ACT-ON-DIP: Study Protocol of a Randomized Controlled Trial of a Home-Based ACTion Observation Tele-RehabilitatioN for Upper Limb in Children with DIPlegic Cerebral Palsy
Abstract
Highlights
- This is the first study applying AOT specifically to promote upper-limb skills in children and adolescents with diplegic CP. The study adopts a home-based AOT protocol, supported by remote clinical supervision, which increases accessibility for families and allows for a more intensive and flexible delivery of the intervention.
- The AOT home intervention could be tailored to the different features of children with diplegic CP, allowing video selection from a large, diversified library of actions, making it adaptable to individual needs.
- This study will contribute in building evidence on the efficacy of AOT for diplegic CP, with possible positive effects on daily-life functional tasks and indirect benefits on postural control and balance. Moreover, demonstrating its feasibility could support the wider adoption of AOT in other neurodevelopmental or motor conditions.
- Through a combination of motor and neuropsychological assessments, the project aims to provide insights into the cognitive components of manual action, such as action planning.
Abstract
1. Introduction
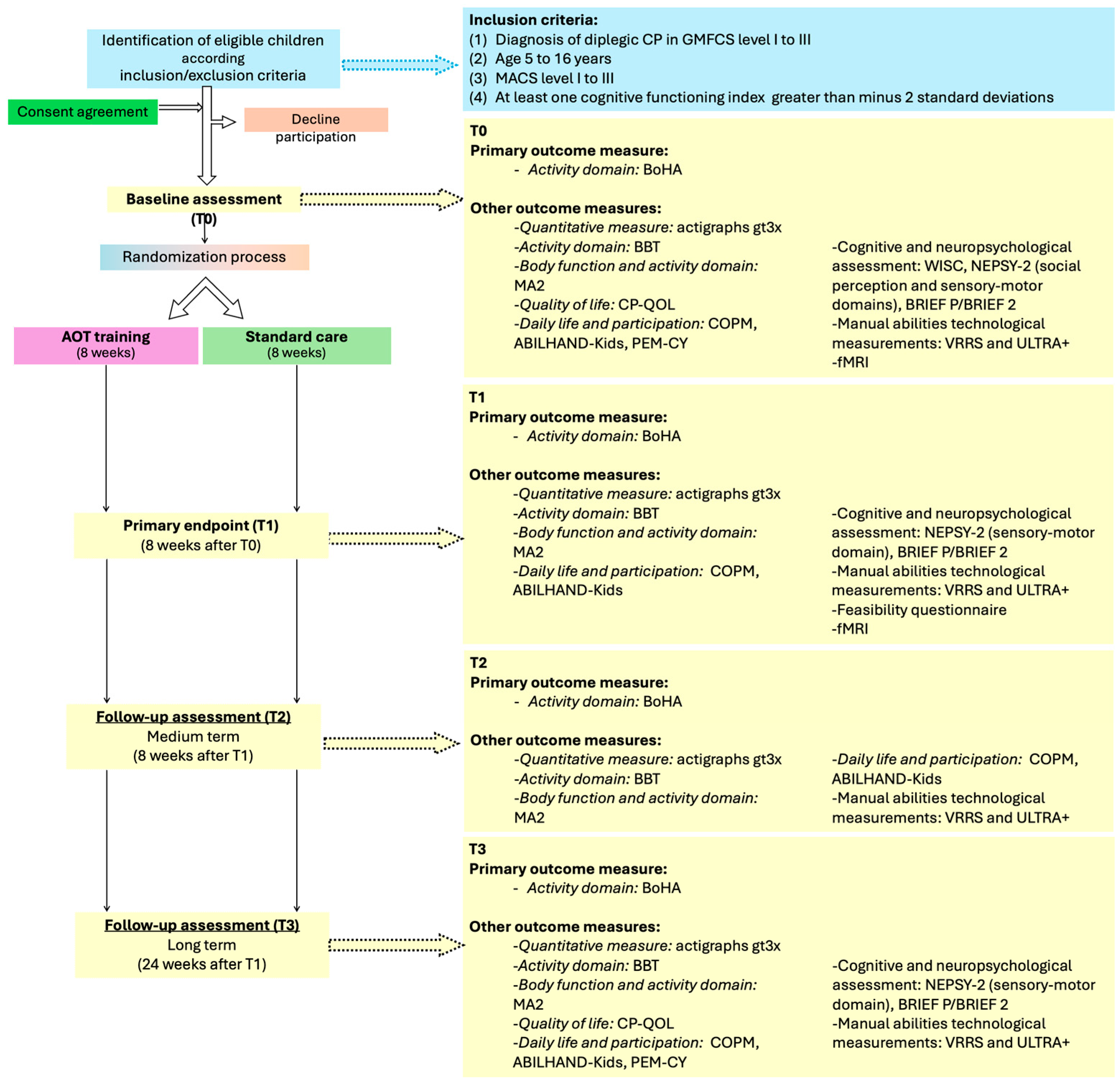
2. Materials and Methods
2.1. Study Design
2.2. Participants
- Confirmed diagnosis of spastic diplegic Cerebral Palsy;
- Age between 5 and 16 years at the time of recruitment;
- Manual Ability Classification System (MACS) levels I–III [39];
- Gross Motor Functional Classification System (GMFCS) levels I–III [40];
- At least one cognitive functioning index greater than −2 standard deviations, assessed with a battery of standardized tests such as WISC-IV or WISC-V, in order to ensure a sufficient level of comprehension and cooperation in the proposed activities.
- Presence of uncontrolled epilepsy;
- Injection of botulinum toxin or orthopaedic surgery in the upper limb carried out in the previous 6 months or planned during the study period.
- They begin an intensive treatment program;
- They require a botulinum toxin injection before the end of the study;
- Other adverse events occur that prevent further participation.
- Insufficient cooperation during approximately 30 min long neuroimaging studies;
- Presence of exclusions for 3T MRI investigations (as metal implants, prostheses, shunts, etc.). These exclusions will be checked using an ad hoc questionnaire.
2.3. Sample Size
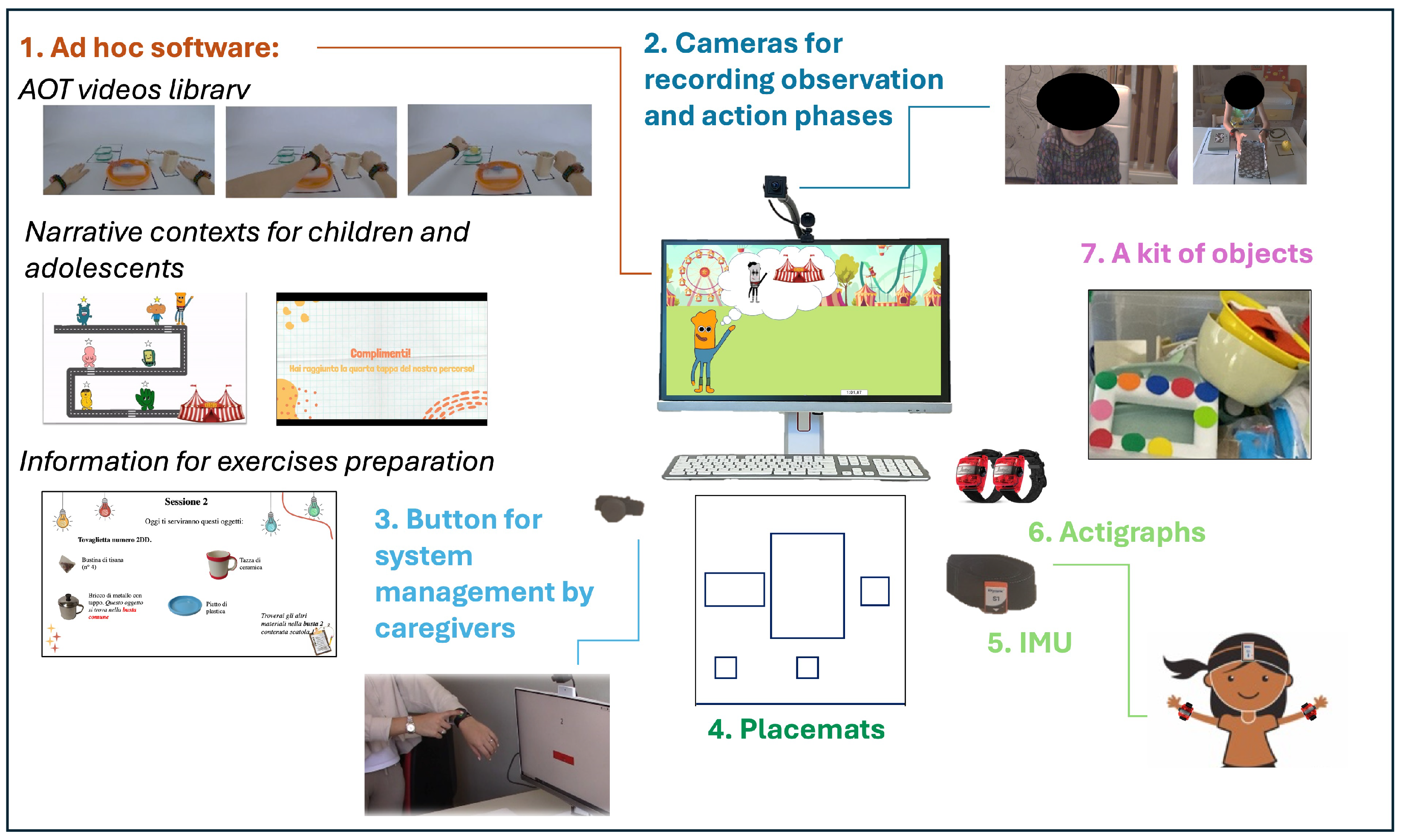
2.4. Experimental Training (AOT)
2.4.1. ACT ON DIP System
2.4.2. AOT Library
2.5. Standard Care
2.6. Outcome Measures
- Both Hands Assessment (BoHA [42]), identified as the primary outcome measure. It is an evaluation tool specifically designed to measure bimanual upper-limb performance in children with bilateral Cerebral Palsy.
- Melbourne Assessment 2 (MA2 [43]). It is a standardized tool designed to assess upper-limb movement quality in children with neurological impairments, aged between 2.5 and 15 years. It is carried out for each UL separately.
- Box and Block Test (BBT [44]). It is a quick and simple assessment tool for evaluating manual dexterity, with the two sides tested separately.
- Corsi Test [45]. This test is used to evaluate visuospatial memory and working memory. It will be administered as part of the assessment process at baseline (T0) and following training at T1 and T3.
- NEPSY II—Imitating Hand Positions subtest [46]. This subtest assesses the child’s ability to observe and replicate hand and finger positions demonstrated by the examiner, using both the dominant and non-dominant hand.
- NEPSY II—Manual Motor Sequences [46]. It involves imitating a series of rhythmic motor sequences using one hand or both.
- ABILHAND-Kids [49]. This brief questionnaire is designed to assess the reported difficulty in 21 key bimanual daily activities.
- Canadian Occupational Performance Measure (COPM [50]). This validated tool is designed to identify rehabilitation needs in daily activities and any changes in performance, as reported directly by the individual or their family members. While this test is intended for caregivers and parents, collaborative children may also participate.
- Cerebral Palsy Quality of Life Questionnaire for Children (CP QOL–Child, 4–12 years [51]) and Cerebral Palsy Quality of Life Questionnaire for Adolescents (CP QOL–Teen, 13–18 years [52]). These questionnaires are designed to assess the quality of life of children and adolescents with Cerebral Palsy. This measure will be taken at T0 and T3. In the case of children older than 9 years, the self-reported form will be administered directly to the child/adolescent in addition to the primary caregiver version.
- Participation and Environment Measure—Children and Youth (PEM-CY [53]). This is a questionnaire that evaluates the child’s participation in the main environments they engage with, such as home, school, and the community. This assessment will be conducted at T0 and T3.
- Questionnaires for training compliance. In order to investigate the feasibility of the system and the compliance of children and their families, ad hoc questionnaires will be administered at the end of the training during the T1 assessment in the experimental group to three different stakeholders: the child, the primary caregiver who assisted the training, and the clinician.
2.7. fMRI Task
2.7.1. Experimental Procedure
2.7.2. Action Observation Task
2.7.3. Motor Task
2.7.4. MRI Data Acquisition
2.7.5. Sample Size Estimation (fMRI Sample)
2.7.6. Sampling for the fMRI Subgroup
2.8. Statistical Analyses
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACT ON DIP | ACTion Observation tele-rehabilitatioN for upper limb in children with DIPlegic Cerebral Palsy |
| AOT | Action Observation Therapy |
| BBT | Box and Block Test |
| BoHA | Both Hands Assessment |
| BRIEF | Behavior Rating Inventory of Executive Function |
| Corsi Test | CORSI block tapping test |
| CG | control group |
| COPM | Canadian Occupational Performance Measure |
| CP | Cerebral Palsy |
| CP-QOL | Cerebral Palsy Quality of Life |
| EG | experimental group |
| EPI | echo-planar imaging |
| FLAIR | fluid-attenuated inversion recovery |
| fMRI | functional magnetic resonance imaging |
| FSE | fast spin-echo |
| FWE | Family-Wise Error |
| GMFCS | Gross Motor Function Classification System |
| LCD | Liquid Crystal Display |
| MA-2 | Melbourne Assessment 2 |
| MACS | Manual Ability Classification System |
| rmANOVA | Repeated Measures Analysis of Variance |
| MNS | Mirror Neuron System |
| NEPSY | NEuroPSYchological assessment |
| PEM-CY | Participation and Environment Measure for Children and Youth |
| PIQ | Performance Intelligence Quotient |
| RCT | randomized controlled trial |
| ROI | Region of Interest |
| UL | upper limb |
| ULTRA+ | Upper Limb TRAcker |
| VIQ | Verbal Intelligence Quotient |
| VRRS | Virtual Reality Rehabilitation System |
References
- McIntyre, S.; Goldsmith, S.; Webb, A.; Ehlinger, V.; Hollung, S.J.; McConnell, K.; Arnaud, C.; Smithers-Sheedy, H.; Oskoui, M.; Khandaker, G.; et al. Global prevalence of cerebral palsy: A systematic analysis. Dev. Med. Child Neurol. 2022, 64, 1494–1506. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar]
- Colver, A.; Fairhurst, C.; Pharoah, P.O.D. Cerebral palsy. Lancet 2014, 383, 1240–1249. [Google Scholar] [CrossRef]
- Cans, C. Surveillance of cerebral palsy in Europe: A collaboration of cerebral palsy surveys and registers. Dev. Med. Child Neurol. 2000, 42, 816–824. [Google Scholar] [CrossRef]
- Hagberg, G.; Hagberg, B.; Olow, I. The changing panorama of cerebral palsy in Sweden 1954-1970. III. The importance of foetal deprivation of supply. Acta Paediatr. 1976, 65, 403–408. [Google Scholar] [CrossRef]
- Balf, C.L.; Ingram, T.T.S. Problems in the Classification of Cerebral Palsy in Childhood. BMJ 1955, 2, 163–166. [Google Scholar] [CrossRef]
- Odding, E.; Roebroeck, M.E.; Stam, H.J. The epidemiology of cerebral palsy: Incidence, impairments and risk factors. Disabil. Rehabil. 2006, 28, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Cimolin, V.; Albertini, G.; Piccinini, L.; Turconi, A.C.; Romkes, J.; Brunner, R. Kinematic analysis of upper limb during walking in diplegic children with Cerebral Palsy. Eur. J. Paediatr. Neurol. 2014, 18, 134–139. [Google Scholar] [CrossRef]
- Johansson, A.-M.; Domellöf, E.; Rönnqvist, L. Timing Training in Three Children with Diplegic Cerebral Palsy: Short- and Long-Term Effects on Upper-Limb Movement Organization and Functioning. Front. Neurol. 2014, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Arner, M.; Eliasson, A.-C.; Nicklasson, S.; Sommerstein, K.; Hägglund, G. Hand Function in Cerebral Palsy. Report of 367 Children in a Population-Based Longitudinal Health Care Program. J. Hand Surg. 2008, 33, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Klevberg, G.L.; Østensjø, S.; Krumlinde-Sundholm, L.; Elkjær, S.; Jahnsen, R.B. Hand Function in a Population-Based Sample of Young Children with Unilateral or Bilateral Cerebral Palsy. Phys. Occup. Ther. Pediatr. 2017, 37, 528–540. [Google Scholar] [CrossRef]
- Plasschaert, V.F.P.; E Vriezekolk, J.; Aarts, P.B.M.; Geurts, A.C.H.; Van den Ende, C.H.M. Interventions to improve upper limb function for children with bilateral cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2019, 61, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Bleyenheuft, Y.; Ebner-Karestinos, D.; Surana, B.; Paradis, J.; Sidiropoulos, A.; Renders, A.; Friel, K.M.; Brandao, M.; Rameckers, E.; Gordon, A.M. Intensive upper- and lower-extremity training for children with bilateral cerebral palsy: A quasi-randomized trial. Dev. Med. Child Neurol. 2017, 59, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.R.P.; Mancini, M.C.; Feitosa, A.M.; Teixeira, C.M.M.F.; Guerzoni, V.P.D.; Elvrum, A.G.; Ferre, C.L.; Gordon, A.M.; Brandão, M.B. Hand–arm bimanual intensive therapy and daily functioning of children with bilateral cerebral palsy: A randomized controlled trial. Dev. Med. Child Neurol. 2020, 62, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Turconi, A.C.; Biffi, E.; Maghini, C.; Peri, E.; Servodio Iammarone, F.; Gagliardi, C. Can new technologies improve upper limb performance in grown-up diplegic children? Eur. J. Phys. Rehabil. Med. 2016, 52, 672–681. [Google Scholar]
- Ferrari, A. From movement to action: A new framework for cerebral palsy. Eur. J. Phys. Rehabil. Med. 2019, 55, 852–861. [Google Scholar] [CrossRef]
- Ego, A.; Lidzba, K.; Brovedani, P.; Belmonti, V.; Gonzalez-Monge, S.; Boudia, B.; Ritz, A.; Cans, C. Visual–perceptual impairment in children with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2015, 57, 46–51. [Google Scholar] [CrossRef]
- Ritterband-Rosenbaum, A.; Christensen, M.S.; Kliim-Due, M.; Petersen, L.Z.; Rasmussen, B.; Nielsen, J.B. Altered sense of Agency in children with spastic cerebral palsy. BMC Neurol. 2011, 11, 150. [Google Scholar] [CrossRef]
- Tieman, B.L.; Palisano, R.J.; Gracely, E.J.; Rosenbaum, P.L. Gross Motor Capability and Performance of Mobility in Children With Cerebral Palsy: A Comparison Across Home, School, and Outdoors/Community Settings. Phys. Ther. 2004, 84, 419–429. [Google Scholar] [CrossRef]
- Bumin, G.; Kayihan, H. Effectiveness of two different sensory-integration programmes for children with spastic diplegic cerebral palsy. Disabil. Rehabil. 2001, 23, 394–399. [Google Scholar] [CrossRef]
- Palisano, R.J.; Tieman, B.L.; Walter, S.D.; Bartlett, D.J.; Rosenbaum, P.L.; Msc, D.R.; E Hanna, S. Effect of environmental setting on mobility methods of children with cerebral palsy. Dev. Med. Child Neurol. 2003, 45, 113–120. [Google Scholar] [CrossRef]
- Fluss, J.; Lidzba, K. Cognitive and academic profiles in children with cerebral palsy: A narrative review. Ann. Phys. Rehabil. Med. 2020, 63, 447–456. [Google Scholar] [CrossRef]
- Ito, J.-I.; Araki, A.; Tanaka, H.; Tasaki, T.; Cho, K. Intellectual status of children with cerebral palsy after elementary education. Pediatr. Rehabil. 1997, 1, 199–206. [Google Scholar] [CrossRef]
- Di Lieto, M.C.; Brovedani, P.; Pecini, C.; Chilosi, A.M.; Belmonti, V.; Fabbro, F.; Urgesi, C.; Fiori, S.; Guzzetta, A.; Perazza, S.; et al. Spastic diplegia in preterm-born children: Executive function impairment and neuroanatomical correlates. Res. Dev. Disabil. 2017, 61, 116–126. [Google Scholar] [CrossRef]
- Pirila, S.; van der Meere, J.J.; Rantanen, K.; Jokiluoma, M.; Eriksson, K. Executive Functions in Youth With Spastic Cerebral Palsy. J. Child Neurol. 2011, 26, 817–821. [Google Scholar] [CrossRef]
- Hoare, B.; Ditchfield, M.; Thorley, M.; Wallen, M.; Bracken, J.; Harvey, A.; Elliott, C.; Novak, I.; Crichton, A. Cognition and bimanual performance in children with unilateral cerebral palsy: Protocol for a multicentre, cross-sectional study. BMC Neurol. 2018, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Fogassi, L. The mirror mechanism: Recent findings and perspectives. Philos. Trans. R. Soc. B: Biol. Sci. 2014, 369, 20130420. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.-H.; Zhu, J.-D.; Chen, C.-C.; Tai, R.-Y.; Lee, C.-Y.; Hsieh, Y.-W. Action observation therapy for improving arm function, walking ability, and daily activity performance after stroke: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 1277–1285. [Google Scholar] [CrossRef]
- Caligiore, D.; Mustile, M.; Spalletta, G.; Baldassarre, G. Action observation and motor imagery for rehabilitation in Parkinson’s disease: A systematic review and an integrative hypothesis. Neurosci. Biobehav. Rev. 2017, 72, 210–222. [Google Scholar] [CrossRef]
- Alamer, A.; Melese, H.; Adugna, B. Effectiveness of Action Observation Training on Upper Limb Motor Function in Children with Hemiplegic Cerebral Palsy: A Systematic Review of Randomized Controlled Trials. Pediatr. Heal. Med. Ther. 2020, 11, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Sgandurra, G.; Ferrari, A.; Cossu, G.; Guzzetta, A.; Fogassi, L.; Cioni, G. Randomized trial of observation and execution of upper extremity actions versus action alone in children with unilateral cerebral palsy. Neurorehabilit. Neural Repair 2013, 27, 808–815. [Google Scholar] [CrossRef]
- Molinaro, A.; Micheletti, S.; Pagani, F.; Garofalo, G.; Galli, J.; Rossi, A.; Fazzi, E.; Buccino, G. Action Observation Treatment in a tele-rehabilitation setting: A pilot study in children with cerebral palsy. Disabil. Rehabil. 2022, 44, 1107–1112. [Google Scholar] [CrossRef]
- Beani, E.; Menici, V.; Sicola, E.; Ferrari, A.; Feys, H.; Klingels, K.; Mailleux, L.; Boyd, R.; Cioni, G.; Sgandurra, G. Effectiveness of the home-based training program Tele-UPCAT (Tele-monitored UPper Limb Children Action Observation Training) in unilateral cerebral palsy: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2023, 59, 554–563. [Google Scholar] [CrossRef]
- Abdelhaleem, N.; Taher, S.; Mahmoud, M.; Hendawy, A.; Hamed, M.; Mortada, H.; Magdy, A.; El-Din, M.R.E.; Zoukiem, I.; Elshennawy, S. Effect of action observation therapy on motor function in children with cerebral palsy: A systematic review of randomized controlled trials with meta-analysis. Clin. Rehabil. 2021, 35, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Sgandurra, G.; Biagi, L.; Fogassi, L.; Sicola, E.; Ferrari, A.; Guzzetta, A.; Tosetti, M.; Cioni, G. Reorganization of the Action Observation Network and Sensory-Motor System in Children with Unilateral Cerebral Palsy: An fMRI Study. Neural Plast. 2018, 2018, 6950547. [Google Scholar] [CrossRef] [PubMed]
- Errante, A.; Beccani, L.; Verzelloni, J.; Maggi, I.; Filippi, M.; Bressi, B.; Ziccarelli, S.; Bozzetti, F.; Costi, S.; Ferrari, A.; et al. Effectiveness of action observation treatment based on pathological model in hemiplegic children: A randomized-controlled trial. Eur. J. Phys. Rehabil. Med. 2024, 60, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Biddiss, E.; Chan-Viquez, D.; Cheung, S.T.; King, G. Engaging children with cerebral palsy in interactive computer play-based motor therapies: Theoretical perspectives. Disabil. Rehabil. 2021, 43, 133–147. [Google Scholar] [CrossRef]
- Kirkpatrick, E.; Pearse, J.; James, P.; Basu, A. Effect of parent-delivered action observation therapy on upper limb function in unilateral cerebral palsy: A randomized controlled trial. Dev. Med. Child Neurol. 2016, 58, 1049–1056. [Google Scholar] [CrossRef]
- Eliasson, A.-C.; Krumlinde-Sundholm, L.; Rösblad, B.; Beckung, E.; Arner, M.; Öhrvall, A.-M.; Rosenbaum, P. The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Dev. Med. Child Neurol. 2006, 48, 549–554. [Google Scholar] [CrossRef]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef]
- Jackman, M.; Lannin, N.; Galea, C.; Sakzewski, L.; Miller, L.; Novak, I. What is the threshold dose of upper limb training for children with cerebral palsy to improve function? A systematic review. Aust. Occup. Ther. J. 2020, 67, 269–280. [Google Scholar] [CrossRef]
- Elvrum, A.-K.G.; Zethræus, B.-M.; Vik, T.; Krumlinde-Sundholm, L. Development and Validation of the Both Hands Assessment for Children with Bilateral Cerebral Palsy. Phys. Occup. Ther. Pediatr. 2018, 38, 113–126. [Google Scholar] [CrossRef]
- Randall, M.; Imms, C.; Carey, L.M.; Pallant, J.F. Rasch analysis of The Melbourne Assessment of Unilateral Upper Limb Function. Dev. Med. Child Neurol. 2014, 56, 665–672. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Federman, S.; Wiemer, D. Box and Block Test of Manual Dexterity: Norms for 6–19 Year Olds. Can. J. Occup. Ther. 1985, 52, 241–245. [Google Scholar] [CrossRef]
- Mammarella, I.; Stefani, F.; Giofrè, D.; Tosco, C. BVS-Corsi-2: Batteria per la Valutazione Della Memoria Di Lavoro, 2nd ed.; Erickson: Trento, Italy, 2008; Available online: https://www.erickson.it/it/test-bvscorsi2 (accessed on 30 April 2025).
- Korkman, M.; Kirk, U.; Kemp, S. NEPSY—II, 2nd ed.; Pearson PsychCorp: San Antonio, TX, USA, 2007. [Google Scholar]
- Gioia, G.; Marano, A. BRIEF 2: Behavior Rating Inventory of Executive Function, 2nd ed.; Hogrefe: Göttingen, Germany, 2016. [Google Scholar]
- Gioia, G.; Espy, K.; Isquith, P.; Marano, A. BRIEF-P: Behavior Rating Inventory of Executive Function-Preschool Version; Hogrefe: Göttingen, Germany, 2018. [Google Scholar]
- Tofani, M.; Blasetti, G.; Lucibello, L.; Berardi, A.; Galeoto, G.; Sabbadini, M.; Santecchia, L.; Castelli, E. An Italian Validation of ABILHAND-Kids for Children With Cerebral Palsy. Percept. Mot. Ski. 2021, 128, 2605–2620. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Baptiste, S.; McColl, M.; Opzoomer, A.; Polatajko, H.; Pollock, N. The Canadian occupational performance measure: An outcome measure for occupational therapy. Can. J. Occup. Ther. 1990, 57, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.; Davis, E.; Mackinnon, A.; Boyd, R.; Graham, H.K.; Lo, S.K.; Wolfe, R.; Stevenson, R.; Bjornson, K.; Blair, E.; et al. Psychometric properties of the quality of life questionnaire for children with CP. Dev. Med. Child Neurol. 2007, 49, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.; Mackinnon, A.; Davern, M.; Boyd, R.; Bohanna, I.; Waters, E.; Graham, H.K.; Reid, S.; Reddihough, D. Description and psychometric properties of the CP QOL-Teen: A quality of life questionnaire for adolescents with cerebral palsy. Res. Dev. Disabil. 2013, 34, 344–352. [Google Scholar] [CrossRef]
- Coster, W.; Law, M.; Bedell, G.; Khetani, M.; Cousins, M.; Teplicky, R. Development of the participation and environment measure for children and youth: Conceptual basis. Disabil. Rehabil. 2012, 34, 238–246. [Google Scholar] [CrossRef]
- Öhrvall, A.; Eliasson, A.; Löwing, K.; Ödman, P.; Krumlinde-Sundholm, L. Self-care and mobility skills in children with cerebral palsy, related to their manual ability and gross motor function classifications. Dev. Med. Child Neurol. 2010, 52, 1048–1055. [Google Scholar] [CrossRef]
- Jeong, Y.-A.; Lee, B.-H. Effect of Action Observation Training on Spasticity, Gross Motor Function, and Balance in Children with Diplegia Cerebral Palsy. Children 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Buccino, G.; Molinaro, A.; Ambrosi, C.; Arisi, D.; Mascaro, L.; Pinardi, C.; Rossi, A.; Gasparotti, R.; Fazzi, E.; Galli, J. Action Observation Treatment Improves Upper Limb Motor Functions in Children with Cerebral Palsy: A Combined Clinical and Brain Imaging Study. Neural Plast. 2018, 2018, 4843985. [Google Scholar] [CrossRef] [PubMed]
| Action | AOT Session | Repetitions |
|---|---|---|
| Action 1: Place an object close to the body |  Observation action 1 Observation action 1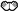 Execution action 1 Execution action 1 Observation action 1 Observation action 1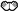 Execution action 1 Execution action 1 | Watch × 7 Perform × 7 Watch × 7 Perform × 7 |
| Action 2: Open a box |  Observation action 2 Observation action 2 Execution action 2 Execution action 2 Observation action 2 Observation action 2 Execution action 2 Execution action 2 | Watch × 7 Perform × 7 Watch × 7 Perform × 7 |
| Action 3: Insert the object in the box |  Observation action 3 Observation action 3 Execution action 3 Execution action 3 Observation action 3 Observation action 3 Execution action 3 Execution action 3 | Watch × 7 Perform × 7 Watch × 7 Perform × 7 |
| Action 4: Place an object close to the body; then open the box, and at the end, insert the object in the box |  Observation action 1 + 2 + 3 Observation action 1 + 2 + 3 Execution action 1 + 2 + 3 Execution action 1 + 2 + 3 Observation action 1 + 2 + 3 Observation action 1 + 2 + 3 Execution action 1 + 2 + 3 Execution action 1 + 2 + 3 | Watch × 7 Perform × 7 Watch × 7 Perform × 7 |
 indicate Observation phase;
indicate Observation phase;  indicate Execution phase.
indicate Execution phase.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beani, E.; Matteucci, E.; Sicola, E.; Martini, G.; Di Lieto, M.C.; Bombonato, C.; Menici, V.; Cotardo, A.; Rizzo, M.; Filogna, S.; et al. ACT-ON-DIP: Study Protocol of a Randomized Controlled Trial of a Home-Based ACTion Observation Tele-RehabilitatioN for Upper Limb in Children with DIPlegic Cerebral Palsy. Children 2025, 12, 1229. https://doi.org/10.3390/children12091229
Beani E, Matteucci E, Sicola E, Martini G, Di Lieto MC, Bombonato C, Menici V, Cotardo A, Rizzo M, Filogna S, et al. ACT-ON-DIP: Study Protocol of a Randomized Controlled Trial of a Home-Based ACTion Observation Tele-RehabilitatioN for Upper Limb in Children with DIPlegic Cerebral Palsy. Children. 2025; 12(9):1229. https://doi.org/10.3390/children12091229
Chicago/Turabian StyleBeani, Elena, Elisa Matteucci, Elisa Sicola, Giada Martini, Maria Chiara Di Lieto, Clara Bombonato, Valentina Menici, Annalisa Cotardo, Marta Rizzo, Silvia Filogna, and et al. 2025. "ACT-ON-DIP: Study Protocol of a Randomized Controlled Trial of a Home-Based ACTion Observation Tele-RehabilitatioN for Upper Limb in Children with DIPlegic Cerebral Palsy" Children 12, no. 9: 1229. https://doi.org/10.3390/children12091229
APA StyleBeani, E., Matteucci, E., Sicola, E., Martini, G., Di Lieto, M. C., Bombonato, C., Menici, V., Cotardo, A., Rizzo, M., Filogna, S., Camuncoli, F., Biagi, L., Cioni, G., Fedeli, F., Gelmini, C., Neviani, R., Vecchi, O., Perazza, S., Faccioli, S., ... Sgandurra, G. (2025). ACT-ON-DIP: Study Protocol of a Randomized Controlled Trial of a Home-Based ACTion Observation Tele-RehabilitatioN for Upper Limb in Children with DIPlegic Cerebral Palsy. Children, 12(9), 1229. https://doi.org/10.3390/children12091229








