Platelet Recovery and Coexisting Conditions in Pediatric Immune Thrombocytopenia: Insights from a Tertiary Care Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- ➢
- Hematologic tests: Complete blood count with differential, including serial platelet count measurements at presentation and throughout the hospital stay to monitor thrombocytopenia severity and recovery.
- ➢
- Serum calcium: Measured to detect any calcium imbalance (e.g., hypocalcemia), given that metabolic disturbances were of interest in this cohort.
- ➢
- Serum iron: Evaluated to assess iron levels and identify any coexistent iron deficiency or anemia.
- ➢
- Liver enzyme panel: Assessment of hepatic function via aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase.
- ➢
- Renal function tests: blood urea, creatinine, and uric acid levels were measured to evaluate kidney function and to rule out any renal impairment or tumor lysis–like syndrome that could contribute to laboratory anomalies.
- ➢
- Serum electrolyte panel: Measurement of key electrolytes (such as sodium, potassium, chloride, and bicarbonate) to detect any electrolyte imbalances.
- ➢
- Urine analysis: A complete urinalysis was performed to screen for hematuria, proteinuria, or signs of infection that might be present alongside ITP.
- ➢
- Fundoscopic examination: An ophthalmologic exam of the ocular fundus (both eyes) was conducted at admission to check for retinal hemorrhages or other abnormalities, as severe thrombocytopenia can predispose to retinal bleeding.
- ➢
- Chest radiograph: A chest X-ray was obtained to identify any pulmonary infiltrates or mediastinal abnormalities, and to evaluate for signs of infection or lymphadenopathy that could suggest a secondary cause of thrombocytopenia.
- ➢
- Viral panel: Screening for viral infections known to trigger secondary ITP was done by testing for serological evidence of the TORCH panel (Toxoplasma, Rubella, Cytomegalovirus, Herpes simplex virus types 1 and 2 (HSV-1, HSV-2)), Epstein–Barr virus (EBV).
- ➢
- Infectious disease markers: Additional serologic tests were performed for hepatitis C virus (anti-HCV antibodies), hepatitis B (hepatitis B surface antigen, HBsAg), and human immunodeficiency virus (HIV-1/2 antibodies) to rule out these chronic infections as contributors to thrombocytopenia.
- ➢
- Lactate dehydrogenase (LDH): Serum LDH levels were measured as a non-specific marker of cell turnover or hemolysis, aiding in the assessment of disease severity and in distinguishing ITP from other hemolytic processes.
2.2. Statistical Analysis
3. Results
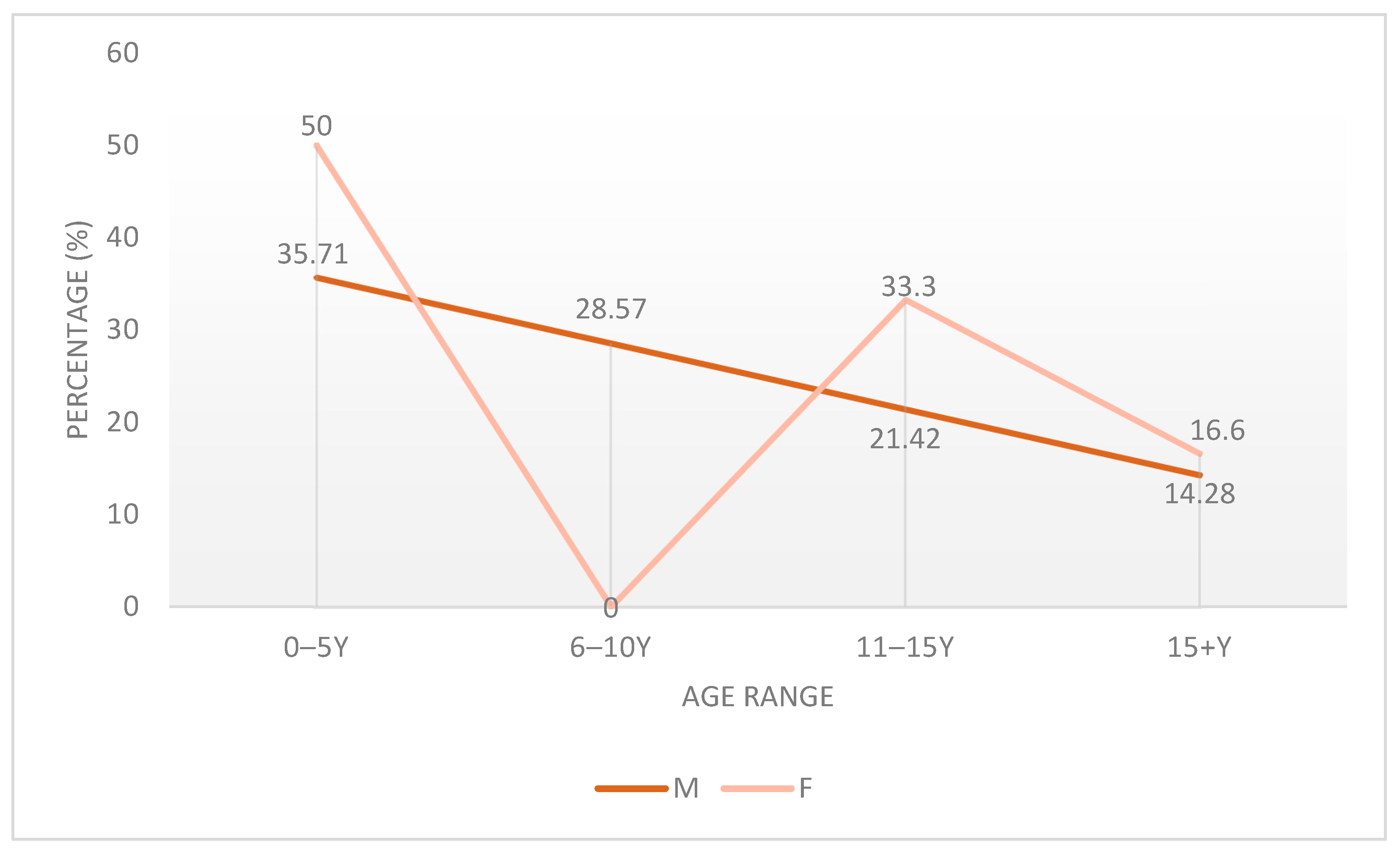
3.1. Age Prevalence
3.2. Patient’s Background
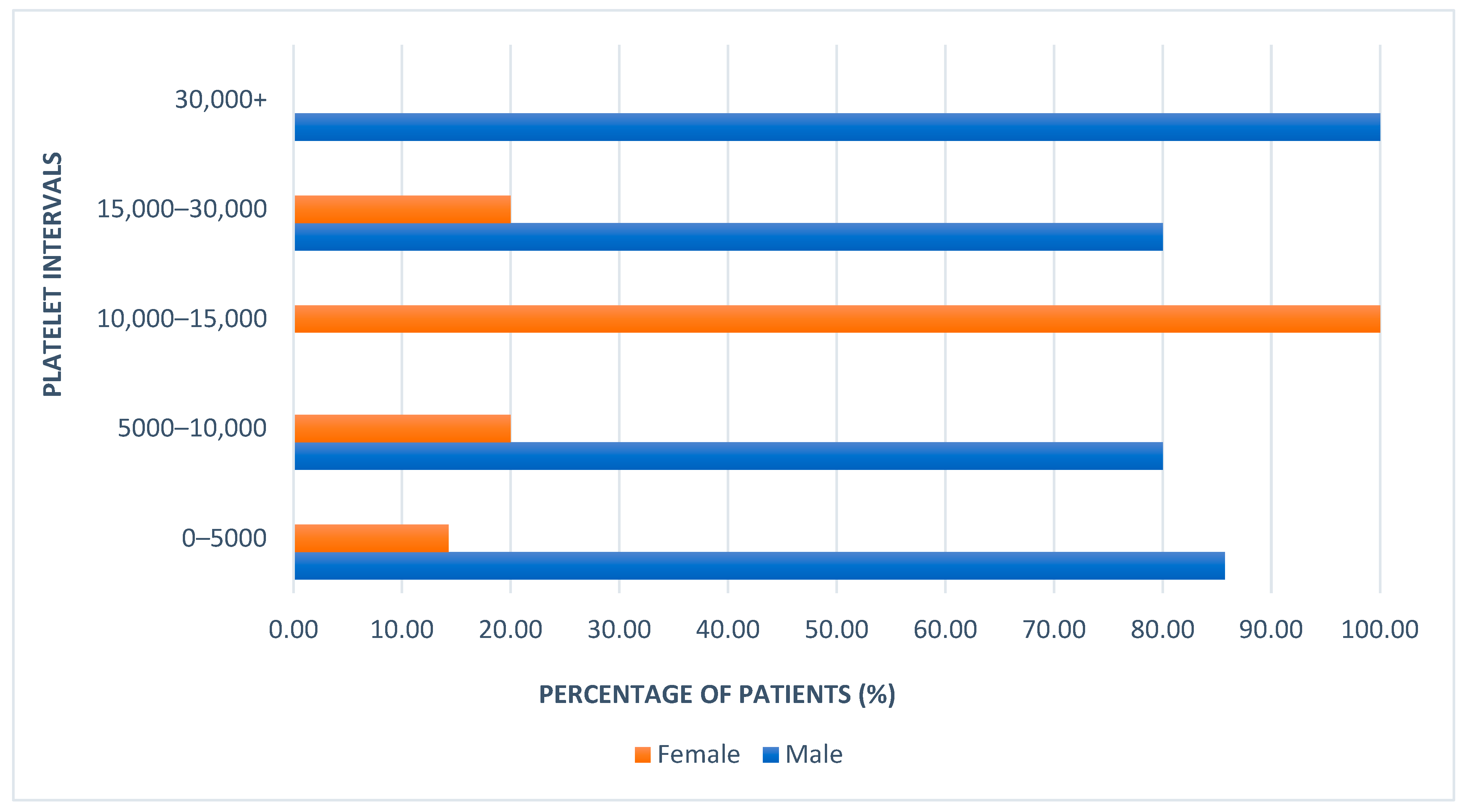
3.3. Platelet Count
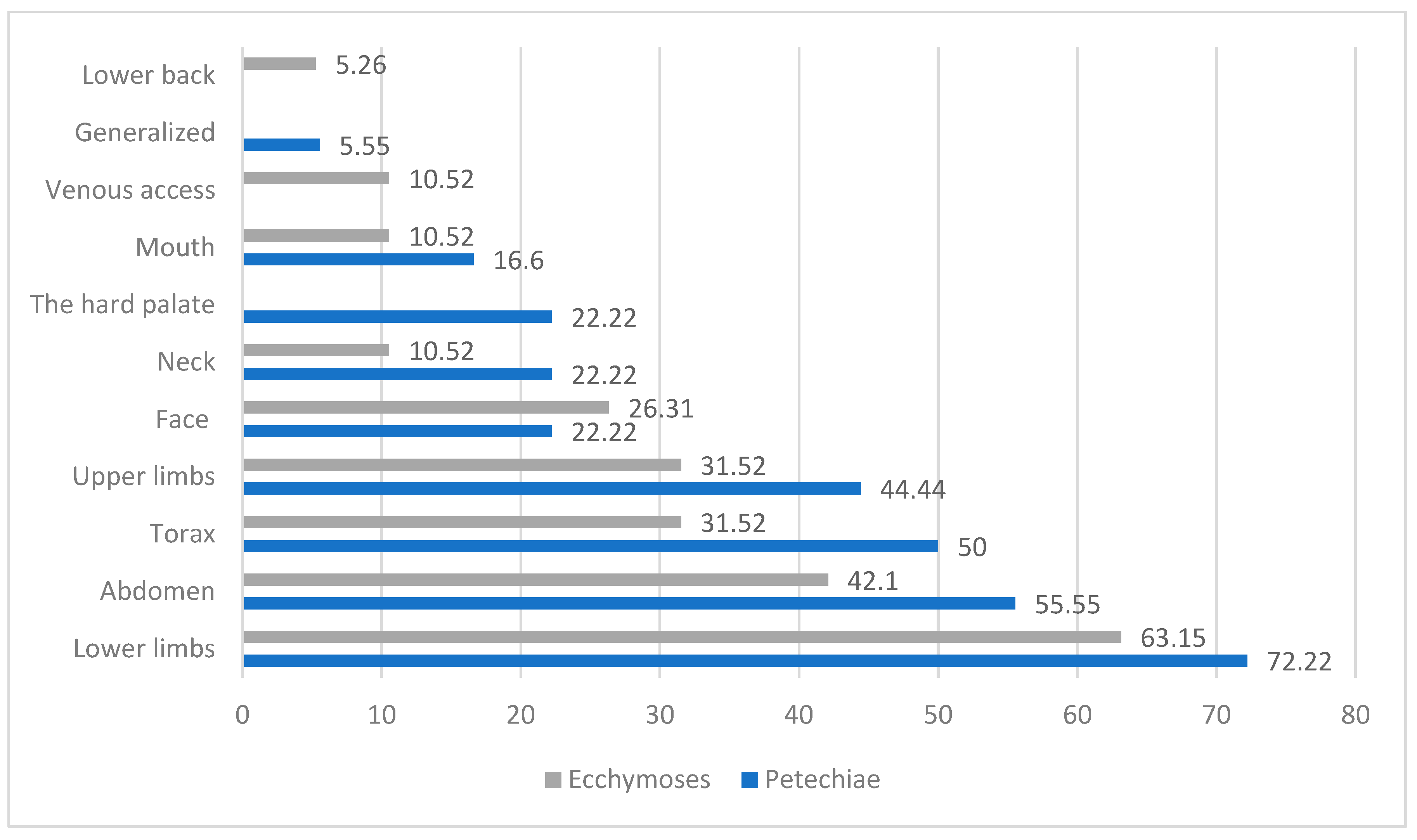
3.4. Symptom Classification
3.5. Treatment
3.6. Diagnosis
4. Discussion
4.1. Demographic Characteristics and Sex-Based Differences
4.2. Clinical Presentation and the Prevalence of Coexisting Clinical Conditions in Pediatric ITP
4.3. Integrated Clinical Management and Prognostic Insights in Pediatric ITP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, G.R. Thrombocytopenia during childhood: What the pediatrician needs to know. Pediatr. Rev. 2005, 26, 401–409. [Google Scholar] [CrossRef]
- Neunert, C.; Lim, W.; Crowther, M.; Cohen, A.; Solberg, L., Jr.; Crowther, M.A. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011, 117, 4190–4207. [Google Scholar] [CrossRef] [PubMed]
- Lev, P.R.; Goette, N.P.; Marta, R.F. Pathophysiological mechanisms leading to low platelet count in immune thrombocyto penia. J. Immunological. Sci. 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Zheng, S.S.; Perdomo, J.S. Desialylation and Apoptosis in Immune Thrombocytopenia: Implications for Pathogenesis and Treatment. Curr. Issues Mol. Biol. 2024, 46, 11942–11956. [Google Scholar] [CrossRef]
- Cines, D.B.; Cuker, A.; Semple, J.W. Pathogenesis of immune thrombocytopenia. Presse Med. 2014, 43 Pt 2, e49–e59. [Google Scholar] [CrossRef]
- Giordano, P.; Lassandro, G.; Barone, A.; Cesaro, S.; Fotzi, I.; Giona, F.; Gorio, C.; Maggio, A.; Miano, M.; Marzollo, A.; et al. Long-Term Use of Eltrombopag in Children with Chronic Immune Thrombocytopenia: Extended Real-Life Retrospective Multicenter Experience of the Italian Association of Pediatric Hematology and Oncology. Front. Med. 2023, 10, 1214308. [Google Scholar] [CrossRef] [PubMed]
- Giordano, P.; Lassandro, G.; Barone, A.; Cesaro, S.; Fotzi, I.; Giona, F.; Ladogana, S.; Miano, M.; Marzollo, A.; Nardi, M.; et al. Use of Eltrombopag in Children with Chronic Immune Thrombocytopenia (ITP): A Real-Life Retrospective Multicenter Experience of the Italian Association of Pediatric Hematology and Oncology (AIEOP). Front. Med. 2020, 7, 66. [Google Scholar] [CrossRef]
- Matzdorff, A.; Alesci, S.R.; Gebhart, J.; Holzhauer, S.; Hütter-Krönke, M.L.; Kühne, T.; Meyer, O.; Ostermann, H.; Pabinger, I.; Rummel, M.; et al. Expert Report on Immune Thrombocytopenia: Current Diagnostics and Treatment—Recommendations from an Expert Group from Austria, Germany, and Switzerland. Oncol. Res. Treat. 2023, 46 (Suppl. S2), 5–44. [Google Scholar] [CrossRef]
- Najaoui, A.; Bakchoul, T.; Stoy, J.; Bein, G.; Rummel, M.J.; Santoso, S.; Sachs, U.J. Autoantibody-mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP). Eur. J. Haematol. 2012, 88, 167–174. [Google Scholar] [CrossRef]
- Sakakura, M.; Wada, H.; Tawara, I.; Nobori, T.; Sugiyama, T.; Sagawa, N.; Shiku, H. Reduced Cd41Cd251 T cells in patients with idiopathic thrombocytopenic purpura. Thromb. Res. 2007, 120, 187–193. [Google Scholar] [CrossRef]
- Li, X.; Zhong, H.; Bao, W.; Boulad, N.; Evangelista, J.; Haider, M.A.; Bussel, J.; Yazdanbakhsh, K. Defective regulatory B-cell compartment in patients with immune thrombocytopenia. Blood 2012, 120, 3318–3325. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Zhao, C.; Li, L.; Peng, J.; Hou, M. CD8+ T cells suppress autologous megakaryocyte apoptosis in idiopathic thrombocytopenic purpura. Br. J. Haematol. 2007, 139, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Vrbensky, J.R.; Nazy, I.; Toltl, L.J.; Ross, C.; Ivetic, N.; Smith, J.W.; Kelton, J.G.; Arnold, D.M. Megakaryocyte apoptosis in immune thrombocytopenia. Platelets 2018, 29, 729–732. [Google Scholar] [CrossRef]
- Olsson, B.; Andersson, P.O.; Jernås, M.; Jacobsson, S.; Carlsson, B.; Carlsson, L.M.S.; Wadenvik, H. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat. Med. 2003, 9, 1123–1124. [Google Scholar] [CrossRef]
- ISO 15189:2022; Medical laboratories—Requirements for Quality and Competence. International Organization for Standardization: Geneva, Switzerland, 2022.
- Provan, D.; Stasi, R.; Newland, A.C.; Blanchette, V.S.; Bolton-Maggs, P.; Bussel, J.B.; Chong, B.H.; Cines, D.B.; Gernsheimer, T.B.; Godeau, B.; et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010, 115, 168–186. [Google Scholar] [CrossRef]
- Neunert, C.E.; Cooper, N. Evidence-Based Management of Immune Thrombocytopenia: ASH Guideline Update. Hematol. Am. Soc. Hematol. Educ. Program. 2018, 2018, 568–575. [Google Scholar] [CrossRef]
- Brîndușe, L.A.; Eclemea, I.; Neculau, A.E.; Păunescu, B.A.; Bratu, E.C.; Cucu, M.A. Rural versus urban healthcare through the lens of health behaviors and access to primary care: A post-hoc analysis of the Romanian health evaluation survey. BMC Health Serv. Res. 2024, 24, 1341. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Kilpatrick, K.; Eisen, M.; Tarantino, M. The incidence and clinical burden of immune thrombocytopenia in pediatric patients in the United States. Platelets 2020, 31, 307–314. [Google Scholar] [CrossRef]
- Consolini, R.; Costagliola, G.; Spatafora, D. The Centenary of Immune Thrombocytopenia—Part 2: Revising Diagnostic and Therapeutic Approach. Front. Pediatr. 2017, 5, 179. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Neunert, C.E. How I treat refractory immune thrombocytopenia. Blood 2016, 128, 1547–1554. [Google Scholar] [CrossRef]
- Cole, C. Lessons in the diagnosis and management of immune thrombocytopenic purpura in children. J. Paediatr. Child Health 2017, 53, 833–835. [Google Scholar] [CrossRef]
- Grace, R.F.; Neunert, C. Second-line therapies in immune thrombocytopenia. Hematol. Am. Soc. Hematol. Educ. Program. 2016, 2016, 698–706. [Google Scholar] [CrossRef]
- Adewoyin, A.S.; Nwogoh, B. Peripheral blood film—A review. Ann. Ib. Postgrad. Med. 2014, 12, 71–79. [Google Scholar]
- Frederiksen, H.; Schmidt, K. The incidence of idiopathic thrombocytopenic purpura in adults increases with age. Blood 1999, 94, 909–913. [Google Scholar] [CrossRef]
- Grimaldi-Bensouda, L.; Nordon, C.; Michel, M.; Viallard, J.-F.; Adoue, D.; Magy Bertrand, N.; Durand, J.-M.; Quittet, P.; Fain, O.; Bonnotte, B.; et al. Immune thrombocytopenia in adults: A prospective cohort study of clinical features and predictors of outcome. Haematologica 2016, 101, 1039. [Google Scholar] [CrossRef]
- Fischer, A.; Provot, J.; Jais, J.P.; Alcais, A.; Mahlaoui, N.; Members of the CEREDIH French PID Study Group. Autoimmune and inflammatory manifestations occur frequently in patients with primary immunodeficiencies. J. Allergy Clin. Immunol. 2017, 140, 1388–1393.e8. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Aladjidi, N.; Fernandes, H.; Members of the French Reference Center for Pediatric Autoimmune Cytopenia; Leverger, G.; Magérus-Chatinet, A.; Mazerolles, F.; Stolzenberg, M.-C.; Jacques, S.; Picard, C.; et al. Pediatric Evans syndrome is associated with a high frequency of potentially damaging variants in immune genes. Blood 2019, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mithoowani, S.; Cervi, A.; Shah, N.; Ejaz, R.; Sirotich, E.; Barty, R.; Li, N.; Nazy, I.; Arnold, D.M. Management of major bleeds in patients with immune thrombocytopenia. J. Thromb. Haemost. 2020, 18, 1783–1790. [Google Scholar] [CrossRef]
- Kohli, R.; Chaturvedi, S. Epidemiology and clinical manifestations of immune thrombocytopenia. Hamostaseologie 2019, 39, 238–249. [Google Scholar] [CrossRef]
- Peng, J.; Friese, P.; Heilmann, E.; George, J.N.; Burstein, S.A.; Dale, G.L. Aged platelets have an impaired response to thrombin as quantitated by P-selectin expression. Blood 1994, 83, 161–166. [Google Scholar] [CrossRef]
- Neunert, C.; Terrell, D.R.; Arnold, D.M.; Buchanan, G.; Cines, D.B.; Cooper, N.; Cuker, A.; Despotovic, J.M.; George, J.N.; Grace, R.F.; et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019, 3, 3829–3866. [Google Scholar] [CrossRef]
- Bruin, M.; Bierings, M.; Uiterwaal, C.; Évész, T.R.; Bode, L.; Wiesman, M.-E.; Kuijpers, T.W.; Tamminga, R.; De Haas, M. Platelet count, previous infection and FCGR2B genotype predict development of chronic disease in newly diagnosed idiopathic thrombocytopenia in childhood: Results of a prospective study. Br. J. Haematol. 2004, 127, 561–567. [Google Scholar] [CrossRef]
- Heitink-Pollé, K.M.; Nijsten, J.; Boonacker, C.W.; De Haas, M.; Bruin, M.C.A. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: A systematic review and meta-analysis. Blood 2014, 124, 3295–3307. [Google Scholar] [CrossRef]
- Tamminga, R.; Berchtold, W.; Bruin, M.; Buchanan, G.R.; Kuehne, T. Possible lower rate of chronic ITP after IVIG for acute childhood ITP: An analysis from registry I of the Intercontinental Cooperative ITP Study Group (ICIS). Br. J. Haematol. 2009, 146, 180–184. [Google Scholar] [CrossRef]
- Arnold, D.M.; Crowther, M.A.; Cook, R.J.; Sigouin, C.; Heddle, N.M.; Molnar, L.; Cook, D.J. Utilization of platelet transfusions in the intensive care unit: Indications, transfusion triggers, and platelet count responses. Transfusion 2006, 46, 1286–1291. [Google Scholar] [CrossRef]
- Rao, M.P.; Boralessa, H.; Morgan, C.; Soni, N.; Goldhill, D.R.; Brett, S.J.; Contreras, M. Blood component use in critically ill patients. Anaesthesia 2002, 57, 530–534. [Google Scholar] [CrossRef]
- van Baarle, F.L.; van de Weerdt, E.K.; van der Velden, W.J.; Ruiterkamp, R.A.; Tuinman, P.R.; Ypma, P.F.; Bergh, W.M.v.D.; Demandt, A.M.; Kerver, E.D.; Jansen, A.G.; et al. Platelet transfusion before CVC placement in patients with thrombocytopenia. N. Engl. J. Med. 2023, 388, 1956–1965. [Google Scholar] [CrossRef]
- Grimaldi-Bensouda, L.; Nordon, C.; Leblanc, T.; Abenhaim, L.; Allali, S.; Armari-Alla, C.; Berger, C.; Courcoux, M.-F.; Fouyssac, F.; Guillaumat, C.; et al. Childhood immune thrombocytopenia: A nationwide cohort study on condition management and outcomes. Pediatr. Blood Cancer 2017, 64, e26389. [Google Scholar] [CrossRef]
- Kime, C.; Klima, J.; Rose, M.J.; O’Brien, S.H. Patterns of inpatient care for newly diagnosed immune thrombocytopenia in US children’s hospitals. Pediatrics 2013, 131, 880–885. [Google Scholar] [CrossRef]
- Jubelirer, S.J.; Harpold, R. The role of the bone marrow examination in the diagnosis of immune thrombocytopenic purpura: Case series and literature review. Clin. Appl. Thromb. Hemost. 2002, 8, 73–76. [Google Scholar] [CrossRef]
- Allegra, A.; Cicero, N.; Mirabile, G.; Giorgianni, C.M.; Gangemi, S. Novel Biomarkers for Diagnosis and Monitoring of Immune Thrombocytopenia. Int. J. Mol. Sci. 2023, 24, 4438. [Google Scholar] [CrossRef]
- Semple, J.W.; Rebetz, J.; Maouia, A.; Kapur, R. An update on the pathophysiology of immune thrombocytopenia. Curr. Opin. Hematol. 2020, 27, 423–429. [Google Scholar] [CrossRef]



| Characteristic | Subcategory | Patients Number |
|---|---|---|
| Age group (years) | 0–5 | 40 |
| 6–10 | 20 | |
| 11–15 | 25 | |
| >15 | 15 | |
| Sex | Male | 70 |
| Female | 30 | |
| Residential background | Urban | 55 |
| Rural | 45 |
| Localization | Petechiae | Ecchymoses | ||
|---|---|---|---|---|
| Sex Distributions | F (Number of Patients/Percentages (%)) | M (Number of Patients/Percentages (%)) | F (Number of Patients/Percentages (%)) | M (Number of Patients/Percentages (%)) |
| Lower limbs | 5 (16.66%) | 60 (85.71%) | 20 (66.66%) | 40 (57.14%) |
| Abdomen | 10 (33.33%) | 50 (71.42%) | 10 (33.33%) | 30 (42.85%) |
| Thorax | 15 (50.00%) | 25 (35.29%) | 5 (16.66%) | 25 (35.71%) |
| Upper limbs | 5 (16.66%) | 29 (41.17%) | 10 (33.33%) | 20 (28.57%) |
| Face | 15 (50.00%) | 4 (5.88%) | 10 (33.33%) | 15 (21.42%) |
| Neck | 5 (16.66%) | 12 (17.64%) | 5 (16.66%) | 5 (7.14%) |
| The hard palate | 5 (16.66%) | 12 (17.64%) | - | - |
| Mouth | 10 (33.30%) | 4 (5.88%) | 0 (0.00%) | 10 (14.28%) |
| Venous access | - | - | 0 (0.00%) | 10 (14.28%) |
| Generalized | 0 (0.00%) | 4 (5.88%) | - | - |
| Lower back | - | - | 5 (16.66%) | 5 (7.14%) |
| Therapeutic Category | Medication | Route of Administration | Pediatric Dosage | Indication |
|---|---|---|---|---|
| First-line therapy | IVIG | Intravenous infusion over 4–6 h | 0.8–1 g/kg/day for 1–2 consecutive days | First-line treatment for newly diagnosed ITP, severe thrombocytopenia, or mucocutaneous bleeding; rapid platelet response within 24–48 h. |
| Prednisone | Oral | 1–2 mg/kg/day for 5–7 days | Used when IVIG was contraindicated, unavailable, or in mild, non-life-threatening bleeding. | |
| Second-line/rescue therapy | Platelet transfusion | Intravenous (slow infusion) | 5–10 mL/kg (single donor unit) | Reserved for active bleeding or platelet count <10 × 109/L, according to institutional protocols. |
| Supportive/adjunctive therapy | Desloratadine | Oral | Age-adjusted standard dosing | Prescribed for allergic symptom control. |
| Calcium gluconate | Oral/Intravenous | 50–100 mg/kg/day (elemental calcium equivalent) | Correction of mild/moderate hypocalcemia frequently observed in ITP patients. | |
| Omeprazole | Oral | 1 mg/kg/day | Gastroprotection during corticosteroid therapy. | |
| Rutoside | Oral | Per manufacturer recommendations | Supportive nutritional therapy. | |
| Cefoperazone–sulbactam/Meropenem | Intravenous | Standard antimicrobial pediatric doses | Used to treat bacterial infections when clinically indicated. | |
| Acetaminophen | Oral | 10–15 mg/kg every 6–8 h as needed | Symptomatic antipyretic/analgesic. | |
| Vitamin C | Oral | Standard pediatric dosing | Antioxidant and supportive therapy. |
| Diagnosis | Total Number of Patients | Number of Male Patients | Number of Female Patients |
|---|---|---|---|
| Thrombocytopenic Purpura (All Patients) | 100 | 70 | 30 |
| Isolated ITP (No coexisting clinical conditions) | 15 | 15 | 0 |
| ITP with coexisting Clinical Conditions | |||
| Thrombocytopenic Purpura + Mild/Moderate Hypocalcemia | 45 | 25 | 20 |
| Thrombocytopenic Purpura + Herpetic Stomatitis | 20 | 20 | 0 |
| Thrombocytopenic Purpura + Rhinosinusitis/Adenoiditis | 20 | 20 | 0 |
| Thrombocytopenic Purpura + Obesity | 20 | 15 | 5 |
| Thrombocytopenic Purpura + Acute Pneumonia | 15 | 5 | 10 |
| Thrombocytopenic Purpura + Epistaxis | 15 | 15 | 0 |
| Thrombocytopenic Purpura + Juvenile Acne | 15 | 10 | 5 |
| Thrombocytopenic Purpura + Iron Deficiency Anemia | 10 | 5 | 5 |
| Thrombocytopenic Purpura + Dental Caries | 10 | 5 | 5 |
| Thrombocytopenic Purpura + Serous Otitis | 10 | 10 | 0 |
| Thrombocytopenic Purpura + Underweight | 10 | 5 | 5 |
| Thrombocytopenic Purpura + Hyponatremia | 5 | 5 | 0 |
| Thrombocytopenic Purpura + Acute Tracheobronchitis | 5 | 0 | 5 |
| Thrombocytopenic Purpura + Eosinophilia | 5 | 0 | 5 |
| Thrombocytopenic Purpura + Tetany | 5 | 0 | 5 |
| Thrombocytopenic Purpura + Epilepsy | 5 | 5 | 0 |
| Thrombocytopenic Purpura + Maxillary Sinusitis | 5 | 5 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, C.E.; Andreea Gabriela, M.; Carmen, S.; Enescu, A.; Gaman, S.; Varut, R.M.; Dalia, D.; Veronica, M.E.; Vintilescu, Ș.B.; Ionovici, N.; et al. Platelet Recovery and Coexisting Conditions in Pediatric Immune Thrombocytopenia: Insights from a Tertiary Care Study. Children 2025, 12, 1482. https://doi.org/10.3390/children12111482
Singer CE, Andreea Gabriela M, Carmen S, Enescu A, Gaman S, Varut RM, Dalia D, Veronica ME, Vintilescu ȘB, Ionovici N, et al. Platelet Recovery and Coexisting Conditions in Pediatric Immune Thrombocytopenia: Insights from a Tertiary Care Study. Children. 2025; 12(11):1482. https://doi.org/10.3390/children12111482
Chicago/Turabian StyleSinger, Cristina Elena, Mocanu Andreea Gabriela, Sîrbuleț Carmen, Anca Enescu, Simina Gaman, Renata Maria Varut, Dop Dalia, Maria Elena Veronica, Ștefănița Bianca Vintilescu, Nina Ionovici, and et al. 2025. "Platelet Recovery and Coexisting Conditions in Pediatric Immune Thrombocytopenia: Insights from a Tertiary Care Study" Children 12, no. 11: 1482. https://doi.org/10.3390/children12111482
APA StyleSinger, C. E., Andreea Gabriela, M., Carmen, S., Enescu, A., Gaman, S., Varut, R. M., Dalia, D., Veronica, M. E., Vintilescu, Ș. B., Ionovici, N., Dorin, P. I., Cristian-Cosmin, A., & Popescu, C. (2025). Platelet Recovery and Coexisting Conditions in Pediatric Immune Thrombocytopenia: Insights from a Tertiary Care Study. Children, 12(11), 1482. https://doi.org/10.3390/children12111482








