Associations of TAS1R2 and TAS2R38 Genetic Variants with Sugar-Sweetened Beverage Intake and Obesity Risk in Kuwaiti Adolescents: A Cross-Sectional Study
Abstract
Highlights
- •
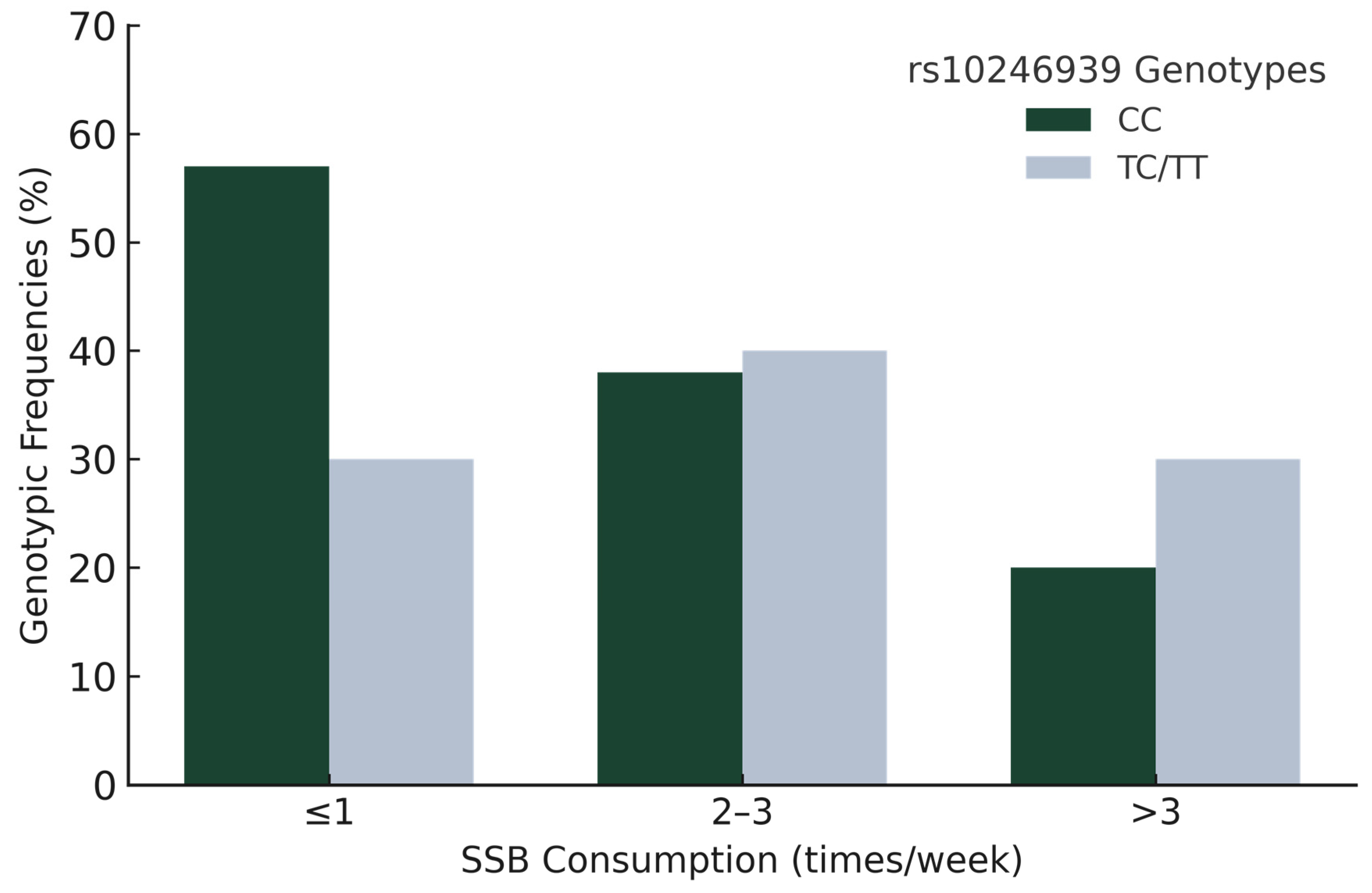
- The rs10246939 CC genotype of TAS2R38 was significantly associated with lower sugar-sweetened beverage consumption (p = 0.018, OR = 0.24, 95% CI = 0.08–0.79).
- •
- The rs713598 SNP in TAS2R38 showed a marginal association with BMI percentiles and z-scores, while no significant associations were observed for TAS1R2 SNPs.
- •
- Genetic variation in TAS2R38 may influence dietary preferences and obesity risk in adolescents.
- •
- These results underscore the importance of considering taste receptor polymorphisms in obesity prevention strategies and personalized nutrition approaches.
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic and Socioeconomic Characteristics
3.2. Genotypic and Allelic Frequencies
3.3. Estimated Dietary Intake and Physical Activity Level, and Validation of SSB Consumption with Blood Glucose Levels
3.4. Anthropometric Measurements and Genotypic Associations
3.5. SSB Consumption and Genotypic Associations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| CDC | Centers for Disease Control and Prevention |
| SSB | sugar-sweetened beverages |
| RCT | Randomized-Controlled Trial |
| TAS1R2 | taste receptor, type1, member 2 |
| TAS2R38 | taste receptor, type2, member 38 |
| CD36 | Cluster of Differentiation |
| TRPM5 | Transient Receptor Potential Subfamily M Member 5 |
| SNP | single-nucleotide polymorphism |
| PAV | Proline–Alanine–Valine |
| AVI | Alanine–Valine–Isoleucine |
| BMI% | body mass index-for-age percentiles |
| BMIz | body mass index z-scores |
| WC | waist circumference |
| WHtR | waist-to-height ratio |
| FFQ | Food Frequency Questionnaire |
| PCR | polymerase chain reaction |
| FDR | false discovery rate |
| BMR | basal metabolic rate |
| PA | physical activity |
| GWAS | genome-wide association studies |
| T2DM | type 2 diabetes mellitus |
| GLP1 | glucagon-like peptide-1 |
| CCK | cholecystokinin |
| MET | Metabolic Equivalent of Task |
| ADA | American Diabetes Association |
| QST | quantitative sensory testing |
References
- Boutari, C.; Mantzoros, C.A. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 May 2025).
- World Health Organization. World Obesity Day 2022—Accelerating Action to Stop Obesity. Available online: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity (accessed on 7 May 2025).
- Al-Taiar, A.; Alqaoud, N.; Ziyab, A.H.; Alanezi, F.; Subhakaran, M.; Sharaf Alddin, R.; Jeng, H.A.; Akpinar-Elci, M. Time trends of overweight and obesity among schoolchildren in Kuwait over a 13-year period (2007–2019): Repeated cross-sectional study. Public Health Nutr. 2021, 24, 5318–5328. [Google Scholar] [CrossRef] [PubMed]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef]
- Seifu, C.N.; Fahey, P.P.; Hailemariam, T.G.; Frost, S.A.; Atlantis, E. Dietary patterns associated with obesity outcomes in adults: An umbrella review of systematic reviews. Public Health Nutr. 2021, 24, 6390–6414. [Google Scholar] [CrossRef]
- Popkin, B.M.; Ng, S.W. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes. Rev. 2021, 23, e13366. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- Magriplis, E.; Michas, G.; Petridi, E.; Chrousos, G.P.; Roma, E.; Benetou, V.; Cholopoulos, N.; Micha, R.; Panagiotakos, D.; Zampelas, A. Dietary sugar intake and its association with obesity in children and adolescents. Children 2021, 8, 676. [Google Scholar] [CrossRef]
- Marriott, B.P.; Hunt, K.J.; Malek, A.M.; Newman, J.C. Trends in intake of energy and total sugar from sugar-sweetened beverages in the United States among children and adults, Nhanes 2003–2016. Nutrients 2019, 11, 2004. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Ding, D.; Mihrshahi, S.; Wu, J.H.Y. Sugar-sweetened beverage consumption and weight gain in children and adults: A systematic review and dose–response meta-analysis. Obes. Rev. 2023, 24, e13544. [Google Scholar] [CrossRef]
- Mao, Z.; Cheng, W.; Li, Z.; Yao, M.; Sun, K. Clinical associations of bitter taste perception and bitter taste receptor variants and the potential for personalized healthcare. Pharmacogenom. Pers. Med. 2023, 16, 121–132. [Google Scholar] [CrossRef]
- Diószegi, J.; Llanaj, E.; Ádány, R. Genetic background of taste perception, taste preferences, and its nutritional implications: A systematic review. Front. Genet. 2019, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- Kourouniotis, S.; Keast, R.S.J.; Riddell, L.J.; Lacy, K.; Thorpe, M.G.; Cicerale, S. The importance of taste on dietary choice, behaviour and intake in a group of young adults. Appetite 2016, 103, 1–7. [Google Scholar] [CrossRef]
- Shyam, S.; Lee, K.X.; Tan, A.S.; Khoo, T.A.; Harikrishnan, S.; Lalani, S.A.; Ramadas, A. Effect of personalized nutrition on dietary, physical activity, and health outcomes: A systematic review of randomized trials. Nutrients 2022, 14, 4104. [Google Scholar] [CrossRef] [PubMed]
- Feeney, E.L.; O’Brien, S.A.; Scannell, A.G.M.; Markey, A.; Gibney, E.R. Suprathreshold measures of taste perception in children—Association with dietary quality and body weight. Appetite 2017, 113, 116–123. [Google Scholar] [CrossRef]
- Keller, K.L.; Olsen, A.; Cravener, T.L.; Bloom, R.; Chung, W.K.; Deng, L.; Lanzano, P.; Meyermann, K. Bitter taste phenotype and body weight predict children’s selection of sweet and savory foods at a palatable test-meal. Appetite 2014, 77, 115–123. [Google Scholar] [CrossRef]
- Primeaux, S.; Weir, A.; Denstel, K.; Martin, C.K.; Hsia, D.; Staiano, A. Role of taste receptor gene variations (SNPs) on diet quality in adolescent females. FASEB J. 2022, 36, R2184. [Google Scholar] [CrossRef]
- Chamoun, E.; Hutchinson, J.; Krystia, O.; Mirotta, J.; Mutch, D.; Buchholz, A.; Duncan, A.; Darlington, G.; Haines, J.; Ma, D. Single nucleotide polymorphisms in taste receptor genes are associated with snacking patterns of preschool-aged children in the Guelph Family Health Study: A pilot study. Nutrients 2018, 10, 153. [Google Scholar] [CrossRef]
- Melo, S.V.; Agnes, G.; Vitolo, M.R.; Mattevi, V.S.; Campagnolo, P.D.B.; Almeida, S. Evaluation of the association between the TAS1R2 and TAS1R3 variants and food intake and nutritional status in children. Genet. Mol. Biol. 2017, 40, 415–420. [Google Scholar] [CrossRef]
- Pioltine, M.; De Melo, M.; Santos, A.; Machado, A.; Fernandes, A.; Fujiwara, C.; Cercato, C.; Mancini, M. Genetic variations in sweet taste receptor gene are related to chocolate powder and dietary fiber intake in obese children and adolescents. J. Pers. Med. 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Chamoun, E.; Carroll, N.A.; Duizer, L.M.; Qi, W.; Feng, Z.; Darlington, G.; Duncan, A.M.; Haines, J.; Ma, D.W.L. The relationship between single nucleotide polymorphisms in taste receptor genes, taste function and dietary intake in preschool-aged children and adults in the Guelph Family Health Study. Nutrients 2018, 10, 990. [Google Scholar] [CrossRef]
- Trius-Soler, M.; Moreno, J.J. Bitter taste receptors: Key target to understand the effects of polyphenols on glucose and body weight homeostasis. Pathophysiological and pharmacological implications. Biochem. Pharmacol. 2024, 228, 116192. [Google Scholar] [CrossRef]
- Chupeerach, C.; Tapanee, P.; On-Nom, N.; Temviriyanukul, P.; Chantong, B.; Reeder, N.; Adegoye, G.; Tolar-Peterson, T. The influence of TAS2R38 bitter taste gene polymorphisms on obesity risk in three racially diverse groups. BioMedicine 2021, 11, 43–49. [Google Scholar] [CrossRef]
- Pawellek, I.; Grote, V.; Rzehak, P.; Xhonneux, A.; Verduci, E.; Stolarczyk, A.; Closa-Monasterolo, R.; Reischl, E.; Koletzko, B. Association of TAS2R38 variants with sweet food intake in children aged 1–6 years. Appetite 2016, 107, 126–134. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.A.; Feeney, E.L.; Scannell, A.G.M.; Markey, A.; Gibney, E.R. Bitter taste perception and dietary intake patterns in Irish children. Lifestyle Genom. 2013, 6, 43–58. [Google Scholar] [CrossRef]
- Hwang, L.-D.; Lin, C.; Gharahkhani, P.; Cuellar-Partida, G.; Ong, J.-S.; An, J.; Gordon, S.D.; Zhu, G.; MacGregor, S.; Lawlor, D.A.; et al. New insight into human sweet taste: A genome-wide association study of the perception and intake of sweet substances. Am. J. Clin. Nutr. 2019, 109, 1724–1737. [Google Scholar] [CrossRef]
- Al-Taiar, A.; Rahman, A.; Al-Sabah, R.; Shaban, L.; Al-Harbi, A. Vitamin D status among adolescents in Kuwait: A cross-sectional study. BMJ Open 2018, 8, e021401. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Child and Teen BMI Categories. Available online: https://www.cdc.gov/bmi/child-teen-calculator/bmi-categories.html (accessed on 16 October 2024).
- Shaw, V. Clinical Paediatric Dietetics; Wiley-Blackwell: Hoboken, NJ, USA, 2020. [Google Scholar]
- Koletzko, B. Basic concepts in nutrition: Nutritional needs of children and adolescents. E-SPEN Eur. E-J. Clin. Nutr. Metab. 2008, 3, e179–e184. [Google Scholar] [CrossRef]
- Joosten, K.; Embleton, N.; Yan, W.; Senterre, T.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. Espghan/Espen/ESPR/CSPEN guidelines on Pediatric Parenteral Nutrition: Energy. Clin. Nutr. 2018, 37, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Al-Hazzaa, H.M.; Al-Sobayel, H.I.; Musaiger, A.O. Convergent validity of the Arab Teens Lifestyle Study (ATLS) physical activity questionnaire. Int. J. Environ. Res. Public Health 2011, 8, 3810–3820. [Google Scholar] [CrossRef]
- Papandreou, D.; Rachaniotis, N.; Lari, M.; Al Mussabi, W. Validation of a food frequency questionnaire for vitamin D and calcium intake in healthy female college students. Food Nutr. Sci. 2014, 5, 2048–2052. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture: Washington, DC, USA, 2020. Available online: https://www.dietaryguidelines.gov/ (accessed on 28 July 2025).
- Zhang, Y.; Xu, P.; Song, Y.; Ma, N.; Lu, J. Association between sugar-sweetened beverage consumption frequency and muscle strength: Results from a sample of Chinese adolescents. BMC Public Health 2023, 23, 1010. [Google Scholar] [CrossRef]
- Grap, M.E.; Hamner, H.C.; Dooyema, C.; Noiman, A.; Park, S. Factors associated with sugar-sweetened beverage intake among young children—United States, 2021. Prev. Chronic Dis. 2024, 21, E17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Zhang, B.; Popkin, B.M.; Du, S. Elevated fat intake increases body weight and the risk of overweight and obesity among Chinese adults: 1991–2015 trends. Nutrients 2020, 12, 3272. [Google Scholar] [CrossRef]
- The American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2020, 44, S15–S33. [Google Scholar] [CrossRef]
- Turner, A.; Veysey, M.; Keely, S.; Scarlett, C.; Lucock, M.; Beckett, E.L. Interactions between bitter taste, diet and dysbiosis: Consequences for appetite and Obesity. Nutrients 2018, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, S.; De Cosmi, V.; Ciappolino, V.; Parazzini, F.; Brambilla, P.; Agostoni, C. Factors influencing children’s eating behaviours. Nutrients 2018, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Fu, O.; Iwai, Y.; Narukawa, M.; Ishikawa, A.W.; Ishii, K.K.; Murata, K.; Yoshimura, Y.; Touhara, K.; Misaka, T.; Minokoshi, Y.; et al. Hypothalamic neuronal circuits regulating hunger-induced taste modification. Nat. Commun. 2019, 10, 4567. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Sun, Q.; Abdurehim, A.; Xu, J.; Xie, J.; Zhang, Y. Taste and its receptors in human physiology: A comprehensive look. Food Front. 2024, 5, 1512–1533. [Google Scholar] [CrossRef]
| Characteristics | Boys (n = 113) | Girls (n = 147) | Total (n = 260) | p-Value | Adjusted p-Value | |
|---|---|---|---|---|---|---|
| Age (yrs) | Mean ± SD | 12.01 ± 0.85 | 11.84 ± 0.90 | 11.92 ± 0.88 | 0.133 1 | 0.266 |
| n (%) | n (%) | n (%) | ||||
| Nationality | Kuwaiti | 59 (52.20) | 127 (86.40) | 186 (71.50) | <0.001 2 | 0.005 |
| Non-Kuwaiti | 54 (47.80) | 20 (13.60) | 74 (28.50) | |||
| Government of Residence | Capital | 17 (15.00) | 14 (9.60) | 31 (12.00) | <0.001 2 | 0.005 |
| Hawally | 23 (20.40) | 7 (4.80) | 30 (11.60) | |||
| Al-Farwaniyah | 10 (8.80) | 2 (1.40) | 12 (4.60) | |||
| Al-Jahra | 28 (24.80) | 28 (19.20) | 56 (21.60) | |||
| Mubarak Al-Kabeer | 4 (3.50) | 26 (17.80) | 30 (11.60) | |||
| Al-Ahmadi | 31 (27.40) | 69 (47.30) | 100 (38.60) | |||
| Father Education | Secondary school or lower | 45 (39.80) | 67 (46.50) | 112 (43.60) | 0.065 2 | 0.217 |
| Diploma | 18 (15.90) | 33 (22.90) | 51 (19.80) | |||
| College degree or higher | 50 (44.20) | 44 (30.60) | 94 (36.60) | |||
| Mother Education | Secondary school or lower | 40 (35.40) | 56 (38.40) | 96 (37.10) | 0.884 2 | 0.887 |
| Diploma | 24 (21.20) | 29 (19.90) | 53 (20.50) | |||
| College degree or higher | 49 (43.40) | 61 (41.80) | 110 (42.50) | |||
| Father Income | <=1000 KD | 31 (27.70) | 50 (34.70) | 81 (31.60) | 0.558 2 | 0.698 |
| 1001–1500 KD | 39 (34.80) | 38 (26.40) | 77 (30.10) | |||
| 1501–2000 KD | 15 (13.40) | 22 (15.30) | 37 (14.50) | |||
| >2000 | 13 (11.60) | 19 (13.20) | 32 (12.50) | |||
| Do Not Know | 14 (12.50) | 15 (10.40) | 29 (11.30) | |||
| Mother Income | <=1000 KD | 48 (57.80) | 68 (55.70) | 116 (56.60) | 0.887 2 | 0.887 |
| 1001–1500 KD | 12 (14.50) | 22 (18.00) | 34 (16.60) | |||
| 1501–2000 KD | 7 (8.40) | 13 (10.70) | 20 (9.80) | |||
| >2000 KD | 4 (4.80) | 4 (3.30) | 8 (3.90) | |||
| Do Not Know | 12 (14.50) | 15 (12.30) | 27 (13.20) | |||
| Weight Status | Not Overweight | 53 (46.90) | 76 (51.70) | 129 (49.60) | 0.261 2 | 0.435 |
| Overweight/Obese | 60 (53.10) | 71 (48.30) | 131 (50.40) | |||
| SSB Consumption | ≤1 | 32 (28.30) | 53 (36.60) | 85 (32.90) | 0.118 2 | 0.266 |
| 2–3 | 40 (35.40) | 56 (38.60) | 96 (37.20) | |||
| >3 | 41 (36.30) | 36 (24.80) | 77 (29.80) | |||
| Parents Smoking Status | No | 72 (63.70) | 97 (66.00) | 169 (65.00) | 0.401 2 | 0.573 |
| Yes | 41 (36.30) | 50 (34.00) | 91 (35.00) | |||
| Gene | SNP | Genotype | n (%) | Expected Frequency n (%) | p-Value | Allele Frequency | |
|---|---|---|---|---|---|---|---|
| TAS1R2 | rs35874116 | CC | 25 (10.00) | 25.3 (10.12) | 0.997 | C | 0.318 |
| TC | 109 (43.60) | 108.4 (43.36) | T | 0.682 | |||
| TT | 116 (46.40) | 116.3 (46.52) | |||||
| Total | 250 (100.00) | 250 (100.00) | |||||
| rs9701796 | CC | 161 (66.30) | 166.5 (68.52) | 0.061 | C | 0.828 | |
| GC | 80 (32.90) | 69.3 (28.52) | G | 0.172 | |||
| GG | 2 (0.80) | 7.2 (2.96) | |||||
| Total | 243 (100.00) | 243 (100.00) | |||||
| TAS2R38 | rs713598 | CC | 99 (39.00) | 100.1 (39.41) | 0.953 | C | 0.628 |
| GC | 121 (47.60) | 118.7 (46.73) | G | 0.372 | |||
| GG | 34 (13.40) | 35.2 (13.86) | |||||
| Total | 254 (100.00) | 254 (100.00) | |||||
| rs1726866 | AA | 38 (15.10) | 40.4 (16.03) | 0.809 | A | 0.401 | |
| GA | 126 (50.00) | 121.0 (48.02) | G | 0.599 | |||
| GG | 88 (34.90) | 90.6 (35.95) | |||||
| Total | 252 (100.00) | 252 (100.00) | |||||
| rs10246939 | CC | 33 (13.40) | 37.5 (15.24) | 0.490 | C | 0.390 | |
| TC | 126 (51.20) | 117.1 (47.60) | T | 0.610 | |||
| TT | 87 (35.40) | 91.4 (37.16) | |||||
| Total | 246 (100.00) | 246 (100.00) | |||||
| Gene | Genotype | Diet | p-Value | Physical Activity | p-Value | ||
|---|---|---|---|---|---|---|---|
| BMR (Kcal/Day) | n (%) | ||||||
| Low | Medium | High | |||||
| TAS1R2 | rs35874116 | 0.577 | 0.309 | ||||
| CC | 1477.93 | 12 (48.00) | 5 (20.00) | 8 (32.00) | |||
| TC/TT | 1457.93 | 79 (35.10) | 76 (33.80) | 70 (31.10) | |||
| rs971796 | 0.705 | 0.922 | |||||
| CC | 1449.14 | 58 (36.00) | 52 (32.30) | 51 (31.70) | |||
| GC/GG | 1469.23 | 30 (36.60) | 28 (34.10) | 24 (29.30) | |||
| TAS2R38 | rs713598 | 0.186 | 0.307 | ||||
| CC | 1473.57 | 36 (36.40) | 37 (37.40) | 26 (26.30) | |||
| GC/GG | 1451.77 | 58 (37.40) | 45 (29.00) | 52 (33.50) | |||
| rs1726866 | 0.293 | 0.468 | |||||
| AA | 1473.02 | 30 (34.10) | 33 (37.50) | 25 (28.40) | |||
| GA/GG | 1452.03 | 63 (38.40) | 49 (29.90) | 52 (31.70) | |||
| rs10246939 | 0.963 | 0.284 | |||||
| CC | 1449.98 | 14 (42.40) | 7 (21.20) | 12 (36.40) | |||
| TC/TT | 1460.54 | 74 (34.70) | 75 (35.20) | 64 (30.00) | |||
| SSB Consumption/Week | n (%) | BG | p-Value | |
|---|---|---|---|---|
| Mean (mmol/L) | Standard Deviation | |||
| ≤1 times | 85 (33.20) | 5.06 | 1.18 | 0.969 |
| 2–3 times | 95 (37.11) | 4.96 | 0.75 | |
| >3 times | 76 (29.69) | 5.59 | 4.29 | |
| Total | 256 (100.00) | 5.18 | 2.48 | |
| Gene | SNP | Genotype | Mean ± SD | 95% CI | Median [25th–75th] | p-Value |
|---|---|---|---|---|---|---|
| BMI% | ||||||
| TAS1R2 | rs35874116 | CC | 72.93 ± 30.48 | (60.35, 85.51) | 87.40 [47.35–97.38] | 0.686 |
| TC/TT | 71.40± 29.41 | (67.65, 75.38) | 83.40 [52.10–95.62] | |||
| rs9701796 | CC | 70.33 ± 30.39 | (65.60, 75.06) | 83.60 [45.55–95.46] | 0.479 | |
| GC/GG | 74.10 ± 27.63 | (68.02, 80.17) | 85.05 [61.73–96.32] | |||
| TAS2R38 | rs713598 | CC | 77.49 ± 24.30 | (72.64, 82.33) | 87.70 [66.80–96.72] | 0.050 |
| GC/GG | 68.59 ± 31.79 | (63.54, 73.63) | 82.80 [43.60–95.70] | |||
| rs1726866 | AA | 76.48 ± 24.98 | (71.18, 81.77) | 87.55 [63.18–97.07] | 0.089 | |
| GA/GG | 69.33 ± 31.21 | (64.51, 74.14) | 83.20 [45.53–95.43] | |||
| rs10246939 | CC | 73.90 ± 29.83 | (63.32, 84.48) | 85.10 [63.40–96.70] | 0.577 | |
| TC/TT | 71.92 ± 28.97 | (68.01, 75.84) | 83.90 [52.35–96.03] | |||
| BMIz | ||||||
| TAS1R2 | rs35874116 | CC | 0.92 ±1.18 | (0.43, 1.40) | 1.15 [−0.07–1.94] | 0.690 |
| TC/TT | 0.88 ± 1.23 | (0.72, 1.04) | 0.97 [0.05–1.72] | |||
| rs9701796 | CC | 0.84 ± 1.25 | (0.64, 1.03) | 0.98 [−0.11–1.69] | 0.482 | |
| GC/GG | 0.97 ± 1.17 | (0.71, 1.22) | 1.04 [0.30–1.79] | |||
| TAS2R38 | rs713598 | CC | 1.12 ± 1.09 | (0.90, 1.34) | 1.16 [−0.43–1.84] | 0.050 |
| GC/GG | 0.76 ± 1.28 | (0.56, 0.96) | 0.95 [−0.16–1.72] | |||
| rs1726866 | AA | 1.09 ± 1.13 | (0.86, 1.34) | 1.16 [0.34–1.89] | 0.088 | |
| GA/GG | 0.77 ± 1.25 | (0.58, 0.97) | 0.96 [−0.11–1.69] | |||
| rs10246939 | CC | 1.03 ± 1.42 | (0.53, 1.54) | 1.04 [0.35–1.87] | 0.577 | |
| TC/TT | 0.88 ± 1.12 | (0.72, 1.04) | 0.99 [0.06–1.76] | |||
| WC | ||||||
| TAS1R2 | rs35874116 | CC | 81.32 ± 15.66 | (74.85, 87.78) | 78.00 [68.50–90.90] | 0.632 |
| TC/TT | 79.22 ± 13.37 | (77.46, 80.98) | 77.00 [68.50–88.00] | |||
| rs9701796 | CC | 78.50 ± 13.30 | (76.47, 80.61) | 77.00 [67.90–88.25] | 0.263 | |
| GC/GG | 80.30 ± 13.26 | (77.41, 83.28) | 78.00 [70.25–89.00] | |||
| TAS2R38 | rs713598 | CC | 81.30 ± 13.23 | (78.68, 83.99) | 78.00 [70.75–90.50] | 0.079 |
| GC/GG | 78.50 ± 13.85 | (76.29, 80.68) | 77.00 [67.50–88.00] | |||
| rs1726866 | AA | 81.12 ± 13.30 | (78.29, 83.96) | 78.00 [70.00–90.50] | 0.129 | |
| GA/GG | 78.38 ± 13.38 | (76.32, 80.45) | 77.00 [68.00–88.00] | |||
| rs10246939 | CC | 79.60 ± 13.80 | (74.70, 84.50) | 78.50 [71.00–86.50] | 0.880 | |
| TC/TT | 79.40± 13.40 | (77.60, 81.22) | 77.00 [68.50–89.00] | |||
| WHtR | ||||||
| TAS1R2 | rs35874116 | CC | 0.54 ± 0.09 | (0.50, 0.58) | 0.51 [0.46–0.61] | 0.358 |
| TC/TT | 0.52 ± 0.09 | (0.51, 0.53) | 0.50 [0.46–0.57] | |||
| rs9701796 | CC | 0.52 ± 0.08 | (0.50, 0.53) | 0.50 [0.45–0.57] | 0.227 | |
| GC/GG | 0.53 ± 0.09 | (0.50, 0.55) | 0.50 [0.46–0.58] | |||
| TAS2R38 | rs713598 | CC | 0.53 ± 0.09 | (0.51, 0.55) | 0.51 [0.47–0.59] | 0.122 |
| GC/GG | 0.52 ± 0.08 | (0.50, 0.53) | 0.49 [0.45–0.57] | |||
| rs1726866 | AA | 0.53 ± 0.09 | (0.51, 0.55) | 0.51 [0.47–0.59] | 0.141 | |
| GA/GG | 0.52 ± 0.08 | (0.50, 0.53) | 0.49 [0.46–0.57] | |||
| rs10246939 | CC | 0.54 ± 0.09 | (0.50, 0.57) | 0.52 [0.47–0.59] | 0.399 | |
| TC/TT | 0.52 ± 0.08 | (0.51, 0.53) | 0.50 [0.46–0.58] | |||
| SSB Consumption/Week | Gene | Predictor | Exp (B) | 95% CI | p-Value | Adjusted p-Value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| 2–3 times vs. ≤1 times | TAS1R2 | rs35874116 CC–TC/TT | 1.97 | 0.71 | 5.45 | 0.192 | 0.603 |
| rs9701796 CC–GC/GG | 0.85 | 0.45 | 1.63 | 0.630 | 0.967 | ||
| TAS2R38 | rs713598 CC–GC/GG | 1.01 | 0.54 | 1.92 | 0.967 | 0.967 | |
| rs1726866 AA–GA/GG | 0.96 | 0.50 | 1.85 | 0.894 | 0.967 | ||
| rs10246939 CC–TC/TT | 0.61 | 0.27 | 1.40 | 0.241 | 0.603 | ||
| >3 times vs. ≤1 times | TAS1R2 | rs35874116 CC–TC/TT | 1.13 | 0.35 | 3.69 | 0.841 | 0.957 |
| rs9701796 CC–GC/GG | 1.02 | 0.51 | 2.06 | 0.957 | 0.957 | ||
| TAS2R38 | rs713598 CC–GC/GG | 1.45 | 0.74 | 2.83 | 0.275 | 0.458 | |
| rs1726866 AA–GA/GG | 1.62 | 0.82 | 3.21 | 0.168 | 0.420 | ||
| rs10246939 CC–TC/TT | 0.24 | 0.08 | 0.79 | 0.018 | 0.090 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousef, R.; Alkazemi, D.U.Z.; Abu-Farha, M.; Abubaker, J.; Devarajan, S.; Rahman, A.; Al-Mulla, F. Associations of TAS1R2 and TAS2R38 Genetic Variants with Sugar-Sweetened Beverage Intake and Obesity Risk in Kuwaiti Adolescents: A Cross-Sectional Study. Children 2025, 12, 1192. https://doi.org/10.3390/children12091192
Yousef R, Alkazemi DUZ, Abu-Farha M, Abubaker J, Devarajan S, Rahman A, Al-Mulla F. Associations of TAS1R2 and TAS2R38 Genetic Variants with Sugar-Sweetened Beverage Intake and Obesity Risk in Kuwaiti Adolescents: A Cross-Sectional Study. Children. 2025; 12(9):1192. https://doi.org/10.3390/children12091192
Chicago/Turabian StyleYousef, Razan, Dalal Usamah Zaid Alkazemi, Mohamed Abu-Farha, Jehad Abubaker, Sriraman Devarajan, Abdur Rahman, and Fahd Al-Mulla. 2025. "Associations of TAS1R2 and TAS2R38 Genetic Variants with Sugar-Sweetened Beverage Intake and Obesity Risk in Kuwaiti Adolescents: A Cross-Sectional Study" Children 12, no. 9: 1192. https://doi.org/10.3390/children12091192
APA StyleYousef, R., Alkazemi, D. U. Z., Abu-Farha, M., Abubaker, J., Devarajan, S., Rahman, A., & Al-Mulla, F. (2025). Associations of TAS1R2 and TAS2R38 Genetic Variants with Sugar-Sweetened Beverage Intake and Obesity Risk in Kuwaiti Adolescents: A Cross-Sectional Study. Children, 12(9), 1192. https://doi.org/10.3390/children12091192










