Steroid Use for Established Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis
Abstract
Highlights
- Steroids have a good rate of success in terms of oxygen decrease. However, more clinically relevant outcomes, such as the total duration of supplemental oxygen, length of hospital stay, and mortality, are not affected by the use of steroids after 28 days of life. No significant side effects are reported in the literature. No clinical variables analyzed in the available studies were associated with the success of the therapy.
- Despite steroid treatment being regularly used for established BPD, only one study reported the incidence of this treatment exclusively after 28 days of life. Two independent, high-level neonatal centers describe steroid administration as standard treatment for severe BPD in order to avoid missing potential responders.
- Identifying patients who would benefit from steroid treatments is a priority in order to design statistically and clinically relevant studies.
- The observed drug safety profile of steroids supports the design of future trials in patients with established BPD.
Abstract
1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Primary and Secondary Outcomes
2.2.1. Efficacy Outcomes
- Short-term respiratory outcomes: Respiratory support step-down and oxygen decrease during treatment.
- Medium-term outcomes: Total duration of oxygen dependency, length of stay, requirement for supplemental oxygen on discharge or home ventilation through a tracheostomy, and feeding difficulties.
- Long-term outcomes: Duration of home oxygen use, number and severity of respiratory symptoms, hospital readmission for any cause and respiratory causes, and function and exercise tests.
2.2.2. Safety Outcomes
2.3. Search Strategy
2.4. Data Management
2.5. Study Selection Process
2.6. Data Extraction
2.7. Data Analysis
2.8. Subgroup Analysis
2.9. Risk of Bias Assessment
2.10. GRADE Assessment and Display of the Results
2.11. Protocol Registration, Amendments, and PRISMA Statement
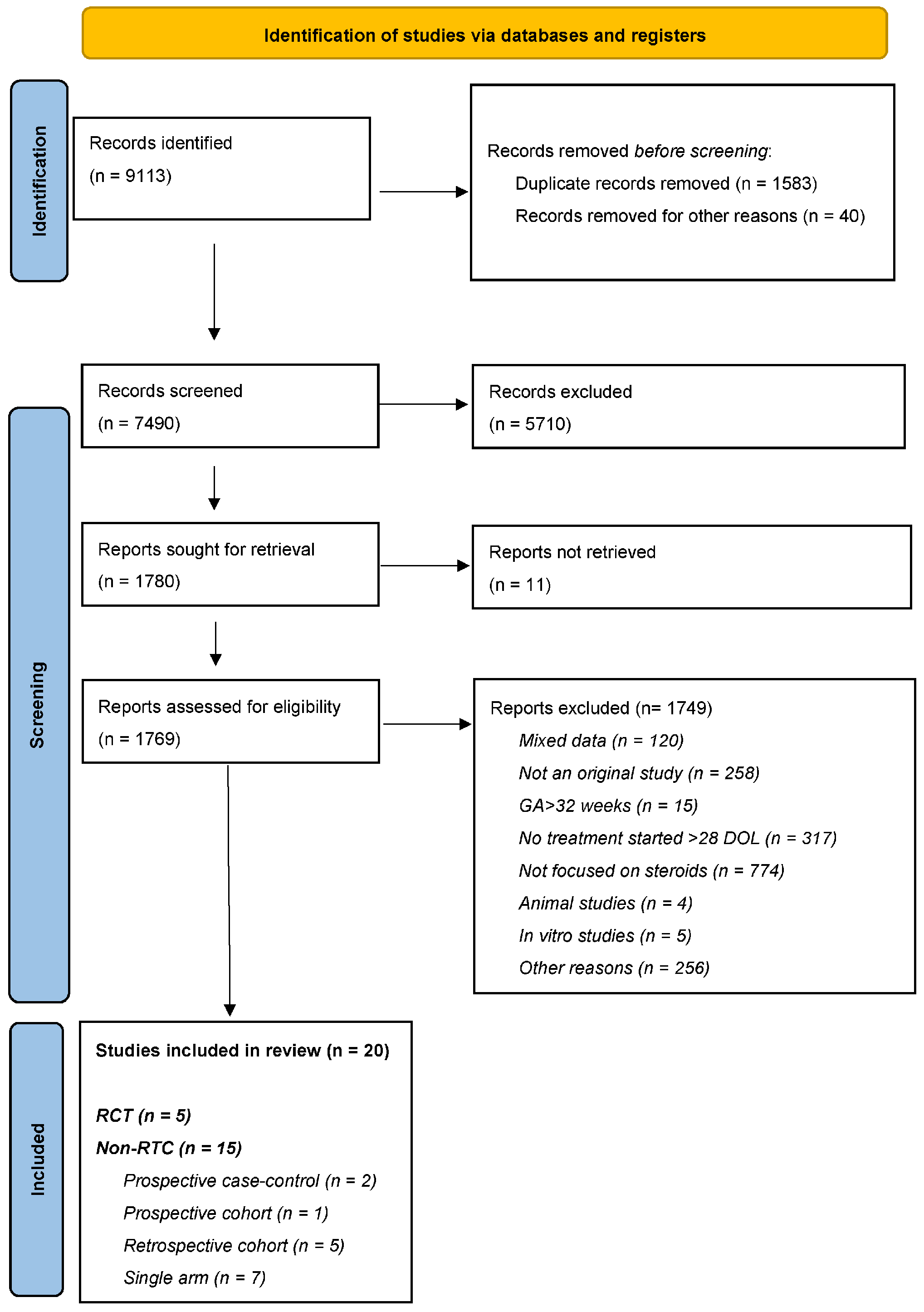
3. Results
3.1. Study Description
3.2. Efficacy and Safety of Steroids for Established BPD: Evidence from RCTs
3.2.1. Efficacy Outcomes
3.2.1.1. Steroid Treatment After 28 Days of Life Probably Improves Short-Term Respiratory Outcomes
3.2.1.2. Steroid Treatment After 28 Days of Life Probably Leads to No or Little Effect on Medium-Term Efficacy Outcomes
3.2.1.3. The Evidence Is Not Enough to Conclude on Long-Term Efficacy Outcomes Following Steroid Commencement After 28 Days of Life
3.2.2. Safety Outcomes
3.2.2.1. Steroid Treatment After 28 Days of Life Probably Increases Blood Pressure
3.2.2.2. Systemic Steroid Treatment After 28 Days of Life Probably Decreases Weight Gain During Treatment
3.2.2.3. Steroid Treatment After 28 Days of Life Probably Has Little or No Effect on Overall Weight Gain During Treatment
3.2.2.4. Quality of Evidence Is Too Low to Make Any Assumption on the Effects of Steroid Treatment After 28 Days of Life with Regards to Adrenal Axis Balance or Hyperglycemia
3.2.2.5. Steroid Treatment After 28 Days of Life May Lead to Little or No Effect on Infection Risk
3.3. Efficacy and Safety of Steroids for Established BPD: Additional Information from Non-RCTs
3.4. Efficacy and Safety of Steroids for Established BPD: Single-Arm Studies
3.5. Factors Associated with the Success and Safety of Steroid Treatment After 28 Days of Life
3.6. Repeat Course of Steroids
3.7. Treatment Timing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeon, G.W.; Oh, M.; Lee, J.; Jun, Y.H.; Chang, Y.S. Comparison of definitions of bronchopulmonary dysplasia to reflect the long term outcomes of extremely preterm infants. Sci. Rep. 2022, 12, 18095. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tarazona, S.; Gomis, G.M.; López, M.P.; Jiménez, C.L.; Pérez-Lara, L. Definitions of Bronchopulmonary Dysplasia: Which One Should We Use? J. Pediatr. 2022, 251, 67. [Google Scholar] [CrossRef] [PubMed]
- Okulu, E.; Kraja, E.; Kostekci, Y.Z.; Aloyeva, R.; Erdeve, O.; Atasay, B.; Arsan, S. Comparison of Definitions for Bronchopulmonary Dysplasia: A Cohort Study. Z. Geburtshilfe Neonatol. 2023, 227, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Balany, J.; Bhandari, V. Understanding the Impact of Infection, Inflammation, and Their Persistence in the Pathogenesis of Bronchopulmonary Dysplasia. Front. Med. 2015, 21, 90. [Google Scholar] [CrossRef]
- Kalikkot Thekkeveedu, R.; Guaman, M.C.; Shivanna, B. Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respir. Med. 2017, 132, 170–177. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef]
- Pierro, M.; Van Mechelen, K.; van Westering-Kroon, E.; Villamor-Martínez, E.; Villamor, E. Endotypes of Prematurity and Phenotypes of Bronchopulmonary Dysplasia: Toward Personalized Neonatology. J. Pers. Med. 2022, 12, 687. [Google Scholar] [CrossRef]
- Abiramalatha, T.; Ramaswamy, V.V.; Bandyopadhyay, T.; Somanath, S.H.; Shaik, N.B.; Pullattayil, A.K.; Weiner, G.M. Interventions to Prevent Bronchopulmonary Dysplasia in Preterm Neonates: An Umbrella Review of Systematic Reviews and Meta-analyses. JAMA Pediatr. 2022, 176, 502–516. [Google Scholar] [CrossRef]
- Doyle, L.W.; Cheong, J.L.; Hay, S.; Manley, B.J.; Halliday, H.L. Late (≥7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2021, 11, CD001145. [Google Scholar] [CrossRef]
- Doyle, L.W.; Cheong, J.L.; Hay, S.; Manley, B.J.; Halliday, H.L. Early (<7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2021, 10, CD001146. [Google Scholar]
- Strashun, S.; Seliga-Siwecka, J.; Chioma, R.; Zielińska, K.; Włodarczyk, K.; Villamor, E.; Philip, R.K.; Al Assaf, N.; Pierro, M. Steroid use for established bronchopulmonary dysplasia: Study protocol for a systematic review and meta-analysis. BMJ Open 2022, 12, e059553. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Patsopoulos, N.A.; Evangelou, E.; Ioannidis, J.P. Sensitivity of between-study heterogeneity in meta-analysis: Proposed metrics and empirical evaluation. Int. J. Epidemiol. 2008, 37, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- GRADE Handbook. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 3 March 2025).
- Harkavy, K.L.; Scanlon, J.W.; Chowdhry, P.K.; Grylack, L.J. Dexamethasone therapy for chronic lung disease in ventilator- and oxygen-dependent infants: A controlled trial. J. Pediatr. 1989, 115, 979–983. [Google Scholar] [CrossRef]
- Noble-Jamieson, C.M.; Regev, R.; Silverman, M. Dexamethasone in neonatal chronic lung disease: Pulmonary effects and intracranial complications. Eur. J. Pediatr. 1989, 148, 356–367. [Google Scholar] [CrossRef]
- Beresford, M.W.; Primhak, R.; Subhedar, N.V.; Shaw, N.J. Randomised double blind placebo controlled trial of inhaled fluticasone propionate in infants with chronic lung disease. Arch. Dis. Child. Fetal Neonatal. Ed. 2002, 87, F62–F63. [Google Scholar] [CrossRef]
- Dugas, M.A.; Nguyen, D.; Frenette, L.; Lachance, C.; St-Onge, O.; Fougères, A.; Bélanger, S.; Caouette, G.; Proulx, E.; Racine, M.-C.; et al. Fluticasone inhalation in moderate cases of bronchopulmonary dysplasia. Pediatrics 2005, 115, e566–e572. [Google Scholar] [CrossRef]
- Kugelman, A.; Peniakov, M.; Zangen, S.; Shiff, Y.; Riskin, A.; Iofe, A.; Shoris, I.; Bader, D.; Arnon, S. Inhaled hydrofluoalkane-beclomethasone dipropionate in bronchopulmonary dysplasia. A double-blind, randomized, controlled pilot study. J. Perinatol. 2017, 37, 197–202. [Google Scholar] [CrossRef]
- Bhandari, A.; Schramm, C.M.; Kimble, C.; Pappagallo, M.; Hussain, N. Effect of a short course of prednisolone in infants with oxygen-dependent bronchopulmonary dysplasia. Pediatrics 2008, 121, e344–e349. [Google Scholar] [CrossRef]
- Bauer, J.; Teufel, U.; Maser-Gluth, C.; Doege, C. Effects of budesonide inhalation on energy expenditure, somatic growth and salivary cortisol levels in preterm infants with chronic lung disease. Horm. Res. 2009, 72, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Cuna, A.; Quiqley, A.; Varghese, K.; Ciccolari-Micaldi, G.; Oliveros, C.; Cheng, A.-L.; Norberg, M.; Truog, W.E. Effectiveness and safety of repeat dexamethasone for bronchopulmonary dysplasia. J. Perinatol. 2021, 41, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Cuna, A.; Lewis, T.; Dai, H.; Nyp, M.; Truog, W.E. Timing of postnatal corticosteroid treatment for bronchopulmonary dysplasia and its effect on outcomes. Pediatr. Pulmonol. 2019, 52, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Harmon, H.M.; Jensen, E.A.; Tan, S.; Chaudhary, A.S.; Slaughter, J.L.; Bell, E.F.; Wyckoff, M.H.; Hensman, A.M.; Sokol, G.M.; DeMauro, S.B.; et al. Timing of postnatal steroids for bronchopulmonary dysplasia: Association with pulmonary and neurodevelopmental outcomes. J. Perinatol. 2020, 40, 616–627. [Google Scholar] [CrossRef]
- Kwok, T.C.; Szatkowski, L.; Sharkey, D. Impact of postnatal dexamethasone timing on preterm mortality and bronchopulmonary dysplasia: A propensity score analysis. Eur. Respir. J. 2023, 62, 2300825. [Google Scholar] [CrossRef]
- Parikh, N.A.; Lasky, R.E.; Kennedy, K.A.; Moya, F.R.; Hochhauser, L.; Romo, S.; Tyson, J.E. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics 2007, 119, 265–272. [Google Scholar] [CrossRef]
- Tiong, N.P.; Peng, C.-C.; Ko, M.H.-J.; Tseng, K.-T.; Chang, J.-H.; Hsu, C.-H.; Sung, Y.-H.; Chang, H.-Y. Impact of inhaled corticosteroids on the neurodevelopmental outcomes in chronically ventilated extremely low birth weight preterm infants. J. Formos. Med. Assoc. 2021, 120, 275–280. [Google Scholar] [CrossRef]
- Linafelter, A.; Cuna, A.; Liu, C.; Quigley, A.; Truog, W.E.; Sampath, V.; Oschman, A. Extended course of prednisolone in infants with severe bronchopulmonary dysplasia. Early Hum. Dev. 2019, 136, 1–6. [Google Scholar] [CrossRef]
- Liviskie, C.; Vesoulis, Z.; Zeller, B.; Rao, R.; McPherson, C. Respiratory effects of prolonged prednisolone use in infants with evolving and established Bronchopulmonary dysplasia. Early Hum. Dev. 2021, 156, 105344. [Google Scholar] [CrossRef]
- Dassios, T.; Kaltsogianni, O.; Greenough, A. Second course of systemic dexamethasone: Efficacy and respiratory function changes. J. Matern. Fetal Neonatal Med. 2022, 35, 1401–1404. [Google Scholar] [CrossRef] [PubMed]
- Tanney, K.; Davis, J.; Halliday, H.L.; Sweet, D.G. Extremely low-dose dexamethasone to facilitate extubation in mechanically ventilated preterm babies. Neonatology 2011, 100, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Tian, Y.; Cheng, H.; Zheng, Y.; Wang, W.; Bao, T.; Wu, R.; Tian, Z. A clinical study on plasma biomarkers for deciding the use of adjuvant corticosteroid therapy in bronchopulmonary dysplasia of premature infants. Int. J. Med. Sci. 2021, 18, 2581–2588. [Google Scholar] [CrossRef]
- Brundage, K.L.; Mohsini, K.G.; Froese, A.B.; Walker, C.R.; Fisher, J.T. Dexamethasone therapy for bronchopulmonary dysplasia: Improved respiratory mechanics without adrenal suppression. Pediatr. Pulmonol. 1992, 12, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, M.; Manabe, C.; Yonetani, M.; Nakao, H.; Uetani, Y. Effect of dexamethasone therapy on pulmonary function in chronic lung disease: A comparison of disease types. Pediatr. Int. 2001, 43, 226–230. [Google Scholar] [CrossRef]
- Yao, S.; Uthaya, S.; Gale, C.; Modi, N.; Battersby, C. Postnatal corticosteroid use for prevention or treatment of bronchopulmonary dysplasia in England and Wales 2012-2019: A retrospective population cohort study. BMJ Open 2022, 12, e063835. [Google Scholar] [CrossRef]
- Santesso, N.; Glenton, C.; Dahm, P.; Garner, P.; Akl, E.A.; Alper, B.; Brignardello-Petersen, R.; Carrasco-Labra, A.; De Beer, H.; Hultcrantz, M.; et al. GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions. J. Clin. Epidemiol. 2020, 119, 126–135. [Google Scholar] [CrossRef]
- Verder, H.; Heiring, C.; Ramanathan, R.; Scoutaris, N.; Verder, P.; Jessen, T.E.; Höskuldsson, A.; Bender, L.; Dahl, M.; Eschen, C.; et al. Bronchopulmonary dysplasia predicted at birth by artificial intelligence. Acta Paediatr. 2021, 110, 503–509. [Google Scholar] [CrossRef]
- Bruno, G.; Chioma, R.; Storti, E.; De Luca, G.; Fantinato, M.; Antonazzo, P.; Pierro, M. Targeted management of evolving and established chronic lung disease of prematurity assisted by cardiopulmonary ultrasound: A case report of four patients. Front. Pediatr. 2023, 10, 1112313. [Google Scholar] [CrossRef]
- Guillot, M.; Guo, T.; Ufkes, S.; Schneider, J.; Synnes, A.; Chau, V.; Grunau, R.E.; Miller, S.P. Mechanical Ventilation Duration, Brainstem Development, and Neurodevelopment in Children Born Preterm: A Prospective Cohort Study. J. Pediatr. 2020, 226, 87–95. [Google Scholar] [CrossRef]
| Study (First Author and Publication Year) | Country | Number of Sites | Study Design | Steroid Regimen | Inclusion Criteria | Number of Patients * (Study/Control) | ||
|---|---|---|---|---|---|---|---|---|
| Drug/Control | Cumulative Dose | Route | ||||||
| Noble-Jamieson, 1989 [18] | UK | Single | Double-blind RCT | Dexamethasone vs. placebo | 5.95 mg/kg, over 21 days | Oral or IV | FiO2 > 0.30 after 4 weeks of age | 18 (9/9) |
| Harkavy, 1989 [17] | USA | Single | Double-blind RCT | Dexamethasone vs. placebo | 8 mg/kg, over at least 2 weeks | Oral or IV | Ventilator and oxygen dependency at 30 days of age | 21 (9/12) |
| Beresford, 2002 [19] | UK | Single | Double-blind RCT | Fluticasone propionate vs. placebo | 182.5 mg, over 1 year | Inhalation | Supplementary oxygen at 36 PMA | 30 (15/15) |
| Dugas, 2005 [20] | Canada | Multi-center (2 centers) | Double-blind RCT | Fluticasone propionate vs. placebo | 6.125 mg over 21 days; dose was doubled for infants >1200 g | Inhalation | 28–60 days of life at treatment, FiO2 ≥ 0.25, pCO2 ≥ 45 mm Hg, chest radiograph compatible with BPD | 32 (16/16) |
| Kugelman, 2017 [21] | Israel | Multi-center (5 centers) | Double-blind RCT | Beclomethasone vs. placebo | 18 mg over 3 months | Inhalation | GA < 32 weeks, FiO2 ≥ 0.30 and/or positive pressure support with any FiO2 at 36 weeks PMA | 38 (18/20) |
| Bhandari, 2008 [22] | USA | Single | Prospective cohort study | Prednisolone vs. no treatment | 16 mg/kg over 14 days | Oral | Oxygen dependence at 36 weeks PMA | 385 (131/254) |
| Bauer, 2009 [23] | Germany | Single | Prospective cohort study | Budesonide vs. no treatment | 2.8 mg/kg, over 4 weeks | Inhalation | GA 25–31 weeks, supplemental oxygen in the first 28 days of life, signs of BPD | 30 (10/20) |
| Cuna, 2018 [25] | USA | Single | Retrospective cohort study | Dexamethasone (late/early treatment) | 0.72–0.89 mg/kg, over 7–10 days | IV | Preterm infants treated with postnatal steroids for BPD | 55 (30/25) |
| Cuna, 2021 [24] | USA | Single | Retrospective cohort study | Dexamethasone (1 course vs. 2 courses) | 0.72–0.89 mg/kg, over 7–10 days | IV | GA < 30 weeks received treatment with 1 or 2 courses of steroids for BPD | 132 (52/80) |
| Harmon, 2020 [26] | USA | Single | Retrospective cohort study | Dexamethasone/hydrocortisone (late vs. early treatment) | Not specified | Not specified | GA < 27 weeks, received steroids between 8 days of life and <36 weeks PMA | 951 (531/420) |
| Kwok, 2023 [27] | UK | Multi-center (185 centers) | Retrospective cohort study | Dexamethasone (late vs. early treatment) | 400 mcg/die for 70.8 ± 44.2 days | Not specified | GA < 32 weeks and received 2 courses of steroids | 1734 (636/10980) |
| Parikh, 2006 [28] | USA | Single | Retrospective case–control study | Dexamethasone vs. no treatment | 2.8 mg/kg, over 7 days | Oral or IV | ELBW, receiving steroids after 4 weeks of life | 41 (11/30) |
| Tiong, 2020 [29] | Taiwan | Single | Retrospective cohort study | Budesonide vs. no treatment | Not specified | Inhalation | ELBW preterm infants, ventilator dependence > 28 days with FiO2 > 0.60/PIP > 14 cmH2O | 115 (64/51) |
| Brundage, 1992 [35] | Canada | Single | Pre–post study | Dexamethasone | 3.5 mg/kg over 7 days | IV | BW < 1250 g with BPD, ventilator dependency at 3 weeks of age and failure to wean | 7 |
| Dassios, 2019 [32] | UK | Single | Retrospective cohort | Dexamethasone | 2.7 mg/kg over 9 days | IV | GA < 30 weeks, ventilator dependency and FiO2 > 0.60 | 15 |
| Linafelter, 2019 [30] | USA | Single | Retrospective cohort | Prednisolone or metylprednisolone | 64.8 ± 41 mg/kg over 77 ± 38.3 days | Oral or IV | Infants dependent on CPAP or mechanical ventilation past 36 weeks PMA who received ≥30 days of therapy | 43 |
| Liviskie, 2021 [31] | USA | Single | Retrospective cohort | Prednisolone or metylprednisolone | Not specified | Oral or IV | Infants who received ≥30 days of therapy | 40 |
| Mizobuchi, 2001 [36] | Japan | Multi-center (2 centers) | Pre–post study | Dexamethasone | 2.3 mg/kg over 7 days | Not specified | Infants dependent on ventilator for 28 days or longer | 22 |
| Tanney, 2011 [33] | UK | Single | Retrospective cohort | Dexamethasone | 0.24 mg/kg over 9 days | Not specified | GA 23–26 weeks, deemed ventilator-dependent | 9 * |
| Zhu H, 2021 [34] | China | Single | Prospective cohort | Dexamethasone | 0.89 mg/kg over 10 days | IV | GA < 32, PMA > 36 w, and oxygen dependency > 28 days | 30 |
| Study (First Author and Publication Year) | GA (Weeks) | BW (Grams) | PMA at Treatment (Weeks) | Days at Treatment | ||||
|---|---|---|---|---|---|---|---|---|
| Study Group | Control Group | Study Group | Control Group | Study Group | Control Group | Study Group | Control Group | |
| Randomized controlled trials | ||||||||
| Noble Jamieson, 1989 [18] | 28.0 ± 1.8 | 27.8 ± 2 | 1090 ± 199 | 1066 ± 196 | 33 ± 1.5 * | 33 ± 1.7 * | 36 ± 8 | 38 ± 10 |
| Harkavy 1989 [17] | 26.1 ± 2.0 | 25.9 ± 1.0 | 857 ± 183 | 772 ± 81 | 31 ± 2.6 * | 30.7 ± 1.5 * | 34.3 ± 2.8 | 34.1 ± 1.8 |
| Beresford, 2002 [19] | 26.8 ± 0.9 | 28.2 ± 0.9 | 885 ± 87 | 1123 ± 175 | 39.8 ± 1.4 | 39.8 ± 1.4 | 91 ± 9.9 * | 81.2 ± 9 * |
| Dugas, 2005 [20] | 27 ± 2.3 | 27.2 ± 1.7 | 995 ± 439 | 926 ± 251 | 33.4 ± 1.6 * | 33.7 ± 1.4 * | 44.8 ± 11 | 45.4 ± 10 |
| Kugelman, 2017 [21] | 26.4 ± 2.1 | 26.9 ± 1.9 | 838 ± 234 | 835 ± 263 | 36.2 ± 0.3 | 36.2 ± 0.4 | 68.6 ± 11 * | 65.1 ± 10 * |
| Non-randomized cohort studies | ||||||||
| Bhandari, 2008 [22] | 26.9 ± 2.2 | 27.2 ± 2.2 | 978 ± 342 | 1005 ± 357 | 38 ± 3.8 | NA | 77 ± 20 * | NA |
| Bauer, 2009 [23] | 28.0 ± 1.9 | 27.0 ± 1.8 | 887 ± 210 | 841 ± 144 | 32.3 ± 7.2 * | NA | 30 ± 1.6 | NA |
| Parikh, 2006 [28] | 25.1 ± 1.0 | 26.2 ± 1.6 | 740 ± 118 | 808 ± 146 | NR | NR | NR | NR |
| Tiong, 2020 [29] | 25.3 ± 1.2 | 25.3 ± 1.2 | 748 ± 133 | 771 ± 117 | NR | NR | NR | NR |
| Single-arm studies | ||||||||
| Brundage, 1992 [35] | 26.9 ± 0.7 | NA | 971 ± 86 | NA | 31.6 ± 0.6 * | NA | 32.9 ± 4.8 | NA |
| Dassios, 2019 [32] | 25.7 ± 0.7 | NA | 795 ± 74.4 | NA | 34.5 ± 1.2 | NA | 61.6 ± 7.1 | NA |
| Linafelter, 2019 [30] | 26.1 ± 2.3 | NA | 729 ± 274 | NA | 43.3 ± 7.8 | NA | 123.2 ± 49 | NA |
| Liviskie, 2021 [31] | 26.5 ± 2.5 | NA | 846 ± 353 | NA | 41.7 ± 5 | NA | 107 ± 35 | NA |
| Mizobuchi, 2001 [36] | 25.8 ± 1.3 | NA | 750 ± 149 | NA | 32.1 ± 1.3 | NA | 45 ± 11 | NA |
| Tanney, 2011 ** [33] | 25.6 ± 1.4 | NA | 690 ± 127 | NA | 30 ± 1.2 | NA | 39 ± 9 | NA |
| Zhu [34] | 31.2 ± 1.4 | NA | 1340 ± 250 | NA | NR | NA | NR | NA |
| Steroid treatment timing: late (after 28 days of life) vs. early (before 28) | ||||||||
| Study (First Author, Publication Year) | GA (Weeks) | BW (Grams) | PMA at Treatment (Weeks) | Days at Treatment | ||||
| Early Steroid | Late Steroid | Early Steroid | Late Steroid | Early Steroid | Late Steroid | Early Steroid | Late Steroid | |
| Cuna, 2018 [25] | 24.9 ± 1.4 | 25.2 ± 1.2 | 729 ± 190 | 751 ± 135 | 28.2 ± 1.5 | 30.2 ± 1.3 | 23 ± 4 | 35 ± 4 |
| Harmon, 2020 [26] | 24.9 ± 1.0 | 24.9 ± 1.0 | 669 ± 132 | 687 ± 136 | 27.7 ± 1.3 | 31.4 ±2.0 | 21 ± 2 | 44 ± 3 |
| Kwok, 2023 [27] | 25.5 ± 0.7 | 24.3 ± 0.6 | 718.7 ± 38.7 | 676.8 ± 30.4 | NR | NR | NR | NR |
| Steroid treatment: repeated dose | ||||||||
| Study (First Author, Publication Year) | GA (Weeks) | BW (Grams) | PMA at Treatment (Weeks) | Days at Treatment | ||||
| One Course | Repeat Course | One Course | Repeat Course | One Course | Repeat Course | One Course | Repeat Course | |
| Cuna 2021 [24] | 25.1 ± 1.3 | 25.4 ± 2.0 | 737.6 ± 186.2 | 710.3 ± 198.0 | 30.5 ± 3 | 35.7 ± 4 | 36.4 ± 18 | 72.4 ± 25 |
| Outcomes | Studies | Statistics | Quality of Evidence | |||
|---|---|---|---|---|---|---|
| Number | RR/OR or MD (95% CI) | I2 | P (RR, OR, MD) | ROB2/ROBIN | Certainty (GRADE) | |
| Daily fall in FiO2 over 10 days # (MD in percentage per day) | 2 RCTs [17,18] | 1.6 (0.25 to 2.95) | 0 | <0.001 |  Some concerns | ⊕⊕⊕⊖ Moderate |
| Oxygen dependency (MD in days) | 5 RCTs [17,18,19,20,21] | 6.4 (−12 to 25.1) | 40 | 0.51 |  Some concerns | ⊕⊕⊕⊖ Moderate |
| Systemic | 2 RCTs [17,18] | −20.8 (−70 to 28.5) | 0 | 0.41 |  Low | ⊕⊕⊕⊖ Moderate |
| Inhaled | 3 RCTs [19,20,21] | 11.3 (−9.3 to 32) | 49 | 0.14 |  Some concerns | ⊕⊕⊕⊖ Moderate |
| Length of stay (MD in days) | 3 RCTs [17,20,21] | 8.2 (−8.5 to 25) | 0 | 0.8 |  Low | ⊕⊕⊕⊖ Moderate |
| Systemic | 1 RCT [17] | −8 (−99.5 to 83.5) | NA | 0.82 |  Low | Insufficient |
| Inhaled | 2 RCTs [20,21] | 8.75 (−8.3 to 25.8) | 35 | 0.7 |  Low | ⊕⊕⊕⊖ Moderate |
| Mortality, RR | 4 RCTs [17,18,20,21] | 0.9 (0.24 to 3.6) | 0 | 0.3 |  Some concerns | ⊕⊕⊕⊖ Moderate |
| Systemic | 2 RCT [17,18] | 0.82 (0.15 to 4.5) | 0 | 0.5 |  Some concerns | ⊕⊕⊕⊖ Moderate |
| Inhaled | 2 RCT [20,21] | 1.16 (0.12 to 10.6) | 0 | 0.4 |  Some concerns | ⊕⊕⊕⊖ Moderate |
| Weight gain during treatment (MD in grams/day) # | 2 RCTs [17,18] | −9.2 (−11.7 to −5.6) | 0 | <0.001 |  Low | ⊕⊕⊕⊖ Moderate |
| Overall weight gain (MD in grams/day) | 3 RCTs [17,20,21] | 2.4 (−0.3 to 6.3) | 0 | 0.078 |  Low | ⊕⊕⊕⊖ Moderate |
| Systemic | 1 RCTs [17] | 3 (−0.4 to 6.4) | 0 | 0.081 |  Low | Insufficient |
| Inhaled | 2 RCTs [20,21] | 1.4 (−3.4 to 6.3) | 0 | 0.56 |  Low | ⊕⊕⊕⊖ Moderate |
| Systolic blood pressure (MD in mmHg) * | 2 RCTs [20,21] | 6.8 (4.63 to 8.9) | 0 | <0.001 |  Low | ⊕⊕⊕⊖ Moderate |
| Adrenal insufficiency (MD in cortisol levels) * | 2 RCTs [20,21] | 2.3 (−43 to 47.6) | 88 | 0.92 |  Low | ⊕⊖⊖⊖ Very low |
| Hyperglycemia, RR | 3 RCTs [17,18,20] | 2.8 (0.5 to 18) | 60 | 0.26 |  Low | ⊕⊖⊖⊖ Very low |
| Systemic | 2 RCT [17,18] | 4 (0.48 to 37) | 48 | 0.2 |  Low | ⊕⊖⊖⊖ Very low |
| Inhaled | 1 RCT [20] | 1.1 (0.04 to 35) | NA | 0.92 |  Low | Insufficient |
| Infections, RR # | 2 RCTs [17,18] | 0.7 (0.26 to 1.92) | 0 | 0.35 |  Low | ⊕⊕⊕⊖ Moderate |
| Total brain volume # (MD in percentage) | 1 case control [28] | −10 (−0.1 to −19) | NA | 0.03 |  High | Insufficient |
| Cerebral palsy, OR *~ | 1 cohort study [29] | 156 (0.89–27399) | NA | 0.6 |  Some concerns | Insufficient |
| NDI, OR *~ | 1 cohort study [29] | 1.70 (0.55 to 5.27) | NA | 0.36 |  Some concerns | Insufficient |
| Reference | Criteria for Steroid Success | Percentage | Success/Total Number |
|---|---|---|---|
| Bhandari, 2008 [22] | Wean off oxygen | 62 | 82/131 |
| Cuna, 2021 [24] | Successful extubation | 37 | 12/34 |
| Dassios, 2019 [32] | Successful extubation | 93 | 14/15 |
| Harkavy, 1989 [17] | Successful extubation | 100 | 9/9 |
| Kwok, 2023 [27] | Successful extubation within 14 days of starting steroids | 78.6 | 591/843 |
| Linafelter, 2019 [30] | Wean off oxygen | 63 | 82/131 |
| Mizobuchi, 2001 [36] | Successful extubation | 100 | 22/22 |
| Tanney, 2011 [33] | Successful extubation | 88.8 | 8/9 |
| Zhu, 2021 [34] | Wean off oxygen/self-ventilating in room air | 33 | 10/30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierro, M.; Chioma, R.; Włodarczyk, K.; Benke, M.; Mangroo, K.; Vetrano, M.C.; Zielińska, K.; O’Keeffe, D.; Seliga-Siwecka, J.; Purtill, H.; et al. Steroid Use for Established Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Children 2025, 12, 1238. https://doi.org/10.3390/children12091238
Pierro M, Chioma R, Włodarczyk K, Benke M, Mangroo K, Vetrano MC, Zielińska K, O’Keeffe D, Seliga-Siwecka J, Purtill H, et al. Steroid Use for Established Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Children. 2025; 12(9):1238. https://doi.org/10.3390/children12091238
Chicago/Turabian StylePierro, Maria, Roberto Chioma, Krzysztof Włodarczyk, Margit Benke, Kaushik Mangroo, Maria Chiara Vetrano, Kinga Zielińska, David O’Keeffe, Joanna Seliga-Siwecka, Helen Purtill, and et al. 2025. "Steroid Use for Established Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis" Children 12, no. 9: 1238. https://doi.org/10.3390/children12091238
APA StylePierro, M., Chioma, R., Włodarczyk, K., Benke, M., Mangroo, K., Vetrano, M. C., Zielińska, K., O’Keeffe, D., Seliga-Siwecka, J., Purtill, H., Al-Assaf, N., Villamor, E., & Philip, R. K. (2025). Steroid Use for Established Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Children, 12(9), 1238. https://doi.org/10.3390/children12091238







