, L.; Chmielecki, J.; Wei, J.S.; Patidar, R.; Rosenberg, M.; Ambrogio, L.; Auclair, D.; Wang, J.; Song, Y.K.; et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014, 4, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Wessel, K.M.; Kaplan, R.N. Targeting tumor microenvironment and metastasis in children with solid tumors. Curr. Opin. Pediatr. 2022, 34, 53–60. [Google Scholar] [CrossRef]
- Singh, S.; Abu-Zaid, A.; Jin, H.; Fang, J.; Wu, Q.; Wang, T.; Feng, H.; Quarni, W.; Shao, Y.; Maxham, L.; et al. Targeting KDM4 for treating PAX3-FOXO1-driven alveolar rhabdomyosarcoma. Sci. Transl. Med. 2022, 14, eabq2096. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e814. [Google Scholar] [CrossRef]
- Emens, L.A.; Romero, P.J.; Anderson, A.C.; Bruno, T.C.; Capitini, C.M.; Collyar, D.; Gulley, J.L.; Hwu, P.; Posey, A.D., Jr.; Silk, A.W.; et al. Challenges and opportunities in cancer immunotherapy: A Society for Immunotherapy of Cancer (SITC) strategic vision. J. Immunother. Cancer 2024, 12, e009063. [Google Scholar] [CrossRef]
- Sherif, S.; Roelands, J.; Mifsud, W.; Ahmed, E.I.; Raynaud, C.M.; Rinchai, D.; Sathappan, A.; Maaz, A.; Saleh, A.; Ozer, E.; et al. The immune landscape of solid pediatric tumors. J. Exp. Clin. Cancer Res. 2022, 41, 199. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.L.; Meyran, D.; Fleuren, E.D.G.; Mayoh, C.; Zhu, J.; Omer, N.; Ziegler, D.S.; Haber, M.; Darcy, P.K.; Trapani, J.A.; et al. Chimeric Antigen Receptor T cell Therapy and the Immunosuppressive Tumor Microenvironment in Pediatric Sarcoma. Cancers 2021, 13, 4704. [Google Scholar] [CrossRef]
- Gomez, S.; Tabernacki, T.; Kobyra, J.; Roberts, P.; Chiappinelli, K.B. Combining epigenetic and immune therapy to overcome cancer resistance. Semin. Cancer Biol. 2020, 65, 99–113. [Google Scholar] [CrossRef]
- De la Nava, D.; Selvi, K.M.; Alonso, M.M. Immunovirotherapy for Pediatric Solid Tumors: A Promising Treatment That is Becoming a Reality. Front. Immunol. 2022, 13, 866892. [Google Scholar] [CrossRef]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Recent Advances and Challenges in the Treatment of Rhabdomyosarcoma. Cancers 2020, 12, 1758. [Google Scholar] [CrossRef]
- Olsen, H.E.; Lynn, G.M.; Valdes, P.A.; Cerecedo Lopez, C.D.; Ishizuka, A.S.; Arnaout, O.; Bi, W.L.; Peruzzi, P.P.; Chiocca, E.A.; Friedman, G.K.; et al. Therapeutic cancer vaccines for pediatric malignancies: Advances, challenges, and emerging technologies. Neuro-Oncol. Adv. 2021, 3, vdab027. [Google Scholar] [CrossRef] [PubMed]
- Morel, V.J.; Rossler, J.; Bernasconi, M. Targeted immunotherapy and nanomedicine for rhabdomyosarcoma: The way of the future. Med. Res. Rev. 2024, 44, 2730–2773. [Google Scholar] [CrossRef] [PubMed]
- Panagi, M.; Pilavaki, P.; Constantinidou, A.; Stylianopoulos, T. Immunotherapy in soft tissue and bone sarcoma: Unraveling the barriers to effectiveness. Theranostics 2022, 12, 6106–6129. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Elkord, E. Regulatory T Cells in the Tumor Microenvironment and Cancer Progression: Role and Therapeutic Targeting. Vaccines 2016, 4, 28. [Google Scholar] [CrossRef]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, M.; Yang, H.; Qu, R.; Qiu, Y.; Hao, J.; Bi, H.; Guo, D. Regulatory Mechanism of M1/M2 Macrophage Polarization in the Development of Autoimmune Diseases. Mediat. Inflamm. 2023, 2023, 8821610. [Google Scholar] [CrossRef] [PubMed]
- DeMartino, J.; Meister, M.T.; Visser, L.L.; Brok, M.; Groot Koerkamp, M.J.A.; Wezenaar, A.K.L.; Hiemcke-Jiwa, L.S.; de Souza, T.; Merks, J.H.M.; Rios, A.C.; et al. Single-cell transcriptomics reveals immune suppression and cell states predictive of patient outcomes in rhabdomyosarcoma. Nat. Commun. 2023, 14, 3074. [Google Scholar] [CrossRef]
- Rutland, C.D.; Gedallovich, J.; Wang, A.; Zdravkovic, S.; Varma, S.; Hornick, J.L.; Charville, G.W. Diagnostic utility of FOXO1 immunohistochemistry for rhabdomyosarcoma classification. Histopathology 2023, 83, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Guo, Y.; Chen, M.; Zhang, Y.; Liu, Z.; Sun, C.; Hu, X.; Lin, C.; Liu, Y.; Wu, Y.; et al. Schwann Cell-Mediated M2-Like Macrophage Polarization in Rhabdomyosarcoma. Oral Dis. 2025, 31, 1140–1153. [Google Scholar] [CrossRef]
- Munisamy, S.; Radhakrishnan, A.K.; Ramdas, P.; Samuel, P.J.; Singh, V.A. Immune Biomarkers in Blood from Sarcoma Patients: A Pilot Study. Curr. Oncol. 2022, 29, 5585–5603. [Google Scholar] [CrossRef]
- Gutkin, D.W.; Shurin, M.R. Clinical evaluation of systemic and local immune responses in cancer: Time for integration. Cancer Immunol. Immunother. 2014, 63, 45–57. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Guo, J.; Jia, R. Treg: A Promising Immunotherapeutic Target in Oral Diseases. Front. Immunol. 2021, 12, 667862. [Google Scholar] [CrossRef]
- Surendran, S.; Aboelkheir, U.; Tu, A.A.; Magner, W.J.; Sigurdson, S.L.; Merzianu, M.; Hicks, W.L., Jr.; Suresh, A.; Kirkwood, K.L.; Kuriakose, M.A. T-Cell Infiltration and Immune Checkpoint Expression Increase in Oral Cavity Premalignant and Malignant Disorders. Biomedicines 2022, 10, 1840. [Google Scholar] [CrossRef]
- Maggi, E.; Munari, E.; Landolina, N.; Mariotti, F.R.; Azzarone, B.; Moretta, L. T cell landscape in the microenvironment of human solid tumors. Immunol. Lett. 2024, 270, 106942. [Google Scholar] [CrossRef] [PubMed]
- Ozaniak, A.; Vachtenheim, J., Jr.; Lischke, R.; Bartunkova, J.; Strizova, Z. Novel Insights into the Immunotherapy of Soft Tissue Sarcomas: Do We Need a Change of Perspective? Biomedicines 2021, 9, 935. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Wei, J.S.; Cheuk, A.T.; Milewski, D.; Zhang, Z.; Kim, Y.Y.; Chou, H.C.; Liu, C.; Badr, S.; Pope, E.G.; et al. CAR T-cells targeting FGFR4 and CD276 simultaneously show potent antitumor effect against childhood rhabdomyosarcoma. Nat. Commun. 2024, 15, 6222. [Google Scholar] [CrossRef]
- Davicioni, E.; Anderson, J.R.; Buckley, J.D.; Meyer, W.H.; Triche, T.J. Gene expression profiling for survival prediction in pediatric rhabdomyosarcomas: A report from the children’s oncology group. J. Clin. Oncol. 2010, 28, 1240–1246. [Google Scholar] [CrossRef]
- Kather, J.N.; Horner, C.; Weis, C.A.; Aung, T.; Vokuhl, C.; Weiss, C.; Scheer, M.; Marx, A.; Simon-Keller, K. CD163+ immune cell infiltrates and presence of CD54+ microvessels are prognostic markers for patients with embryonal rhabdomyosarcoma. Sci. Rep. 2019, 9, 9211. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Wong, H.Y.; Zeng, Q.; Le Lin, J.; Cheng, M.S.; Kuick, C.H.; Chang, K.T.E.; Loh, A.H.P.; Schwarz, H. Ectopic CD137 expression by rhabdomyosarcoma provides selection advantages but allows immunotherapeutic targeting. Oncoimmunology 2021, 10, 1877459. [Google Scholar] [CrossRef]
- Preglej, T.; Ellmeier, W. CD4(+) Cytotoxic T cells—Phenotype, Function and Transcriptional Networks Controlling Their Differentiation Pathways. Immunol. Lett. 2022, 247, 27–42. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef]
- Belgiovine, C.; Mebelli, K.; Raffaele, A.; De Cicco, M.; Rotella, J.; Pedrazzoli, P.; Zecca, M.; Riccipetitoni, G.; Comoli, P. Pediatric Solid Cancers: Dissecting the Tumor Microenvironment to Improve the Results of Clinical Immunotherapy. Int. J. Mol. Sci. 2024, 25, 3225. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sameshima, J.; Yokomizo, S.; Sueyoshi, T.; Nagano, H.; Miyahara, Y.; Sakamoto, T.; Fujii, S.; Kiyoshima, T.; Guy, T.; et al. Expansion of CD4+ cytotoxic T lymphocytes with specific gene expression patterns may contribute to suppression of tumor immunity in oral squamous cell carcinoma: Single-cell analysis and in vitro experiments. Front. Immunol. 2023, 14, 1305783. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- He, S.; Zheng, L.; Qi, C. Myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment and their targeting in cancer therapy. Mol. Cancer 2025, 24, 5. [Google Scholar] [CrossRef]
- Youn, J.I.; Nagaraj, S.; Collazo, M.; Gabrilovich, D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008, 181, 5791–5802. [Google Scholar] [CrossRef]
- Highfill, S.L.; Cui, Y.; Giles, A.J.; Smith, J.P.; Zhang, H.; Morse, E.; Kaplan, R.N.; Mackall, C.L. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 2014, 6, 237ra267. [Google Scholar] [CrossRef]
- Nomikos, J.; Wunker, C.; Waspe, A.C.; Babichev, Y.; Piorkowska, K.; Wong, S.; Foltz, W.; Gerstle, J.T.; Demicco, E.G.; Gupta, A.A.; et al. Abstract A064 Characterizing the immune microenvironment and examining the effect of tumour-targeted MRgHIFU mediated hyperthermia in combination with thermosensitive liposomal doxorubicin in a mouse model of embryonal rhabdomyosarcoma. Cancer Res. 2024, 84 (Suppl. S17), A064. [Google Scholar] [CrossRef]
- Chen, L.; Oke, T.; Siegel, N.; Cojocaru, G.; Tam, A.J.; Blosser, R.L.; Swailes, J.; Ligon, J.A.; Lebid, A.; Morris, C.; et al. The Immunosuppressive Niche of Soft-Tissue Sarcomas is Sustained by Tumor-Associated Macrophages and Characterized by Intratumoral Tertiary Lymphoid Structures. Clin. Cancer Res. 2020, 26, 4018–4030. [Google Scholar] [CrossRef]
- Bien, E.; Krawczyk, M.; Izycka-Swieszewska, E.; Trzonkowski, P.; Kazanowska, B.; Adamkiewicz-Drozynska, E.; Balcerska, A. Deregulated systemic IL-10/IL-12 balance in advanced and poor prognosis paediatric soft tissue sarcomas. Biomarkers 2013, 18, 204–215. [Google Scholar] [CrossRef]
- Sato, T.; Terai, M.; Tamura, Y.; Alexeev, V.; Mastrangelo, M.J.; Selvan, S.R. Interleukin 10 in the tumor microenvironment: A target for anticancer immunotherapy. Immunol. Res. 2011, 51, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Morris, J.C. Transforming growth factor-beta: A therapeutic target for cancer. Hum. Vaccines Immunother. 2017, 13, 1741–1750. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, X.; Guo, J.; Zhang, F.; Qian, Y.; Wang, G.; Duan, M.; Wang, Y.; Zhao, H.; Yang, Z.; et al. TA-MSCs, TA-MSCs-EVs, MIF: Their crosstalk in immunosuppressive tumor microenvironment. J. Transl. Med. 2022, 20, 320. [Google Scholar] [CrossRef]
- Zhang, Y.; Katkhada, K.; Meng, L.Z.; Zhao, B.; Tong, S.; Chaabane, W.; Kallai, A.; Tobin, N.P.; Ostman, A.; Mega, A.; et al. Myogenic IGFBP5 levels in rhabdomyosarcoma are nourished by mesenchymal stromal cells and regulate growth arrest and apoptosis. Cell Commun. Signal. 2025, 23, 184. [Google Scholar] [CrossRef]
- Drouillard, D.; Craig, B.T.; Dwinell, M.B. Physiology of chemokines in the cancer microenvironment. Am. J. Physiol. Cell Physiol. 2023, 324, C167–C182. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, M.; Niu, M.; Mei, Q.; Wu, K. Myeloid-derived suppressor cells: An emerging target for anticancer immunotherapy. Mol. Cancer 2022, 21, 184. [Google Scholar] [CrossRef]
- Dahmani, A.; Delisle, J.S. TGF-β in T Cell Biology: Implications for Cancer Immunotherapy. Cancers 2018, 10, 194. [Google Scholar] [CrossRef]
- Quamine, A.E.; Olsen, M.R.; Cho, M.M.; Capitini, C.M. Approaches to Enhance Natural Killer Cell-Based Immunotherapy for Pediatric Solid Tumors. Cancers 2021, 13, 2796. [Google Scholar] [CrossRef] [PubMed]
- Milewski, D.; Tian, M.; Kim, Y.; Wei, J.; Khan, J. Abstract 6736: Suppression of antigen presentation is a hallmark of pediatric rhabdomyosarcoma. Cancer Res. 2023, 83 (Suppl. S7), 6736. [Google Scholar] [CrossRef]
- Gabrych, A.; Peksa, R.; Kunc, M.; Krawczyk, M.; Izycka-Swieszewska, E.; Biernat, W.; Bien, E. The PD-L1/PD-1 axis expression on tumor-infiltrating immune cells and tumor cells in pediatric rhabdomyosarcoma. Pathol. Res. Pract. 2019, 215, 152700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pang, Y.; Yi, L.; Wang, X.; Wei, P.; Wang, H.; Lin, S. Epigenetic regulators combined with tumour immunotherapy: Current status and perspectives. Clin. Epigenetics 2025, 17, 51. [Google Scholar] [CrossRef]
- Lavoie, R.R.; Gargollo, P.C.; Ahmed, M.E.; Kim, Y.; Baer, E.; Phelps, D.A.; Charlesworth, C.M.; Madden, B.J.; Wang, L.; Houghton, P.J.; et al. Surfaceome Profiling of Rhabdomyosarcoma Reveals B7-H3 as a Mediator of Immune Evasion. Cancers 2021, 13, 4528. [Google Scholar] [CrossRef] [PubMed]
- Getu, A.A.; Tigabu, A.; Zhou, M.; Lu, J.; Fodstad, O.; Tan, M. New frontiers in immune checkpoint B7-H3 (CD276) research and drug development. Mol. Cancer 2023, 22, 43. [Google Scholar] [CrossRef]
- Miwa, S.; Nishida, H.; Tsuchiya, H. Current status of immunotherapy for sarcomas. Immunotherapy 2017, 9, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M.; Surana, R.; Wang, S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010, 10, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Tsuchikama, K.; Anami, Y.; Ha, S.Y.Y.; Yamazaki, C.M. Exploring the next generation of antibody–drug conjugates. Nat. Rev. Clin. Oncol. 2024, 21, 203–223. [Google Scholar] [CrossRef]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P.; Yang, J.C.; Morgan, R.A.; Dudley, M.E. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 2008, 8, 299–308. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Wang, L.; Geng, H.; Liu, Y.; Liu, L.; Chen, Y.; Wu, F.; Liu, Z.; Ling, S.; Wang, Y.; Zhou, L. Hot and cold tumors: Immunological features and the therapeutic strategies. MedComm 2023, 4, e343. [Google Scholar] [CrossRef]
- Ren, X.; Guo, S.; Guan, X.; Kang, Y.; Liu, J.; Yang, X. Immunological Classification of Tumor Types and Advances in Precision Combination Immunotherapy. Front. Immunol. 2022, 13, 790113. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Gröbner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Brien, G.L.; Stegmaier, K.; Armstrong, S.A. Targeting chromatin complexes in fusion protein-driven malignancies. Nat. Rev. Cancer 2019, 19, 255–269. [Google Scholar] [CrossRef]
- Dyson, K.A.; Stover, B.D.; Grippin, A.; Mendez-Gomez, H.R.; Lagmay, J.; Mitchell, D.A.; Sayour, E.J. Emerging trends in immunotherapy for pediatric sarcomas. J. Hematol. Oncol. 2019, 12, 78. [Google Scholar] [CrossRef]
- Casey, D.L.; Cheung, N.V. Immunotherapy of Pediatric Solid Tumors: Treatments at a Crossroads, with an Emphasis on Antibodies. Cancer Immunol. Res. 2020, 8, 161–166. [Google Scholar] [CrossRef]
- Long, A.H.; Morgenstern, D.A.; Leruste, A.; Bourdeaut, F.; Davis, K.L. Checkpoint Immunotherapy in Pediatrics: Here, Gone, and Back Again. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology (ASCO): Alexandria, VA, USA, 2022; pp. 781–794. [Google Scholar] [CrossRef]
- Chowdhury, F.; Dunn, S.; Mitchell, S.; Mellows, T.; Ashton-Key, M.; Gray, J.C. PD-L1 and CD8+PD1+ lymphocytes exist as targets in the pediatric tumor microenvironment for immunomodulatory therapy. OncoImmunology 2015, 4, e1029701. [Google Scholar] [CrossRef]
- Wang, Y.; Shelton, S.E.; Kastrunes, G.; Barbie, D.A.; Freeman, G.J.; Marasco, W.A. Preclinical models for development of immune-oncology therapies. Immuno-Oncol. Insights 2022, 3, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yang, Q.; Zhang, S.; Millar, D.G.; Alpert, E.J.; Do, D.; Veloso, A.; Brunson, D.C.; Drapkin, B.J.; Stanzione, M.; et al. Single-cell imaging of T cell immunotherapy responses in vivo. J. Exp. Med. 2021, 218, e20210314. [Google Scholar] [CrossRef] [PubMed]
- Rytlewski, J.; Milhem, M.M.; Monga, V. Turning ‘Cold’ tumors ‘Hot’: Immunotherapies in sarcoma. Ann. Transl. Med. 2021, 9, 1039. [Google Scholar] [CrossRef]
- Jacob, W.; James, I.; Hasmann, M.; Weisser, M. Clinical development of HER3-targeting monoclonal antibodies: Perils and progress. Cancer Treat. Rev. 2018, 68, 111–123. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Hubert, P.; Amigorena, S. Antibody-dependent cell cytotoxicity in monoclonal antibody-mediated tumor immunotherapy. Oncoimmunology 2012, 1, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Levine, J.H.; Cogdill, A.P.; Zhao, Y.; Anang, N.A.S.; Andrews, M.C.; Sharma, P.; Wang, J.; Wargo, J.A.; Pe’er, D.; et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 2017, 170, 1120–1133.e1117. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Saerens, M.; Brusselaers, N.; Rottey, S.; Decruyenaere, A.; Creytens, D.; Lapeire, L. Immune checkpoint inhibitors in treatment of soft-tissue sarcoma: A systematic review and meta-analysis. Eur. J. Cancer 2021, 152, 165–182. [Google Scholar] [CrossRef]
- Bertolini, G.; Bergamaschi, L.; Ferrari, A.; Renne, S.L.; Collini, P.; Gardelli, C.; Barisella, M.; Centonze, G.; Chiaravalli, S.; Paolino, C.; et al. PD-L1 assessment in pediatric rhabdomyosarcoma: A pilot study. BMC Cancer 2018, 18, 652. [Google Scholar] [CrossRef]
- Kim, C.; Kim, E.K.; Jung, H.; Chon, H.J.; Han, J.W.; Shin, K.H.; Hu, H.; Kim, K.S.; Choi, Y.D.; Kim, S.; et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer 2016, 16, 434. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Bessede, A.; Pulido, M.; Bompas, E.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Bertucci, F.; Toulmonde, M.; Bellera, C.; et al. Pembrolizumab in soft-tissue sarcomas with tertiary lymphoid structures: A phase 2 PEMBROSARC trial cohort. Nat. Med. 2022, 28, 1199–1206. [Google Scholar] [CrossRef]
- Davis, K.L.; Fox, E.; Merchant, M.S.; Reid, J.M.; Kudgus, R.A.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): A multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020, 21, 541–550. [Google Scholar] [CrossRef]
- Merchant, M.S.; Wright, M.; Baird, K.; Wexler, L.H.; Rodriguez-Galindo, C.; Bernstein, D.; Delbrook, C.; Lodish, M.; Bishop, R.; Wolchok, J.D.; et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, P.; Gong, F.; Tian, Y.; Zhao, X. Case Report: A PD-L1-Positive Patient With Pleomorphic Rhabdomyosarcoma Achieving an Impressive Response to Immunotherapy. Front. Immunol. 2022, 13, 815598. [Google Scholar] [CrossRef]
- Timpanaro, A.; Piccand, C.; Uldry, A.C.; Bode, P.K.; Dzhumashev, D.; Sala, R.; Heller, M.; Rössler, J.; Bernasconi, M. Surfaceome Profiling of Cell Lines and Patient-Derived Xenografts Confirm FGFR4, NCAM1, CD276, and Highlight AGRL2, JAM3, and L1CAM as Surface Targets for Rhabdomyosarcoma. Int. J. Mol. Sci. 2023, 24, 2601. [Google Scholar] [CrossRef] [PubMed]
- Kendsersky, N.M.; Lindsay, J.; Kolb, E.A.; Smith, M.A.; Teicher, B.A.; Erickson, S.W.; Earley, E.J.; Mosse, Y.P.; Martinez, D.; Pogoriler, J.; et al. The B7-H3-Targeting Antibody-Drug Conjugate m276-SL-PBD Is Potently Effective Against Pediatric Cancer Preclinical Solid Tumor Models. Clin. Cancer Res. 2021, 27, 2938–2946. [Google Scholar] [CrossRef]
- Rasic, P.; Jeremic, M.; Jeremic, R.; Dusanovic Pjevic, M.; Rasic, M.; Djuricic, S.M.; Milickovic, M.; Vukadin, M.; Mijovic, T.; Savic, D. Targeting B7-H3—A Novel Strategy for the Design of Anticancer Agents for Extracranial Pediatric Solid Tumors Treatment. Molecules 2023, 28, 3356. [Google Scholar] [CrossRef]
- Troitskaya, O.; Varlamov, M.; Nushtaeva, A.; Richter, V.; Koval, O. Recombinant Lactaptin Induces Immunogenic Cell Death and Creates an Antitumor Vaccination Effect in Vivo with Enhancement by an IDO Inhibitor. Molecules 2020, 25, 2804. [Google Scholar] [CrossRef]
- De Giovanni, C.; Nanni, P.; Landuzzi, L.; Ianzano, M.L.; Nicoletti, G.; Croci, S.; Palladini, A.; Lollini, P.L. Immune targeting of autocrine IGF2 hampers rhabdomyosarcoma growth and metastasis. BMC Cancer 2019, 19, 126. [Google Scholar] [CrossRef]
- Phelps, M.P.; Yang, H.; Patel, S.; Rahman, M.M.; McFadden, G.; Chen, E. Oncolytic Virus-Mediated RAS Targeting in Rhabdomyosarcoma. Mol. Ther. Oncolytics 2018, 11, 52–61. [Google Scholar] [CrossRef]
- Merchant, M.S.; Bernstein, D.; Amoako, M.; Baird, K.; Fleisher, T.A.; Morre, M.; Steinberg, S.M.; Sabatino, M.; Stroncek, D.F.; Venkatasan, A.M.; et al. Adjuvant Immunotherapy to Improve Outcome in High-Risk Pediatric Sarcomas. Clin. Cancer Res. 2016, 22, 3182–3191. [Google Scholar] [CrossRef]
- Krishnadas, D.K.; Shusterman, S.; Bai, F.; Diller, L.; Sullivan, J.E.; Cheerva, A.C.; George, R.E.; Lucas, K.G. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol. Immunother. 2015, 64, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C. Cellular immunotherapies for cancer. Ir. J. Med. Sci. 2021, 190, 41–57. [Google Scholar] [CrossRef]
- Lin, H.; Cheng, J.; Mu, W.; Zhou, J.; Zhu, L. Advances in Universal CAR-T Cell Therapy. Front. Immunol. 2021, 12, 744823. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Jung, H.; Noh, J.Y. Emerging Approaches for Solid Tumor Treatment Using CAR-T Cell Therapy. Int. J. Mol. Sci. 2021, 22, 12126. [Google Scholar] [CrossRef]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Chimeric antigen receptor T (CAR-T) cell immunotherapy for sarcomas: From mechanisms to potential clinical applications. Cancer Treat. Rev. 2020, 82, 101934. [Google Scholar] [CrossRef]
- Kulczycka, M.; Derlatka, K.; Tasior, J.; Lejman, M.; Zawitkowska, J. CAR T-Cell Therapy in Children with Solid Tumors. J. Clin. Med. 2023, 12, 2326. [Google Scholar] [CrossRef] [PubMed]
- Timpanaro, A.; Piccand, C.; Dzhumashev, D.; Anton-Joseph, S.; Robbi, A.; Moser, J.; Rössler, J.; Bernasconi, M. CD276-CAR T cells and Dual-CAR T cells targeting CD276/FGFR4 promote rhabdomyosarcoma clearance in orthotopic mouse models. J. Exp. Clin. Cancer Res. 2023, 42, 293. [Google Scholar] [CrossRef]
- Lake, J.A.; Woods, E.; Hoffmeyer, E.; Schaller, K.L.; Cruz-Cruz, J.; Fernandez, J.; Tufa, D.; Kooiman, B.; Hall, S.C.; Jones, D.; et al. Directing B7-H3 chimeric antigen receptor T cell homing through IL-8 induces potent antitumor activity against pediatric sarcoma. J. Immunother. Cancer 2024, 12, e009221. [Google Scholar] [CrossRef]
- Gattenloehner, S.; Vincent, A.; Leuschner, I.; Tzartos, S.; Müller-Hermelink, H.K.; Kirchner, T.; Marx, A. The fetal form of the acetylcholine receptor distinguishes rhabdomyosarcomas from other childhood tumors. Am. J. Pathol. 1998, 152, 437–444. [Google Scholar]
- Gattenlöhner, S.; Marx, A.; Markfort, B.; Pscherer, S.; Landmeier, S.; Juergens, H.; Müller-Hermelink, H.K.; Matthews, I.; Beeson, D.; Vincent, A.; et al. Rhabdomyosarcoma lysis by T cells expressing a human autoantibody-based chimeric receptor targeting the fetal acetylcholine receptor. Cancer Res. 2006, 66, 24–28. [Google Scholar] [CrossRef]
- Simon-Keller, K.; Paschen, A.; Eichmüller, S.; Gattenlöhner, S.; Barth, S.; Koscielniak, E.; Leuschner, I.; Stöbel, P.; Hombach, A.; Abken, H.; et al. Adoptive T-cell therapy of rhabdomyosarcoma. Pathologe 2010, 31 (Suppl. S2), 215–220. [Google Scholar] [CrossRef]
- Simon-Keller, K.; Paschen, A.; Hombach, A.A.; Ströbel, P.; Coindre, J.M.; Eichmüller, S.B.; Vincent, A.; Gattenlöhner, S.; Hoppe, F.; Leuschner, I.; et al. Survivin blockade sensitizes rhabdomyosarcoma cells for lysis by fetal acetylcholine receptor-redirected T cells. Am. J. Pathol. 2013, 182, 2121–2131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, X.; Park, H.; Greene, J.; Pao, J.; Mulvey, E.; Zhou, S.X.; Albert, C.M.; Moy, F.; Sachdev, D.; Yee, D.; et al. IGF1R- and ROR1-Specific CAR T Cells as a Potential Therapy for High Risk Sarcomas. PLoS ONE 2015, 10, e0133152. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, J.; Wen, X.; Xu, B.; Que, Y.; Yu, K.; Xu, L.; Zhao, J.; Pan, Q.; Zhou, P.; et al. Chimeric antigen receptor-modified T-cell therapy for platelet-derived growth factor receptor α-positive rhabdomyosarcoma. Cancer 2020, 126 (Suppl. S9), 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H.; Yagyu, S.; Nakamura, K.; Yamashima, K.; Tomida, A.; Kikuchi, K.; Iehara, T.; Nakazawa, Y.; Hosoi, H. Development of non-viral, ligand-dependent, EPHB4-specific chimeric antigen receptor T cells for treatment of rhabdomyosarcoma. Mol. Ther. Oncolytics 2021, 20, 646–658. [Google Scholar] [CrossRef]
- Shivaprasad, N.; Xiong, Y.; Yohe, M.; Schneider, D.; Shern, J.; Baskar, S.; Dimitrov, D.; Sorenson, P.; Orentas, R.; Khan, J. 649. Developing FGFR4 Chimeric Antigen Receptor CAR T Cell Therapy Against Rhabdomyosarcoma. Mol. Ther. 2016, 24 (Suppl. S1), S257–S258. [Google Scholar] [CrossRef]
- Alijaj, N.; Moutel, S.; Gouveia, Z.L.; Gray, M.; Roveri, M.; Dzhumashev, D.; Weber, F.; Meier, G.; Luciani, P.; Rössler, J.K.; et al. Novel FGFR4-Targeting Single-Domain Antibodies for Multiple Targeted Therapies against Rhabdomyosarcoma. Cancers 2020, 12, 3313. [Google Scholar] [CrossRef]
- Sullivan, P.M.; Kumar, R.; Li, W.; Hoglund, V.; Wang, L.; Zhang, Y.; Shi, M.; Beak, D.; Cheuk, A.; Jensen, M.C.; et al. FGFR4-Targeted Chimeric Antigen Receptors Combined with Anti-Myeloid Polypharmacy Effectively Treat Orthotopic Rhabdomyosarcoma. Mol. Cancer Ther. 2022, 21, 1608–1621. [Google Scholar] [CrossRef]
- Tian, M.; Wei, J.S.; Shivaprasad, N.; Highfill, S.L.; Gryder, B.E.; Milewski, D.; Brown, G.T.; Moses, L.; Song, H.; Wu, J.T.; et al. Preclinical development of a chimeric antigen receptor T cell therapy targeting FGFR4 in rhabdomyosarcoma. Cell Rep. Med. 2023, 4, 101212. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Xu, L.; Wang, J.; Yu, K.; Xu, B.; Que, Y.; Zhao, J.; Pan, Q.; Gao, C.; Zhou, P.; et al. FGFR4-specific CAR-T cells with inducible caspase-9 suicide gene as an approach to treat rhabdomyosarcoma. Cancer Gene Ther. 2024, 31, 1571–1584. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, W.; Qin, M.; Jin, M.; Chang, L.; Ma, X. CD56-chimeric antigen receptor T-cell therapy for refractory/recurrent rhabdomyosarcoma: A 3.5-year follow-up case report. Medicine 2019, 98, e17572. [Google Scholar] [CrossRef]
- Shum, T.; Omer, B.; Tashiro, H.; Kruse, R.L.; Wagner, D.L.; Parikh, K.; Yi, Z.; Sauer, T.; Liu, D.; Parihar, R.; et al. Constitutive Signaling from an Engineered IL7 Receptor Promotes Durable Tumor Elimination by Tumor-Redirected T Cells. Cancer Discov. 2017, 7, 1238–1247. [Google Scholar] [CrossRef]
- Del Bufalo, F.; De Angelis, B.; Caruana, I.; Del Baldo, G.; De Ioris, M.A.; Serra, A.; Mastronuzzi, A.; Cefalo, M.G.; Pagliara, D.; Amicucci, M.; et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. N. Engl. J. Med. 2023, 388, 1284–1295. [Google Scholar] [CrossRef]
- Pezzella, M.; Quintarelli, C.; Quadraccia, M.C.; Sarcinelli, A.; Manni, S.; Iaffaldano, L.; Ottaviani, A.; Ciccone, R.; Camera, A.; D’Amore, M.L.; et al. Tumor-derived G-CSF induces an immunosuppressive microenvironment in an osteosarcoma model, reducing response to CAR.GD2 T-cells. J. Hematol. Oncol. 2024, 17, 127. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Mechtersheimer, G.; Staudter, M.; Majdic, O.; Dörken, B.; Moldenhauer, G.; Möller, P. Expression of HLA-A,B,C, beta 2-microglobulin (beta 2m), HLA-DR, -DP, -DQ and of HLA-D-associated invariant chain (Ii) in soft-tissue tumors. Int. J. Cancer 1990, 46, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, H.G.; Kärre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Cho, D.; Shook, D.R.; Shimasaki, N.; Chang, Y.H.; Fujisaki, H.; Campana, D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin. Cancer Res. 2010, 16, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef]
- Boerman, G.H.; van Ostaijen-ten Dam, M.M.; Kraal, K.C.; Santos, S.J.; Ball, L.M.; Lankester, A.C.; Schilham, M.W.; Egeler, R.M.; van Tol, M.J. Role of NKG2D, DNAM-1 and natural cytotoxicity receptors in cytotoxicity toward rhabdomyosarcoma cell lines mediated by resting and IL-15-activated human natural killer cells. Cancer Immunol. Immunother. 2015, 64, 573–583. [Google Scholar] [CrossRef]
- Wagner, J.; Pfannenstiel, V.; Waldmann, A.; Bergs, J.W.J.; Brill, B.; Huenecke, S.; Klingebiel, T.; Rödel, F.; Buchholz, C.J.; Wels, W.S.; et al. A Two-Phase Expansion Protocol Combining Interleukin (IL)-15 and IL-21 Improves Natural Killer Cell Proliferation and Cytotoxicity against Rhabdomyosarcoma. Front. Immunol. 2017, 8, 676. [Google Scholar] [CrossRef]
- Rademacher, M.J.; Cruz, A.; Faber, M.; Oldham, R.A.A.; Wang, D.; Medin, J.A.; Schloemer, N.J. Sarcoma IL-12 overexpression facilitates NK cell immunomodulation. Sci. Rep. 2021, 11, 8321. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, H.; Thiede, M.; Weiss, J.; Biele, E.; Flohe, L.; Lachermaier, H.; Prexler, C.; Evdokimova, V.; Radvanyi, L.; Akhtar, I.; et al. Cytokine screening identifies TNF to potentially enhance immunogenicity of pediatric sarcomas. Front. Immunol. 2024, 15, 1347404. [Google Scholar] [CrossRef] [PubMed]
- Vela, M.; Bueno, D.; González-Navarro, P.; Brito, A.; Fernández, L.; Escudero, A.; Valentín, J.; Mestre-Durán, C.; Arranz-Álvarez, M.; Pérez de Diego, R.; et al. Anti-CXCR4 Antibody Combined With Activated and Expanded Natural Killer Cells for Sarcoma Immunotherapy. Front. Immunol. 2019, 10, 1814. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.; Pfeiffer, M.; Müller, I.; Schumm, M.; Ebinger, M.; Koscielniak, E.; Feuchtinger, T.; Föll, J.; Martin, D.; Handgretinger, R. Haploidentical stem cell transplantation in patients with pediatric solid tumors: Preliminary results of a pilot study and analysis of graft versus tumor effects. Klin. Padiatr. 2006, 218, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Llosa, N.J.; Cooke, K.R.; Chen, A.R.; Gamper, C.J.; Klein, O.R.; Zambidis, E.T.; Luber, B.; Rosner, G.; Siegel, N.; Holuba, M.J.; et al. Reduced-Intensity Haploidentical Bone Marrow Transplantation with Post-Transplant Cyclophosphamide for Solid Tumors in Pediatric and Young Adult Patients. Biol. Blood Marrow Transplant. 2017, 23, 2127–2136. [Google Scholar] [CrossRef]
- Pérez-Martínez, A.; Leung, W.; Muñoz, E.; Iyengar, R.; Ramírez, M.; Vicario, J.L.; Lassaletta, A.; Sevilla, J.; González-Vicent, M.; Madero, L.; et al. KIR-HLA receptor-ligand mismatch associated with a graft-versus-tumor effect in haploidentical stem cell transplantation for pediatric metastatic solid tumors. Pediatr. Blood Cancer 2009, 53, 120–124. [Google Scholar] [CrossRef]
- Marofi, F.; Abdul-Rasheed, O.F.; Rahman, H.S.; Budi, H.S.; Jalil, A.T.; Yumashev, A.V.; Hassanzadeh, A.; Yazdanifar, M.; Motavalli, R.; Chartrand, M.S.; et al. CAR-NK cell in cancer immunotherapy; A promising frontier. Cancer Sci. 2021, 112, 3427–3436. [Google Scholar] [CrossRef]
- Imai, C.; Iwamoto, S.; Campana, D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 2005, 106, 376–383. [Google Scholar] [CrossRef]
- Gossel, L.D.H.; Heim, C.; Pfeffermann, L.M.; Moser, L.M.; Bönig, H.B.; Klingebiel, T.E.; Bader, P.; Wels, W.S.; Merker, M.; Rettinger, E. Retargeting of NK-92 Cells against High-Risk Rhabdomyosarcomas by Means of an ERBB2 (HER2/Neu)-Specific Chimeric Antigen Receptor. Cancers 2021, 13, 1443. [Google Scholar] [CrossRef]
- Lam, P.Y.; Omer, N.; Wong, J.K.M.; Tu, C.; Alim, L.; Rossi, G.R.; Victorova, M.; Tompkins, H.; Lin, C.Y.; Mehdi, A.M.; et al. Enhancement of anti-sarcoma immunity by NK cells engineered with mRNA for expression of a EphA2-targeted CAR. Clin. Transl. Med. 2025, 15, e70140. [Google Scholar] [CrossRef]
- Heim, C.; Hartig, L.; Weinelt, N.; Moser, L.M.; Salzmann-Manrique, E.; Merker, M.; Wels, W.S.; Tonn, T.; Bader, P.; Klusmann, J.H.; et al. Bortezomib promotes the TRAIL-mediated killing of resistant rhabdomyosarcoma by ErbB2/Her2-targeted CAR-NK-92 cells via DR5 upregulation. Mol. Ther. Oncol. 2024, 32, 200802. [Google Scholar] [CrossRef]
- Schmidt-Wolf, I.G.; Negrin, R.S.; Kiem, H.P.; Blume, K.G.; Weissman, I.L. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J. Exp. Med. 1991, 174, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Leuci, V.; Donini, C.; Grignani, G.; Rotolo, R.; Mesiano, G.; Fiorino, E.; Gammaitoni, L.; D’Ambrosio, L.; Merlini, A.; Landoni, E.; et al. CSPG4-Specific CAR.CIK Lymphocytes as a Novel Therapy for the Treatment of Multiple Soft-Tissue Sarcoma Histotypes. Clin. Cancer Res. 2020, 26, 6321–6334. [Google Scholar] [CrossRef] [PubMed]
- Merker, M.; Pfirrmann, V.; Oelsner, S.; Fulda, S.; Klingebiel, T.; Wels, W.S.; Bader, P.; Rettinger, E. Generation and characterization of ErbB2-CAR-engineered cytokine-induced killer cells for the treatment of high-risk soft tissue sarcoma in children. Oncotarget 2017, 8, 66137–66153. [Google Scholar] [CrossRef] [PubMed]
- Merker, M.; Wagner, J.; Kreyenberg, H.; Heim, C.; Moser, L.M.; Wels, W.S.; Bonig, H.; Ivics, Z.; Ullrich, E.; Klingebiel, T.; et al. ERBB2-CAR-Engineered Cytokine-Induced Killer Cells Exhibit Both CAR-Mediated and Innate Immunity Against High-Risk Rhabdomyosarcoma. Front. Immunol. 2020, 11, 581468. [Google Scholar] [CrossRef]
- Moser, L.M.; Heim, C.; Koschade, S.E.; Wendel, P.; Bozkurt, S.; Harenkamp, S.; Kreyenberg, H.; Merker, M.; Munch, C.; Gradhand, E.; et al. CAR-CIK vs. CAR-T: Benchmarking novel cytokine-induced killer cells as solid tumor immunotherapy in ErbB2+ rhabdomyosarcoma. Front. Immunol. 2025, 16, 1485817. [Google Scholar] [CrossRef]
- Gupta, A.; Cripe, T.P. Immunotherapies for Pediatric Solid Tumors: A Targeted Update. Paediatr. Drugs 2022, 24, 1–12. [Google Scholar] [CrossRef]
- Wedekind, M.F.; Denton, N.L.; Chen, C.Y.; Cripe, T.P. Pediatric Cancer Immunotherapy: Opportunities and Challenges. Paediatr. Drugs 2018, 20, 395–408. [Google Scholar] [CrossRef]
- Babu, S.; Krishnan, M. Catalysts of change: Immunotherapy’s frontier in oral oncology. Oral Oncol. Rep. 2024, 11, 100601. [Google Scholar] [CrossRef]
- Wood, G.E.; Meyer, C.; Petitprez, F.; D’Angelo, S.P. Immunotherapy in Sarcoma: Current Data and Promising Strategies. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology (ASCO): Alexandria, VA, USA, 2024; Volume 44, p. e432234. [Google Scholar] [CrossRef]
- Pilavaki, P.; Panagi, M.; Arifi, S.; Jones, R.L.; Stylianopoulos, T.; Constantinidou, A. Exploring the landscape of immunotherapy approaches in sarcomas. Front. Oncol. 2022, 12, 1069963. [Google Scholar] [CrossRef]
- Peng, L.; Sferruzza, G.; Yang, L.; Zhou, L.; Chen, S. CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumors. Cell. Mol. Immunol. 2024, 21, 1089–1108. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.V.; Bender, J.L.G. Delays in Pediatric Evaluation of New and Relevant Cancer Therapies. J. Pediatr. 2024, 265, 113826. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Cancer Clinical Trial Eligibility Criteria: Minimum Age Considerations for Inclusion of Pediatric Patients. Guidance for Industry and IRBs. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-minimum-age-considerations-inclusion-pediatric-patients (accessed on 14 May 2025).
- Yan, A.P.; Venkatramani, R.; Bradley, J.A.; Lautz, T.B.; Urla, C.I.; Merks, J.H.M.; Oberoi, S. Clinical Characteristics, Treatment Considerations, and Outcomes of Infants with Rhabdomyosarcoma. Cancers 2023, 15, 2296. [Google Scholar] [CrossRef]
- Makimoto, A. Optimizing Rhabdomyosarcoma Treatment in Adolescents and Young Adults. Cancers 2022, 14, 2270. [Google Scholar] [CrossRef] [PubMed]
- Vo, K.T.; Parsons, D.W.; Seibel, N.L. Precision Medicine in Pediatric Oncology. Surg. Oncol. Clin. N. Am. 2020, 29, 63–72. [Google Scholar] [CrossRef]
- Heipertz, A.E.; Pajtler, K.W.; Pfaff, E.; Schramm, K.; Blattner-Johnson, M.; Milde, T.; Jones, B.C.; Zuliani, C.; Hutter, C.; Lohi, O.; et al. Outcome of Children and Adolescents With Relapsed/Refractory/Progressive Malignancies Treated With Molecularly Informed Targeted Drugs in the Pediatric Precision Oncology Registry INFORM. JCO Precis. Oncol. 2023, 7, e2300015. [Google Scholar] [CrossRef]
- McCabe, M.G.; Geoerger, B.; Chesler, L.; Hargrave, D.; Parsons, D.W.; van Tilburg, C.M.; Schleiermacher, G.; Hickman, J.A.; George, S.L. Precision Medicine for Childhood Cancer: Current Limitations and Future Perspectives. JCO Precis. Oncol. 2024, 8, e2300117. [Google Scholar] [CrossRef]
| Modality | Role in RMS Treatment | Limitations |
|---|---|---|
| Surgery | Local tumor control by complete excision. | Often limited by anatomical complexity in the oral cavity; may affect function and appearance. |
| Chemotherapy | Systemic disease control; commonly includes vincristine, actinomycin D, and cyclophosphamide (VAC regimen). | Systemic toxicity, resistance in high-risk subtypes. |
| Radiotherapy | Local control for unresectable or residual disease. | Growth inhibition, craniofacial deformities, cognitive effects in children. |
| Targeted Therapy | Includes inhibitors of IGF1R, FGFR4, and other molecular targets relevant to RMS biology. | Target specificity and resistance; mostly in experimental stages. |
| Immunotherapy | Emerging approaches include CAR-T, checkpoint inhibitors, and oncolytic viruses; efficacy in pediatric RMS under investigation. | Limited clinical data in children; immune ‘coldness’ of tumors poses challenge. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Meligy, O.A.; Elemam, N.M.; Hassan, W.A.; Talaat, I.M. Tumor–Immune Interactions in Pediatric Oral Rhabdomyosarcoma: A Narrative Review on Immuno-Oncology and Emerging Therapies. Children 2025, 12, 1249. https://doi.org/10.3390/children12091249
El Meligy OA, Elemam NM, Hassan WA, Talaat IM. Tumor–Immune Interactions in Pediatric Oral Rhabdomyosarcoma: A Narrative Review on Immuno-Oncology and Emerging Therapies. Children. 2025; 12(9):1249. https://doi.org/10.3390/children12091249
Chicago/Turabian StyleEl Meligy, Omar A., Noha M. Elemam, Wael A. Hassan, and Iman M. Talaat. 2025. "Tumor–Immune Interactions in Pediatric Oral Rhabdomyosarcoma: A Narrative Review on Immuno-Oncology and Emerging Therapies" Children 12, no. 9: 1249. https://doi.org/10.3390/children12091249
APA StyleEl Meligy, O. A., Elemam, N. M., Hassan, W. A., & Talaat, I. M. (2025). Tumor–Immune Interactions in Pediatric Oral Rhabdomyosarcoma: A Narrative Review on Immuno-Oncology and Emerging Therapies. Children, 12(9), 1249. https://doi.org/10.3390/children12091249







_Talaat.jpg)

