Abstract
Macrophage activation syndrome (MAS), a hyperinflammatory condition driven by uncontrolled immune activation, is widely recognized as a critical complication in pediatric septic shock. This syndrome shares pathophysiological features with hemophagocytic lymphohistiocytosis (HLH) and other cytokine storm syndromes, and it contributes to significant morbidity and mortality in pediatric and adult patients. Hyperferritinemia—a hallmark of MAS—is not only a diagnostic clue but also a prognostic marker for poor outcomes in sepsis. High ferritin levels are strongly suggestive of MAS, yet even moderate elevations in combination with the trend of ferritin levels can be indicative of heightened mortality risk. Distinguishing MAS from severe sepsis or other hyperinflammatory syndromes in children (such as multisystem inflammatory syndrome in children (MIS-C)) can be challenging, as clinical features often overlap. However, early recognition and timely immunomodulatory therapy, particularly corticosteroids and targeted biologic agents, can be life-saving. Recent advances emphasize a syndromic approach to diagnosing MAS within the spectrum of hyperferritinemic sepsis, using scoring tools or MAS-specific criteria adapted to sepsis or MIS-C contexts. Ongoing studies aim to refine biomarker-based stratification and therapeutic algorithms. This review synthesizes current knowledge on MAS as a complication of sepsis, including the diagnostic importance of ferritin levels, differential diagnosis with other cytokine storm syndromes, and the latest therapeutic approaches. It underscores the importance of early suspicion and intervention to reverse immune dysregulation and improve outcomes in critically ill pediatric patients.
1. Introduction
Sepsis remains a leading cause of mortality in both pediatric and adult intensive care units worldwide [1]. Current treatments focus on eliminating the source of infection and supporting organ failure, while the proceeding inflammatory disease processes and pathobiology are still unclear. In recent years, attention has turned towards a subset of patients who develop an exaggerated immune response marked by hyperinflammation and multi-organ dysfunction [2]. Among these hyperinflammatory syndromes, macrophage activation syndrome (MAS)—a form of secondary hemophagocytic lymphohistiocytosis (HLH)—has emerged as a pivotal driver of disease severity and poor outcomes in septic shock [3]. MAS is characterized by uncontrolled activation of macrophages and T-lymphocytes, resulting in a cytokine storm, hemophagocytosis, and high circulating ferritin levels [4]. Ferritin, in this context, serves as both a biomarker and a pathogenic mediator, and its measurement can aid in both early detection and prognostic stratification of septic patients at risk of MAS [5].
Despite its clinical importance, MAS remains under-recognized in sepsis, partly due to overlapping features with other inflammatory conditions such as severe bacterial infections, MIS-C, or autoimmune flares [4]. Furthermore, diagnostic criteria for MAS and HLH were originally developed in pediatric oncology or rheumatology populations, making them imperfect tools for sepsis-related presentations [6,7]. As our understanding of cytokine storm syndromes expands, there is a growing consensus that MAS in sepsis should be conceptualized as part of a broader spectrum of hyperferritinemic conditions, each requiring timely immunomodulatory therapy [8,9]. This review explores the pathophysiology, diagnostic role of ferritin, differential diagnoses, and therapeutic strategies for MAS in septic contexts, with special attention to recent advances in pediatric clinical practice.
2. Pathophysiology of MAS in the Context of Sepsis
MAS is an extreme immune response wherein excessive activation of T-lymphocytes and macrophages leads to a cytokine storm and multi-organ damage. In the context of septic shock, a subset of patients develops a “sepsis-associated HLH/MAS” phenotype—sometimes termed macrophage activation-like syndrome (MALS)—characterized by persistent fever, cytopenias, coagulopathy, hepatosplenomegaly, and hyperferritinemia [10]. Phenotypes based on the inflammatory profile of children with sepsis-induced multiple organ failure have been described, where MALS represents the final common pathway of uncontrolled inflammation [11]. The driving immunopathology involves uncontrolled cytokine release (e.g., IL-1β, IL-6, IL-18, IFN-γ) and impaired cytotoxic cell function, resulting in the accumulation of activated macrophages that perform hemophagocytosis (engulfing blood cells in bone marrow and spleen) [12] (Figure 1). These macrophages release massive amounts of ferritin—an acute phase reactant—under the influence of inflammatory cytokines such as TNF, IL-1, and IL-6, which upregulate ferritin synthesis via NF-κB pathways [13]. Autopsy studies of adults who died of sepsis have directly linked hemophagocytic macrophage infiltration in organs to the pattern of multi-organ failure (particularly hepatic dysfunction and disseminated intravascular coagulation (DIC)) seen in fatal septic shock [12]. Notably, such patients had exceedingly high circulating levels of IL-1, IL-6, IL-8, and IL-10, reflecting a cytokine storm indistinguishable from classical MAS [12]. Thus, in septic shock (both pediatric and adult), hyperferritinemia can be a biomarker of a MAS-like hyperinflammatory state driving organ failure. This hyperinflammatory syndrome is often referred to collectively as a “Cytokine Storm Syndrome” (CSS), a category that includes MAS, secondary HLH, and other hypercytokinemic conditions [10]. Importantly, the syndrome feeds on itself: high cytokine levels both result from and further activate macrophages and lymphocytes, unless interrupted by therapy. The end result, if untreated, is progressive multiple organ dysfunction, immune paralysis, and death in a significant fraction of cases [3,13].
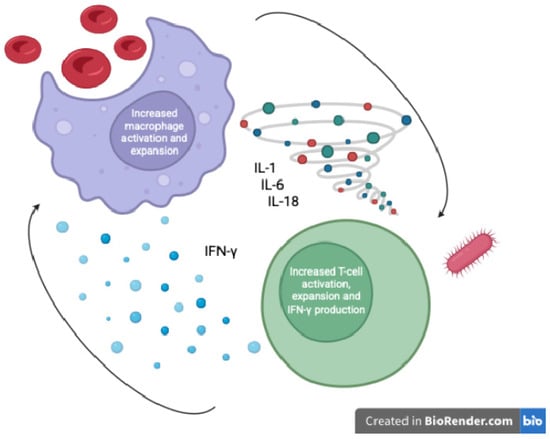
Figure 1.
Macrophage activation and hyperferritinemia in pediatric septic shock (author-created schematic).
3. Diagnostic Role and Prognostic Value of Serum Ferritin
Serum ferritin is a central diagnostic and prognostic marker in HLH/MAS and is often the first red flag for hyperinflammation in a septic patient. Marked hyperferritinemia is included as a criterion in all HLH/MAS diagnostic frameworks [14] (Table 1). The conventional HLH-2004 criteria were originally developed by the Histiocyte Society and consider ferritin ≥ 500 ng/mL as one of eight diagnostic criteria aiming to detect familial (primary) HLH [6]. In 2016, the Paediatric Rheumatology International Trials Organisation (PRINTO) introduced updated classification criteria for MAS associated with systemic juvenile idiopathic arthritis (sJIA). These criteria use a higher ferritin cutoff (>684 ng/mL) [4,14] and have also been proven helpful in identifying MAS in the context of other pediatric inflammatory conditions such as Kawasaki disease (KD) and juvenile dermatomyositis (JDM) [7]. In 2014, Fardet et al. invented a scoring system (HScore) different from the HLH-2004 criteria by incorporating variables such as aspartate aminotransferase (AST) levels and underlying immunosuppression, while excluding assessments of NK cell function and sCD25 levels. HScore has 93% sensitivity and 86% specificity for diagnosing MAS and has been widely used since [15]. Lately, Avrusin et al. in 2024 elaborated diagnostic criteria allowing for the differentiation of MAS in MIS-C patients with a sensitivity of 100% and a specificity of 94.9% [16,17]. These oncology- and rheumatology-derived tools have important limitations in septic shock, with substantial risk of misclassification in children; sepsis-adapted, pediatric-specific criteria are needed.
In practice, patients with true MAS in sepsis usually manifest far higher levels: ferritin > 1000 ng/mL, and extreme hyperferritinemia (often into the several thousands) is common [4]. Critically ill children presenting with sepsis and MAS manifestations demonstrated markedly elevated ferritin levels (623–28,931 ng/mL) prior to intervention, as documented in a recent study [18]. Pediatric rheumatology data indicate that ferritin levels > 10,000 ng/mL are >90% sensitive and specific for MAS in children, often considered virtually pathognomonic of HLH/MAS [4]. Adult studies similarly note that ferritin value above 3000–5000 ng/mL in the context of septic shock or inflammatory illness is highly concerning for a hyperferritinemic syndrome (MAS/HLH) [12]. Indeed, one adult series coined the term “hyperferritinemic syndrome” for patients with ferritin above 3000 ng/mL presenting with features of MAS (such as those related to adult-onset Still’s disease, catastrophic antiphospholipid syndrome, or septic shock) [12].
Beyond its role in diagnosis, ferritin provides prognostic information in septic shock. Multiple studies over the past two years have reinforced that even moderate elevations (e.g., >500–1000 ng/mL) are associated with worse outcomes in sepsis [12], and recent studies in pediatric populations with severe sepsis set lower cutoffs for ferritin levels (500–705 ng/mL) to predict mortality risk [19,20]. In a multicenter pediatric cohort, children with “hyperferritinemic sepsis” (ferritin > 500 ng/mL) had a fourfold higher odds of mortality than those without hyperferritinemia (23% vs. 5.7% mortality) [12]. Notably, mortality rose stepwise with ferritin strata: children with ferritin ≥ 10,000 had extremely poor outcomes [12]. Similarly, extreme ferritin elevations in adults with septic shock correlate with a high risk of death; one report found the highest ferritin values (often >9000 ng/mL) predominantly in non-survivors [16]. Moreover, Carcillo et al. proposed the use of combination of ferritin and C-reactive protein to assess the risk of mortality and the treatment effect of sepsis in children [11] Apart from a one-time cutoff, ferritin kinetics are important: a sharp rise in ferritin during the course of sepsis can herald impending MAS [12], whereas a decline with therapy often signals response. In the PROVIDE trial, ferritin concentration remained significantly elevated, even after immunomodulatory treatment (anakinra) was discontinued in critically ill adult patients with MALS [17]. Based on this evidence, extended immunomodulatory therapy until normalization of ferritin levels was proposed. Serial ferritin tracking is therefore useful to monitor disease activity and therapeutic response, and a failure of ferritin to fall (or a secondary spike) should prompt escalation of immunomodulatory treatment [4]. That said, clinicians must remember that ferritin is an acute phase reactant and not specific to HLH—it can be elevated in typical bacterial sepsis, liver failure, malignancy, or trauma. For example, one retrospective analysis of 163 hospitalized children with ferritin above the HLH-2004 cutoff found that only ~5% had HLH, and even when ferritin level was above 10,000 ng/mL the positive predictive value for HLH was only 18% [18]. Thus, hyperferritinemia should always prompt inclusion of HLH/MAS in the differential (especially when very high), but diagnosis still requires compatible clinical features and lab constellation [19]. In practice, a threshold like ferritin > 500 or >1000 ng/mL in septic shock should trigger a systematic evaluation for HLH/MAS (e.g., applying an HScore (Table 1) or checking other criteria such as cytopenias, fibrinogen, soluble IL-2 receptor) [4]. Values climbing into the thousands demand urgent consideration of immunosuppressive therapy, even in parallel with infectious workup.

Table 1.
Diagnostic criteria for MAS/HLH in children and ferritin levels.
Table 1.
Diagnostic criteria for MAS/HLH in children and ferritin levels.
| Citeria Set | Ferritin (ng/mL) | Other Key Criteria | Diagnosis | Notes |
|---|---|---|---|---|
| HLH-2004 | ≥500 | Cytopenias, splenomegaly, hypertriglyceridemia, hemophagocytosis, low NK cell activity, high sCD25 (sIL-2R) | 5 out of 8 key criteria | Not specific for MAS; used in oncology [6] |
| MAS-2016 (sJIA) | >684 | Platelets ≤ 181 × 109/L, AST > 48, TG > 156, Fibrinogen ≤ 360 | High ferritin + ≥2/4 criteria | Validated in sJIA; bedside criteria [7] |
| MIS-C MAS (2024) | >469 | Platelets < 114 × 109/L, splenomegaly, CNS symptoms, hypotension | Ferritin > 469 AND Platelets < 114 × 109/L, Exclude other shock syndromes | Preliminary; tailored to MIS-C context [21] |
| HScore (Points) | >2000 ng/mL | Cytopenias, hepatosplenomegaly, fever, hypertriglyceridemia, elevated AST, low fibrinogen, hemophagocytosis, known immunosuppression | Points assigned to features; Sum of points ≥ 169 | 93% sensitivity and 86% specificity for diagnosing MAS [22] |
MAS, macrophage activation syndrome; HLH, hemophagocytic lymphohistiocytosis; HScore, hemophagocytic syndrome diagnostic score; MIS-C, multisystem inflammatory syndrome in children; CNS, central nervous system; AST, aspartate aminotransferase; TG, triglycerides; sCD25 (sIL-2R), soluble interleukin-2 receptor; NK, natural killer.
4. Differentiating MAS, HLH, and Other Cytokine Storm Syndromes
MAS and secondary HLH are overlapping entities—some experts use the terms interchangeably when referring to hyperinflammatory syndromes. Technically, HLH is the broad syndrome of hemophagocytic lymphohistiocytosis, which can be familial (primary) due to genetic mutations or secondary to triggers like infection, malignancy, or autoimmune disease [4]. MAS is often used to describe HLH arising in the context of rheumatologic disease (classically systemic JIA in children or adult-onset Still’s disease), but mechanistically it is a form of secondary HLH [23]. In septic shock, the hyperinflammatory state has been called “sepsis-associated HLH” by hematologists and simultaneously labeled “MAS” by rheumatologists—underscoring that these syndromes lie on the same spectrum [24]. Indeed, a severe case of hyperferritinemic septic shock with DIC, hepatobiliary dysfunction, cytopenias, and high fevers might meet diagnostic criteria for HLH and MAS simultaneously, even though historically HLH criteria were designed for pediatric oncology settings and MAS criteria for rheumatic disease flares. Differentiating an HLH/MAS flare from “just severe sepsis” can be challenging; they often mimic each other, and in fact may coexist. Key clues favoring HLH/MAS include disproportionately high ferritin levels, trilineage cytopenias not explained by dilution or DIC alone, hypofibrinogenemia (fibrinogen consumption from hemophagocytosis), and evidence of immune dysregulation such as low NK cell activity or very high soluble IL-2 receptor (sCD25) [10]. In children, the presentation of primary viral HLH or MAS can initially appear like severe septic shock with multi-organ dysfunction syndrome (MODS), but such patients often fail to improve with standard antiviral/supportive care. A useful bedside prompt from recent guidelines is to remember the “three Fs” when standard therapies are not working: fever, falling blood counts, and high ferritin [23]. The presence of all three should raise suspicion for HLH/MAS in a deteriorating patient, either pediatric or adult, and should expedite consultation with hematology/rheumatology and initiation of HLH-directed workup.
It is also important to distinguish MAS/HLH from other cytokine storm syndromes that share features but have different contexts. For instance, cytokine release syndrome (CRS) in CAR-T cell therapy or severe COVID-19 can cause fever, ferritin elevation, and organ dysfunction, but the management might differ (tocilizumab is first-line in CAR-T CRS, whereas HLH requires broader immunosuppression). Likewise, Kawasaki disease or toxic shock syndrome in children can present with hyperinflammation and even hemophagocytosis in some cases [14], yet these conditions are not primarily disorders of immune cell cytotoxicity. Overlap is frequent—for example, a child with severe Kawasaki disease or Epstein–Barr virus infection can demonstrate secondary HLH, and conversely, a patient with genetic HLH may be tipped into a flare by a common infection. Thus, clinicians must maintain a high index of suspicion and often pursue parallel diagnoses, e.g., treating presumed sepsis and investigating HLH simultaneously.
Modern scoring systems like the HScore (which assigns points for fever, organomegaly, cytopenias, ferritin level, triglycerides, fibrinogen, etc.) are used in adults to estimate the probability of HLH in critically ill patients [3,12]. In children, updated classification criteria (MAS-2016 for sJIA, etc.) help identify MAS earlier, and they intentionally exclude findings like hemophagocytosis (which may or may not be present on bone marrow biopsy) because waiting for a marrow aspirate can delay life-saving treatment [4]. In short, MAS, HLH, and related cytokine storm syndromes should be thought of as a spectrum of hyperinflammation, with the specific label varying with context (infection triggered, malignancy triggered, or autoimmune triggered). In the bottom line, they all share common pathways and benefit from prompt immunomodulation. Differentiating among them is less crucial than recognizing the syndrome and treating it early, while also addressing any underlying cause.
5. Overlaps with MIS-C and Pediatric Hyperinflammation
The COVID-19 pandemic revealed that viral infections can serve as potent triggers for HLH pathogenesis, with numerous patients developing secondary HLH/MAS, which worsened prognosis [25]. Specifically, MIS-C is a post-infectious hyperinflammatory condition associated with COVID-19 that has pathophysiologic overlap with MAS/HLH. Children with MIS-C often present with fever, shock or hypotension, elevated inflammatory markers (CRP, D-dimer, ferritin), and multi-organ involvement—features that can fulfill criteria for MAS. Indeed, MAS has been reported as a complication in MIS-C cases, contributing to more severe courses [16]. However, MIS-C is a distinct entity with specific diagnostic criteria (e.g., history of SARS-CoV-2 exposure, marked cardiac involvement like ventricular dysfunction or coronary aneurysms, etc.), so it is important not to mislabel every MIS-C case as HLH. The two syndromes can be concomitant: one analysis found that about 10–15% of MIS-C patients clearly exhibit MAS by expert review, and these children tended to be older and had more profound shock, cytopenias, and coagulopathy [16]. Because traditional HLH/MAS criteria (HLH-2004, MAS-2005, MAS-2016) were developed in other populations, they may not fully capture MIS-C-associated MAS. A 2024 study proposed preliminary, tailored criteria for MAS in MIS-C (Table 1): notably, ferritin > 469 μg/L and platelet count < 114×109/L were identified as simple cutoffs that discriminated MIS-C with MAS (sensitivity 100%, specificity 94.9%) [16]. This low ferritin threshold (≈500) reflects that even moderate hyperferritinemia in MIS-C could indicate MAS on top of the baseline inflammation. Other signs like hypotension, splenomegaly, and CNS dysfunction were also more common in MIS-C patients with MAS [16]. The overlap of MIS-C and MAS has practical implications: both conditions respond to immunomodulatory therapy. In fact, standard MIS-C treatment already includes intravenous immunoglobulin (IVIG) and glucocorticoids, and in refractory cases, IL-1 blockade (anakinra) has been used, which is the same armamentarium used for HLH/MAS. Thus, clinicians managing MIS-C should be vigilant for signs of macrophage activation (e.g., dropping platelets with rising ferritin), since this may prompt escalation to high-dose steroids or second-line agents sooner [14,16]. From a research perspective, MIS-C provides a unique window into cytokine storm syndromes, as it shares features with classic HLH/MAS (e.g., hyperferritinemia, cytopenias) but also has differences (e.g., profound IL-17A and IL-10 elevations, frequent cardiac involvement). Ongoing studies are comparing the cytokine and proteomic profiles of MIS-C versus other hyperferritinemic syndromes [14], which may refine our diagnostic and therapeutic approaches. For now, MIS-C is best thought of as part of the hyperinflammation spectrum; when a child with MIS-C is critically ill or not improving, clinicians should strongly consider MAS and treat accordingly, rather than viewing MIS-C and HLH/MAS as mutually exclusive diagnoses.
MAS in the Context of Zoonotic and Viral Infections
Beyond bacterial sepsis and MIS-C, zoonotic and viral pathogens can trigger MAS, including Crimean–Congo hemorrhagic fever, brucellosis, Q fever, rickettsioses, and influenza. Management balances urgent immunomodulation (e.g., corticosteroids, anakinra) with targeted antimicrobial/antiviral therapy and source control. Recognizing these associations—particularly in endemic regions—can expedite diagnosis and tailored care [25,26,27,28].
6. Therapeutic Approaches and Recent Advances
Despite the extensive research conducted over the past years on the host’s dysregulated immune response in sepsis, immunomodulating agents have failed to show clear therapeutic success. This lack of benefit is primarily due to marked heterogeneity of the clinical and immunological phenotypes of sepsis. Early characterization of precise immune dysregulation could enable personalized treatment strategies using adjunctive immunotherapeutic approaches [17]. Particularly managing MAS/HLH in septic shock, prompt immunomodulatory therapy is the cornerstone, alongside optimal supportive care and treatment of the inciting cause. Given the life-threatening nature of HLH/MAS, therapies are often initiated empirically (based on high ferritin and clinical suspicion) without waiting for complete diagnostic confirmation [4]. Key elements of management include the following (Table 2):

Table 2.
Pediatric-specific summary of MAS in septic shock.
- (i)
- Concurrent Infection Control: In sepsis-associated HLH, appropriate antibiotics or antivirals must be administered to address the trigger, even if immunosuppressive therapy is started [4]. The 2024 consensus is that controlling the known or suspected trigger (e.g., broad-spectrum antibiotics for bacterial sepsis) should happen in parallel with HLH treatment, not in sequence [47]. Similarly, removal of potential triggers (draining abscesses, stopping culprit drugs, or treating malignancy if present) is crucial.
- (ii)
- Supportive Care: Patients often require ICU support for organ dysfunction (vasoactive support in shock, ventilation for acute respiratory distress syndrome (ARDS), dialysis for acute kidney impairment (AKI)). Coagulopathy and cytopenias in MAS may necessitate blood product transfusions [43]. Aggressive supportive care cannot replace immunotherapy in HLH, but it can buy crucial time for it to take effect. Prognostic discussions should reflect that early immunomodulation has the potential to dramatically reverse even severe organ failure.
- (iii)
- Corticosteroids: High-dose corticosteroids remain the backbone therapy for both pediatric and adult HLH/MAS [13]. Steroids have broad anti-inflammatory effects and speed the suppression of cytokine production. Typical regimens include IV methylprednisolone (for example, 1–2 mg/kg up to pulse doses ~30 mg/kg/day for 3–5 days in severe cases) [5]. In critically ill patients, steroids are often started at diagnosis; if HLH is confirmed, a dexamethasone-based regimen (as per HLH-94 protocol) may be continued. Rapid improvement in fever spikes, ferritin levels, and clinical status often follows steroid initiation if HLH/MAS is the correct diagnosis.
- (iv)
- Intravenous Immunoglobulin (IVIG): IVIG is commonly given in pediatric hyperinflammatory syndromes, such as Kawasaki disease and MIS-C, and has been used in infection-associated HLH as well. IVIG can help neutralize pathogens and provide immune modulation. Some protocols incorporate IVIG 1–2 g/kg, especially when an underlying infection like EBV is suspected, or in MAS complicating Kawasaki disease or sepsis [14]. However, IVIG is not recommended as a standard treatment in sepsis treatment guidelines due to its lack of therapeutic benefit [33]. More research is needed on this field in septic patiets with MALS. While IVIG alone is usually insufficient to control full-blown HLH, it may serve as a useful adjunct in milder cases or as a temporary bridge in resource-limited settings.
- (v)
- Etoposide-Based Therapy: Etoposide, a chemotherapeutic agent, is a main component of the standard HLH protocol (HLH-94 and HLH-2004), as it induces apoptosis of overactive immune cells (particularly T cells). In patients with established HLH (especially familial or malignancy-associated HLH), etoposide + dexamethasone therapy significantly improves survival and is considered definitive therapy [35,47]. However, in sepsis-associated HLH, the decision to use etoposide is nuanced due to concerns about severe myelosuppression and infection risk in an already septic patient. Recent practice has been trending towards using biologic immunomodulators (like anakinra) first and reserving etoposide for refractory cases [4]. Nonetheless, if a patient fails to respond to first-line therapy or if genetic HLH is strongly suspected, experts will initiate etoposide even in adults. The 2024 HLH consensus guidelines affirm that HLH-94 therapy can be life-saving in adults with secondary HLH, despite historically being a pediatric regimen [35]. Thus, etoposide remains in the arsenal, but newer therapies have thankfully reduced the frequency with which it is needed in sepsis/MAS scenarios.
- (vi)
- Cyclosporine A (CSA): CSA, a calcineurin inhibitor, is another conventional HLH/MAS treatment that suppresses T-cell activation. In rheumatology, CSA is often combined with steroids as first-line for MAS in sJIA. In infection-associated HLH, CSA can be added early, especially if there is only a partial steroid response. However, practice is shifting—some centers favor IL-1 blockade (anakinra) instead of CSA up front, due to CSA’s renal/hepatic toxicities and slower onset [48]. For example, many institutions in the United States now start anakinra with high-dose steroids as initial therapy and reserve cyclosporine for later [4]. CSA may still be used as an adjunct if needed (e.g., ongoing MAS features after anakinra and steroids), at doses ~2–7 mg/kg/day, aiming for therapeutic trough levels.
- (vii)
- IL-1 Blockade (Anakinra): Interleukin-1 is a key pro-inflammatory cytokine in MAS, and anakinra (recombinant IL-1 receptor antagonist) has emerged as a front-line therapy for hyperferritinemic syndromes. Anakinra has the advantages of a quick onset, short half-life, and a favorable safety profile even in infection. It blocks IL-1-mediated inflammation without broadly suppressing adaptive immunity [49]. A pivotal post hoc analysis in adults with septic shock and secondary HLH features showed that adding anakinra reduced mortality, especially in those with DIC and hepatobiliary dysfunction [12]. Moreover, the PROVIDE trial demonstrated that, based on specific immune phenotypes along with other parameters in septic adults, anakinra treatment led to improved clinical outcomes [50]. In pediatric MAS, case series have reported rapid remission of hyperinflammation with anakinra, including refractory cases where steroids and CSA had failed [4,18,39]. By 2024, consensus recommendations frequently list anakinra as the preferred second-line agent for HLH/MAS if there is an inadequate response to steroids [49]. Many experts will initiate anakinra concurrently with steroids in fulminant cases, given the high lethality of MAS [51]. Dosing is higher than typical rheumatoid dosing—often 4–8 mg/kg/day, divided 2–4 times daily or given as a continuous infusion in critically ill patients [49]. High-dose anakinra has been used safely in septic shock trials (even up to 48 mg/kg/day IV) [49]. The only real drawbacks are logistical (need for repeated dosing or infusion) and cost. Overall, anakinra has become a pillar of therapy, bridging the gap between traditional immunosuppressants and targeted biologics. Early use of anakinra in sepsis-associated HLH can halt the progression of the cytokine storm and has been associated with recovery of organ function in many reports [12].
- (viii)
- IL-6 Inhibition: Tocilizumab (anti-IL-6 receptor) is well-known for treating cytokine storms in CAR-T cell therapy and severe COVID-19. IL-6 is often markedly elevated in MAS; although IL-1 blockade is usually favored, tocilizumab has been used in refractory MAS. Case reports suggest it can be effective when ferritin remains high despite anakinra [41,42]. Caution is warranted, as IL-6 inhibition can mask fever and cause paradoxical increases in serum ferritin (making clinical monitoring tricky). Currently, evidence from clinical trials remains insufficient to support the use of tocilizumab in sepsis treatment.
- (ix)
- Interferon-γ Neutralization: Emapalumab, a monoclonal antibody against IFN-γ, was approved for primary pediatric HLH and has shown activity in secondary HLH as well. IFN-γ is a central driver of macrophage activation, so its blockade can dampen the HLH cascade [44]. Due to limited availability and cost, emapalumab is generally reserved for refractory cases or considered in known familial HLH [44].
- (x)
- JAK Inhibitors: Ruxolitinib, a JAK1/2 inhibitor, can broadly suppress the cytokine circuit by interfering with signaling of multiple interleukins and interferons. Emerging evidence (case series and phase II trials) indicates that ruxolitinib can salvage patients with refractory HLH [45]. It has been incorporated into some clinical protocols (e.g., the HLH-94 “RUXO” experimental arm) [45] and is being studied in combination with steroids in secondary HLH. As of 2025, ruxolitinib is not yet standard first-line therapy but is a promising option for severe cases not responding to IL-1/IL-6 blockers and steroids.
- (xi)
- Other Targets: In specific scenarios, additional therapies are considered. For Epstein–Barr virus-driven HLH (often seen in teens/adults), rituximab (anti-CD20 monoclonal) can help clear EBV-infected B cells and has been added to HLH regimens [4]. Intravenous etoposide, as noted, remains a key option for cytotoxic debulking of the immune response. Experimental approaches like anti-IL-18 (tadekinig alfa) are under investigation given the extraordinarily high IL-18 levels in some MAS patients [10]. Lastly, extracorporeal blood purification therapies are a group of treatments that may modulate the host’s inflammatory response by removing inflammatory mediators and/or circulating bacterial toxins. However, significant practical challenges remain and require consideration. Plasmapheresis has been used in hyperferritinemic sepsis to remove inflammatory mediators—some centers report stabilization in otherwise refractory cases, though evidence is not yet definitive [48].
Clinical Course and Outcomes: With these therapies, survival in HLH/MAS has improved markedly over the past two decades. Where historically HLH was 95% fatal, recent multicenter experiences report survival rates on the order of 60–80% even in critical illness. Nonetheless, in the septic shock population, MAS confers significant mortality risk. As mentioned, pediatric septic shock with MAS had ~4-fold increased mortality [12], and adult septic shock patients meeting HLH criteria have reported death rates of 50% or higher despite ICU care. Early recognition and treatment are therefore vital. In 2024, an international consensus (HiHASC Collaboration) published guidelines stressing a systematic approach to recognize HLH/MAS early, using the 3-F mnemonic and urgent diagnostic evaluation, and to institute therapy promptly even while awaiting confirmatory tests [23] (Table 3). Very recent ongoing trials are testing some of the above biologics (e.g., anakinra, emapalumab, ruxolitinib) in controlled settings for sepsis-associated hyperinflammation [52]. The hope is that by tamping down the “friendly fire” of the immune system in septic shock, we can improve outcomes without compromising infection control. The current paradigm is multi-disciplinary management involving intensivists, hematologists, rheumatologists, and infectious disease specialists to tailor treatment for each patient. Every septic shock patient with unexplained cytopenias, organ failure, and high ferritin represents a race against time to diagnose MAS/HLH. The latest literature reinforces that vigilance and early immunomodulation save lives in this hyperferritinemic subset of sepsis [12,38,53] (Figure 2).

Table 3.
Therapeutic Options for HLH/MAS in Septic Shock.
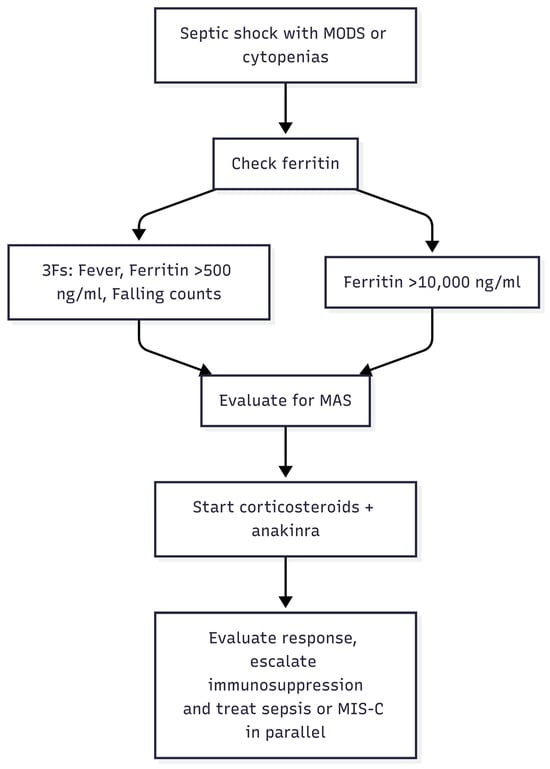
Figure 2.
Recognition and Treatment Algorithm for MAS in Septic Shock. MODS, multiorgan dysfunction syndrome; MAS, macrophage activation syndrome; MIS-C, multisystem inflammatory syndrome in children.
Table 4. Sepsis-adapted prompts and diagnostic pathway (HiHASC 2023 ‘3-F’ mnemonic—fever, falling blood counts, ferritin), with suggested escalation steps for pediatric septic shock complicated by MAS (Cox et al., 2024 [23]).

Table 4.
Sepsis-adapted prompts and diagnostic pathway (HiHASC 2023 ‘3-F’ mnemonic).
7. Conclusions and Future Directions
Hyperinflammatory syndromes in children are life-threatening manifestations of immune dysregulation, and their overlap with severe infections, autoimmune flares, and MIS-C continues to obscure timely recognition. Despite increasing awareness, progress in diagnosis and treatment has been fragmented, and most tools remain extrapolated from adult or oncology-based HLH cohorts.
Future priorities must include the development of pediatric-specific, sepsis-adapted diagnostic criteria, informed by ferritin kinetics and integrated with cytokine and immunophenotyping signatures. Equally urgent is the design of biomarker-driven, prospective clinical trials to define when and how to use corticosteroids, biologics, and emerging targeted agents in children.
To translate these advances into survival benefit, the field must adopt risk-stratification algorithms and protocolized treatment pathways, tested in real-world pediatric critical care settings and disseminated globally. Education of pediatric intensivists and frontline teams is critical to ensure early recognition and uniform care.
The next phase requires a shift from merely recognizing MAS to delivering precision immunotherapy within standardized sepsis care protocols—a step that can transform outcomes for critically ill children worldwide. Achieving this transition—from descriptive recognition to precision, protocolized immunotherapy—represents the most important opportunity in decades to change the natural history of hyperinflammatory shock in children.
Author Contributions
Conceptualization, E.B.; methodology, E.B. and N.S.; investigation, E.B.; resources, E.B.; writing—original draft preparation, E.B.; writing—review and editing, N.S. and S.I.; visualization (tables and figures), N.S.; supervision, S.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| MAS | Macrophage Activation Syndrome |
| HLH | Hemophagocytic Lymphohistiocytosis |
| MIS-C | Multisystem Inflammatory Syndrome in Children |
| MALS | Macrophage Activation-Like Syndrome |
| DIC | Disseminated Intravascular Coagulation |
| IVIG | Intravenous Immune Globulin |
| MODS | Multi-Organ Dysfunction Syndrome |
| CSA | Cyclosporine A |
| NK | Natural Killer |
| CRS | Cytokine Release Syndrome |
| CSS | Cytokine Storm Syndrome |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| IV | Intravenous |
| IL-1 | Interleukin 1 |
| IL-6 | Interleukin 6 |
| IFN-γ | Interferon Gamma |
References
- Kernan, K.F.; Ghaloul-Gonzalez, L.; Shakoory, B.; Kellum, J.A.; Angus, D.C.; Carcillo, J.A. Adults with Septic Shock and Extreme Hyperferritinemia Exhibit Pathogenic Immune Variation. Genes Immun. 2019, 20, 520–526. [Google Scholar] [CrossRef]
- Pal, P.; Bathia, J.; Giri, P.P.; Roy, M.; Nandi, A. Macrophage Activation Syndrome in Pediatrics: 10 Years Data from an Indian Center. Int. J. Rheum. Dis. 2020, 23, 1412–1416. [Google Scholar] [CrossRef]
- Benlamkaddem, S.; Doughmi, D.; Tlamçani, I.; Berdai, M.A.; Harandou, M. The Challenging Aspect of Macrophage Activation Syndrome in the Setting of Sepsis or Systemic Inflammatory Response Syndrome (SIRS). Cureus 2023, 15, e36228. [Google Scholar] [CrossRef]
- Lee, J.; Bae, K.S.; Rhim, J.W.; Lee, S.-Y.; Jeong, D.C.; Kang, J.H. Macrophage Activation Syndrome in Children: Update on Diagnosis and Treatment. Children 2024, 11, 755. [Google Scholar] [CrossRef]
- Valerie, I.C.; Prabandari, A.A.S.M.; Wati, D.K. Ferritin in Pediatric Critical Illness: A Scoping Review. Clin. Exp. Pediatr. 2023, 66, 98–109. [Google Scholar] [CrossRef]
- Henter, J.-I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and Therapeutic Guidelines for Hemophagocytic Lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef]
- Ravelli, A.; Minoia, F.; Davì, S.; Horne, A.; Bovis, F.; Pistorio, A.; Aricò, M.; Avcin, T.; Behrens, E.M.; De Benedetti, F.; et al. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. 2016, 68, 566–576. [Google Scholar] [CrossRef]
- Schuster, F.S.; Nyvlt, P.; Heeren, P.; Spies, C.; Adam, M.F.; Schenk, T.; Brunkhorst, F.M.; Janka, G.; La Rosée, P.; Lachmann, C.; et al. Differential Diagnosis of Hyperferritinemia in Critically Ill Patients. J. Clin. Med. 2022, 12, 192. [Google Scholar] [CrossRef]
- Tang, S.; Li, S.; Zheng, S.; Ding, Y.; Zhu, D.; Sun, C.; Hu, Y.; Qiao, J.; Fang, H. Understanding of Cytokines and Targeted Therapy in Macrophage Activation Syndrome. Semin. Arthritis Rheum. 2021, 51, 198–210. [Google Scholar] [CrossRef]
- Carol, H.A.; Mayer, A.S.; Zhang, M.S.; Dang, V.; Varghese, J.; Martinez, Z.; Schneider, C.; Baker, J.; Tsoukas, P.; Behrens, E.M.; et al. Hyperferritinemia Screening to Aid Identification and Differentiation of Patients with Hyperinflammatory Disorders. J. Clin. Immunol. 2025, 45, 4. [Google Scholar] [CrossRef]
- Carcillo, J.A.; Berg, R.A.; Wessel, D.; Pollack, M.; Meert, K.; Hall, M.; Newth, C.; Lin, J.C.; Doctor, A.; Shanley, T.; et al. A Multicenter Network Assessment of Three Inflammation Phenotypes in Pediatric Sepsis-Induced Multiple Organ Failure. Pediatr. Crit. Care Med. 2019, 20, 1137–1146. [Google Scholar] [CrossRef]
- Fan, Z.; Kernan, K.F.; Qin, Y.; Canna, S.; Berg, R.A.; Wessel, D.; Pollack, M.M.; Meert, K.; Hall, M.; Newth, C.; et al. Hyperferritinemic Sepsis, Macrophage Activation Syndrome, and Mortality in a Pediatric Research Network: A Causal Inference Analysis. Crit. Care 2023, 27, 347. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Kim, Y.T.; Jeong, G.; Jin, M. Immunopathology of and Potential Therapeutics for Secondary Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome: A Translational Perspective. Exp. Mol. Med. 2024, 56, 559–569. [Google Scholar] [CrossRef]
- Inguscio, G.; Romano, S.; Mastrolia, M.V.; Simonini, G.; Giani, T. Macrophage Activation Syndrome in Kawasaki Disease: Insights from a Systematic Literature Review on Diagnosis, Clinical Features, and Treatment. Children 2025, 12, 349. [Google Scholar] [CrossRef]
- Fardet, L.; Galicier, L.; Lambotte, O.; Marzac, C.; Aumont, C.; Chahwan, D.; Coppo, P.; Hejblum, G. Development and Validation of the HScore, a Score for the Diagnosis of Reactive Hemophagocytic Syndrome. Arthritis Rheumatol. 2014, 66, 2613–2620. [Google Scholar] [CrossRef]
- Avrusin, I.S.; Bregel, L.V.; Efremova, O.S.; Kostik, M.M. Development of Preliminary Criteria of Macrophage Activation Syndrome in Multisystem Inflammatory Syndrome Associated with COVID-19 in Children. Biomedicines 2024, 12, 2868. [Google Scholar] [CrossRef]
- Day-Lewis, M.; Berbert, L.; Baker, A.; Dionne, A.; Newburger, J.W.; Son, M.B.F. Updated Case Definition of MIS-C and Implications for Clinical Care. Pediatrics 2024, 153, e2023063259. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Kruse, K.; Kovey, K.; Davis, A.T.; Hassan, N.E.; Ndika, A.N.; Zuiderveen, S.; Birmingham, J. Therapeutic Role of Anakinra, an Interleukin-1 Receptor Antagonist, in the Management of Secondary Hemophagocytic Lymphohistiocytosis/Sepsis/Multiple Organ Dysfunction/Macrophage Activating Syndrome in Critically Ill Children. Pediatr. Crit. Care Med. 2014, 15, 401–408. [Google Scholar] [CrossRef]
- Lal, M.; Goel, M.; Shrivastava, N.; Pal, P.K.; Datta, M. Serum Ferritin Levels as a Prognostic Marker for Predicting Outcomes in Children with Severe Sepsis and Their Correlation With Pediatric Sequential Organ Failure Assessment Score. Cureus 2025, 17, e84436. [Google Scholar] [CrossRef]
- Pai, K.; Angurana, S.K.; Nallasamy, K.; Muralidharan, J.; Bhatia, P.; Rawat, A. Serum Ferritin Levels in Critically Ill Children with Severe Sepsis and Their Relationship with Mortality: A Prospective Observational Study (FerriPedS Study). Pediatr. Infect. Dis. J. 2025. [Google Scholar] [CrossRef]
- Khoury, M. Multisystem Inflammatory Syndrome in Children—Emerging Insights from Large Datasets. JAMA Netw. Open 2025, 8, e2456229. [Google Scholar] [CrossRef]
- Knaak, C.; Nyvlt, P.; Schuster, F.S.; Spies, C.; Heeren, P.; Schenk, T.; Balzer, F.; La Rosée, P.; Janka, G.; Brunkhorst, F.M.; et al. Hemophagocytic Lymphohistiocytosis in Critically Ill Patients: Diagnostic Reliability of HLH-2004 Criteria and HScore. Crit. Care 2020, 24, 244. [Google Scholar] [CrossRef]
- Cox, M.F.; Mackenzie, S.; Low, R.; Brown, M.; Sanchez, E.; Carr, A.; Carpenter, B.; Bishton, M.; Duncombe, A.; Akpabio, A.; et al. Diagnosis and Investigation of Suspected Haemophagocytic Lymphohistiocytosis in Adults: 2023 Hyperinflammation and HLH Across Speciality Collaboration (HiHASC) Consensus Guideline. Lancet Rheumatol. 2024, 6, e51–e62. [Google Scholar] [CrossRef]
- PulmCrit- Sepsis 4.0: Understanding Sepsis-HLH Overlap Syndrome. Available online: https://emcrit.org/pulmcrit/sepsis-hlh-overlap-syndrome-shlhos/ (accessed on 25 May 2025).
- Papageorgiou, D.; Gogos, C.; Akinosoglou, K. Macrophage Activation Syndrome in Viral Sepsis. Viruses 2024, 16, 1004. [Google Scholar] [CrossRef]
- Frank, M.G.; Weaver, G.; Raabe, V. Crimean-Congo Hemorrhagic Fever Virus for Clinicians—Epidemiology, Clinical Manifestations, and Prevention. Emerg. Infect. Dis. 2024, 30, 854–863. [Google Scholar] [CrossRef]
- Shi, L.; Wang, B.; Peng, D.; Zhang, K.; Wang, Y. Brucella-Associated Hemophagocytic Syndrome: Case Report of a Potentially Life-Threatening Condition and Literature Review. Front. Immunol. 2025, 16, 1592089. [Google Scholar] [CrossRef]
- Jevtic, D.; da Silva, M.D.; Haylock, A.B.; Nordstrom, C.W.; Oluic, S.; Pantic, N.; Nikolajevic, M.; Nikolajevic, N.; Kotseva, M.; Dumic, I. Hemophagocytic Lymphohistiocytosis (HLH) in Patients with Tick-Borne Illness: A Scoping Review of 98 Cases. Infect. Dis. Rep. 2024, 16, 154–169. [Google Scholar] [CrossRef]
- Demirkol, D.; Yildizdas, D.; Bayrakci, B.; Karapinar, B.; Kendirli, T.; Koroglu, T.F.; Dursun, O.; Erkek, N.; Gedik, H.; Citak, A.; et al. Hyperferritinemia in the Critically Ill Child with Secondary Hemophagocytic Lymphohistiocytosis/Sepsis/Multiple Organ Dysfunction Syndrome/Macrophage Activation Syndrome: What Is the Treatment? Crit. Care Lond. Engl. 2012, 16, R52. [Google Scholar] [CrossRef]
- Bursa, D.; Bednarska, A.; Pihowicz, A.; Paciorek, M.; Horban, A. Analysis of the Occurrence of Hemophagocytic Lymphohistiocytosis (HLH) Features in Patients with Sepsis: A Prospective Study. Sci. Rep. 2021, 11, 10529. [Google Scholar] [CrossRef]
- Canna, S.W.; Marsh, R.A. Pediatric Hemophagocytic Lymphohistiocytosis. Blood 2020, 135, 1332–1343. [Google Scholar] [CrossRef]
- Chinnici, A.; Beneforti, L.; Pegoraro, F.; Trambusti, I.; Tondo, A.; Favre, C.; Coniglio, M.L.; Sieni, E. Approaching Hemophagocytic Lymphohistiocytosis. Front. Immunol. 2023, 14, 1210041. [Google Scholar] [CrossRef]
- Weiss, S.L.; Peters, M.J.; Alhazzani, W.; Agus, M.S.D.; Flori, H.R.; Inwald, D.P.; Nadel, S.; Schlapbach, L.J.; Tasker, R.C.; Argent, A.C.; et al. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr. Crit. Care Med. 2020, 21, e52–e106. [Google Scholar] [CrossRef]
- Bergsten, E.; Horne, A.; Aricó, M.; Astigarraga, I.; Egeler, R.M.; Filipovich, A.H.; Ishii, E.; Janka, G.; Ladisch, S.; Lehmberg, K.; et al. Confirmed Efficacy of Etoposide and Dexamethasone in HLH Treatment: Long-Term Results of the Cooperative HLH-2004 Study. Blood 2017, 130, 2728–2738. [Google Scholar] [CrossRef]
- La Rosée, P.; Horne, A.; Hines, M.; von Bahr Greenwood, T.; Machowicz, R.; Berliner, N.; Birndt, S.; Gil-Herrera, J.; Girschikofsky, M.; Jordan, M.B.; et al. Recommendations for the Management of Hemophagocytic Lymphohistiocytosis in Adults. Blood 2019, 133, 2465–2477. [Google Scholar] [CrossRef]
- Naymagon, L.; Roehrs, P.; Hermiston, M.; Connelly, J.; Bednarski, J.; Boelens, J.-J.; Chandrakasan, S.; Dávila Saldaña, B.; Henry, M.M.; Satwani, P.; et al. Perspectives on the Current Diagnostic and Treatment Paradigms in Secondary Hemophagocytic Lymphohistiocytosis (HLH). Orphanet J. Rare Dis. 2025, 20, 200. [Google Scholar] [CrossRef]
- Böhm, S.; Wustrau, K.; Pachlopnik Schmid, J.; Prader, S.; Ahlmann, M.; Yacobovich, J.; Beier, R.; Speckmann, C.; Behnisch, W.; Ifversen, M.; et al. Survival in Primary Hemophagocytic Lymphohistiocytosis, 2016 to 2021: Etoposide Is Better than Its Reputation. Blood 2024, 143, 872–881. [Google Scholar] [CrossRef]
- Karakike, E.; Giamarellos-Bourboulis, E.J. Macrophage Activation-Like Syndrome: A Distinct Entity Leading to Early Death in Sepsis. Front. Immunol. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Shakoory, B.; Carcillo, J.A.; Chatham, W.W.; Amdur, R.L.; Zhao, H.; Dinarello, C.A.; Cron, R.Q.; Opal, S.M. Interleukin-1 Receptor Blockade Is Associated with Reduced Mortality in Sepsis Patients with Features of the Macrophage Activation Syndrome: Re-Analysis of a Prior Phase III Trial. Crit. Care Med. 2016, 44, 275–281. [Google Scholar] [CrossRef]
- Hall, M. Targeted Reversal of Inflammation in Pediatric Sepsis-Induced MODS (TRIPS). 2025. Available online: https://clinicaltrials.gov/study/NCT05267821?cond=(MUSCULAR%20DYSTROPHY,%20CONGENITAL,%20DAVIGNON-CHAUVEAU%20TYPE)%20OR%20(trips)&checkSpell=&rank=3 (accessed on 1 June 2025).
- Paranga, T.G.; Mitu, I.; Pavel-Tanasa, M.; Rosu, M.F.; Miftode, I.-L.; Constantinescu, D.; Obreja, M.; Plesca, C.E.; Miftode, E. Cytokine Storm in COVID-19: Exploring IL-6 Signaling and Cytokine-Microbiome Interactions as Emerging Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 11411. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Interleukin-6 in SARS-CoV-2 Induced Disease: Interactions and Therapeutic Applications. Biomed. Pharmacother. 2022, 145, 112419. [Google Scholar] [CrossRef]
- Slaney, E.D.; Modica, R.; Woolnough, L.; Kafisheh, D.; Bell-Brunson, D.H.; Elder, M. Case Report: Refractory Macrophage Activation Syndrome Requiring High-Dose Anakinra, Emapalumab, and Etoposide Therapy in Early-Onset Systemic Juvenile Idiopathic Arthritis Associated with Adenoviremia. Front. Pediatr. 2023, 11, 1336554. [Google Scholar] [CrossRef]
- Garonzi, C.; Chinello, M.; Cesaro, S. Emapalumab for Adult and Pediatric Patients with Hemophagocytic Lymphohistiocytosis. Expert Rev. Clin. Pharmacol. 2021, 14, 527–534. [Google Scholar] [CrossRef]
- Huarte, E.; Peel, M.T.; Verbist, K.; Fay, B.L.; Bassett, R.; Albeituni, S.; Nichols, K.E.; Smith, P.A. Ruxolitinib, a JAK1/2 Inhibitor, Ameliorates Cytokine Storm in Experimental Models of Hyperinflammation Syndrome. Front. Pharmacol. 2021, 12, 650295. [Google Scholar] [CrossRef]
- Guo, S.-Y.; Han, J.-Y.; Qiu, K.-Y.; Wang, J.; Fang, J.-P.; Zhou, D.-H. Retrospective Analysis of Ruxolitinib as Induction Treatment in Pediatric Hemophagocytic Lymphohistiocytosis. Hematol. Amst. Neth. 2025, 30, 2526124. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, X.; Kang, K.; Yang, Y.; Li, H.; Zhao, A.; Niu, T. Hemophagocytic Lymphohistiocytosis: Current Treatment Advances, Emerging Targeted Therapy and Underlying Mechanisms. J. Hematol. Oncol. 2024, 17, 106. [Google Scholar] [CrossRef]
- Lin, C.I.; Yu, H.H.; Lee, J.H.; Wang, L.C.; Lin, Y.T.; Yang, Y.H.; Chiang, B.L. Clinical Analysis of Macrophage Activation Syndrome in Pediatric Patients with Autoimmune Diseases. Clin. Rheumatol. 2012, 31, 1223–1230. [Google Scholar] [CrossRef]
- EMCrit Project. Hemophagocytic LymphoHistiocytosis (HLH). Available online: https://emcrit.org/ibcc/hlh/ (accessed on 1 June 2025).
- Leventogiannis, K.; Kyriazopoulou, E.; Antonakos, N.; Kotsaki, A.; Tsangaris, I.; Markopoulou, D.; Grondman, I.; Rovina, N.; Theodorou, V.; Antoniadou, E.; et al. Toward Personalized Immunotherapy in Sepsis: The PROVIDE Randomized Clinical Trial. Cell Rep. Med. 2022, 3, 100817. [Google Scholar] [CrossRef]
- Mehta, P.; Cron, R.Q.; Hartwell, J.; Manson, J.J.; Tattersall, R.S. Silencing the Cytokine Storm: The Use of Intravenous Anakinra in Haemophagocytic Lymphohistiocytosis or Macrophage Activation Syndrome. Lancet Rheumatol. 2020, 2, e358–e367. [Google Scholar] [CrossRef]
- Home|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ (accessed on 10 July 2025).
- Kyriazopoulou, E.; Leventogiannis, K.; Norrby-Teglund, A.; Dimopoulos, G.; Pantazi, A.; Orfanos, S.E.; Rovina, N.; Tsangaris, I.; Gkavogianni, T.; Botsa, E.; et al. Macrophage Activation-like Syndrome: An Immunological Entity Associated with Rapid Progression to Death in Sepsis. BMC Med. 2017, 15, 172. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).