1. Introduction
Duchenne Muscular Dystrophy (DMD) is an X-linked inherited disorder characterized by progressive muscle weakness and degeneration, primarily observed in male children. Epidemiologically, DMD is one of the most common neuromuscular disorders of childhood, with an estimated incidence of approximately 1 in 3500–5000 live male births worldwide, corresponding to a prevalence of about 1 in 10,000–20,000 boys [
1,
2,
3]. The etiology of DMD is linked to a genetic defect resulting in absent or dysfunctional production of the protein dystrophin. This deficiency leads to muscle fiber damage and subsequent loss of muscle function. However, DMD is not limited to skeletal muscle involvement; its systemic effects also contribute to impairment of the cardiovascular system. While both skeletal and cardiac dysfunction worsen over time, their progression is often not parallel; some patients with advanced skeletal muscle weakness may have relatively preserved cardiac function, whereas others may develop significant cardiac involvement even in earlier disease stages [
4].
Cardiovascular involvement is a frequently overlooked but critical component of DMD. Most children with DMD develop cardiac muscle weakness (cardiomyopathy) that becomes more pronounced with advancing age. This cardiomyopathy mainly manifests as left ventricular dysfunction, which can eventually progress to heart failure [
5]. Additionally, arrhythmias and other electrophysiological abnormalities play a significant role in disease progression and contribute to increased mortality rates in these patients [
6].
Non-invasive diagnostic tools such as electrocardiography (ECG) enable early detection of cardiovascular involvement in DMD. ECG provides valuable information on cardiac status; commonly observed abnormalities in children with DMD include QRS complex widening, PR interval prolongation, T-wave inversions, and pathological Q waves [
7].
Recently, systemic inflammatory indices have gained prominence as novel markers for predicting cardiovascular disease prognosis and quantifying inflammation. Indices such as neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), systemic inflammatory response index (SIRI: neutrophils × monocytes/lymphocytes), systemic immune-inflammation index (SII: neutrophils × platelets/lymphocytes), and pan-immune inflammation value (PIV: neutrophils × monocytes × platelets/lymphocytes) are simple, low-cost parameters derived from routine blood tests that reflect the extent of systemic inflammation. Their prognostic values have been demonstrated in various cardiovascular, neurovascular, oncological, and metabolic disorders [
8,
9,
10].
Early identification and management of cardiovascular complications in children with Duchenne Muscular Dystrophy may improve disease prognosis. In this context, further research is required to clarify whether systemic inflammatory indices can serve as predictors of cardiovascular involvement in this population. This study aims to investigate the cardiovascular effects and electrocardiographic findings of DMD and to evaluate the potential utility of novel inflammatory indices in guiding cardiac monitoring and treatment strategies.
2. Materials and Methods
This retrospective study evaluated data from 25 patients diagnosed with Duchenne Muscular Dystrophy (DMD) who were under follow-up in the pediatric cardiology outpatient clinic between 1 January 2021 and 1 July 2024. All eligible patients under 18 years of age during this period were included. The diagnosis of Duchenne Muscular Dystrophy in all patients was confirmed based on clinical features, persistently elevated serum creatine kinase (CK) levels, and genetic testing demonstrating dystrophin gene mutations. Muscle biopsy was not required since molecular genetic confirmation was available for all patients. The control group consisted of an equal number of healthy male children aged 2 to 18 years, randomly selected from individuals presenting to the pediatric outpatient clinic who had undergone complete blood counts as part of their routine clinical evaluation. The number of controls was determined based on an a priori power analysis. Assuming a two-tailed α of 0.05, a power of 80%, and a large effect size (Cohen’s d = 0.80), the analysis indicated that a minimum of 25 participants per group would be sufficient; therefore, 25 controls were included.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Non-Interventional Research Ethics Committee of Ordu University (protocol code 2025/68; date of approval: 7 March 2025). As this was a retrospective study, informed consent could not be obtained directly from the patients or from the individuals in the control group; however, all data were collected and analyzed in accordance with ethical guidelines, and patient confidentiality was strictly maintained.
The variables assessed included patient age, age at diagnosis, wheelchair dependence, tracheostomy status, treatment regimens, hematological parameters, NLR, MLR, PLR, SIRI, SII, PIV, ECG, and ECHO parameters. Complete blood count and biochemical parameters were obtained as part of the patients’ routine clinical follow-up visits in the pediatric cardiology outpatient clinic. These assessments were performed at the time of presentation for regular monitoring, and not during acute illness or intercurrent infection. Additional metrics, including Pro-BNP, ECG, and ECHO, were collected as part of the standard cardiological evaluation protocol for patients with DMD. In our center, ECG is typically performed at each annual follow-up visit to monitor electrical abnormalities such as conduction defects or arrhythmias, while echocardiography is performed annually or more frequently if clinically indicated, in line with current recommendations for the surveillance of cardiac function in DMD patients.
Statistical Analysis
All statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) for Windows, version 26 (IBM Corp., Armonk, NY, USA), with statistical significance set at p < 0.05. The normality of continuous variables was assessed using the Kolmogorov–Smirnov test and skewness–kurtosis measures. Since the data were normally distributed, parametric tests were applied.
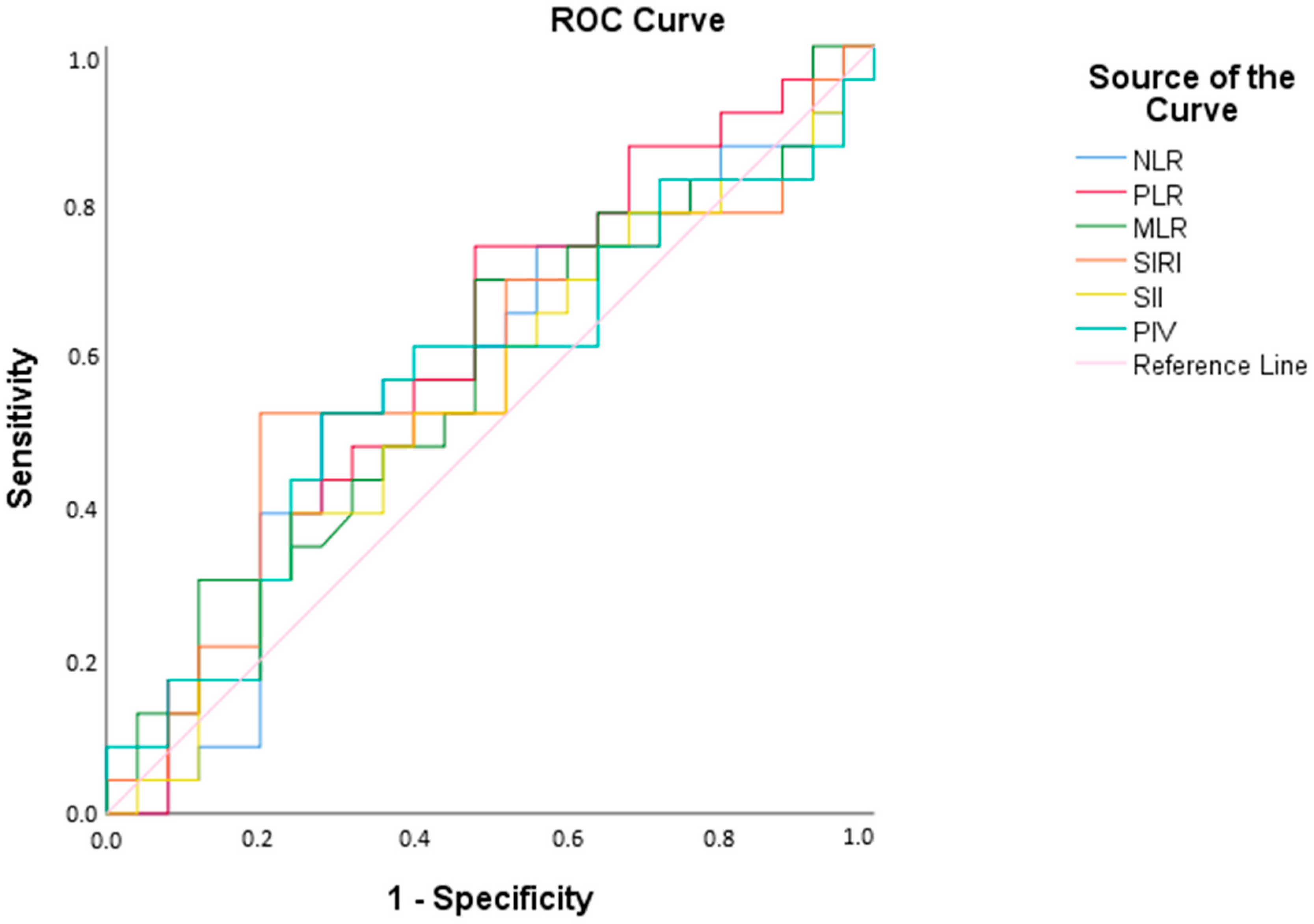
Descriptive statistics were presented as mean, standard deviation, count (n), and percentage (%). Independent samples t-test was used for comparisons between groups. Receiver operating characteristic (ROC) curve analysis was conducted to determine optimal cut-off values for variables within the patient group, calculating area under the curve (AUC), sensitivity, and specificity.
Pearson correlation coefficient was employed to evaluate relationships between continuous variables.
4. Discussion
This study examined the clinical, biochemical, and cardiological data of 25 patients diagnosed with DMD. The findings provide valuable insights into the clinical course, treatment strategies, and cardiac complications associated with DMD.
All patients included in the study were male, which is consistent with the expected inheritance pattern of DMD as an X-linked genetic disorder [
1]. The median age of patients was 12 years, and the mean age at diagnosis was 4.8 ± 2.6 years. This finding emphasizes that DMD is typically diagnosed during childhood, highlighting the importance of early diagnosis in monitoring disease progression [
11]. The diagnosis of DMD is commonly established between ages 2 and 5, and early diagnosis can shorten the time to treatment initiation, aiding in the preservation of motor functions [
12]. In our cohort, the patient age range was 5–18 years, whereas controls were defined between 2–18 years of age to reflect the full pediatric spectrum in which DMD typically manifests and is clinically monitored. While this difference in lower age limits might raise concern for potential confounding, the median age did not differ significantly between groups, suggesting that age-related bias was unlikely to have influenced the main findings.
Regarding physical status and needs, the rate of wheelchair use was found to be 16%. The median age of our cohort (12 years) is clinically meaningful, as this period coincides with the expected onset of key disease milestones such as loss of ambulation (~13 years) [
13] This indicates significant motor function decline with advancing age, with wheelchair use typically required in later stages. None of the patients required home mechanical ventilation or tracheostomy.
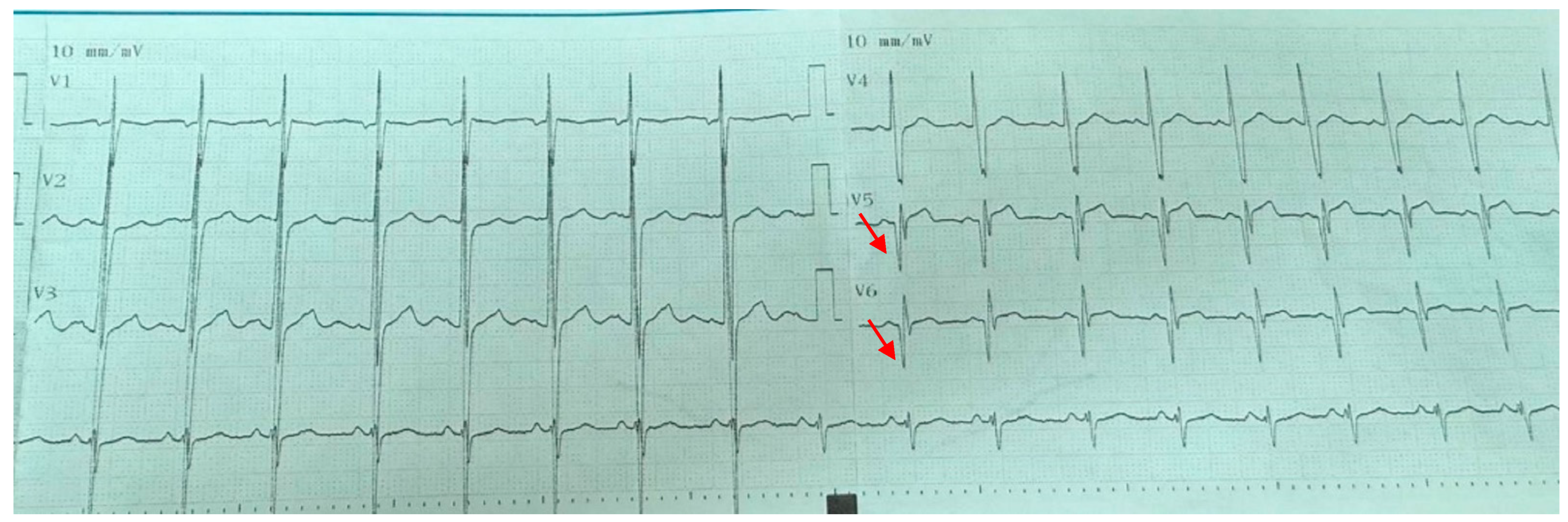
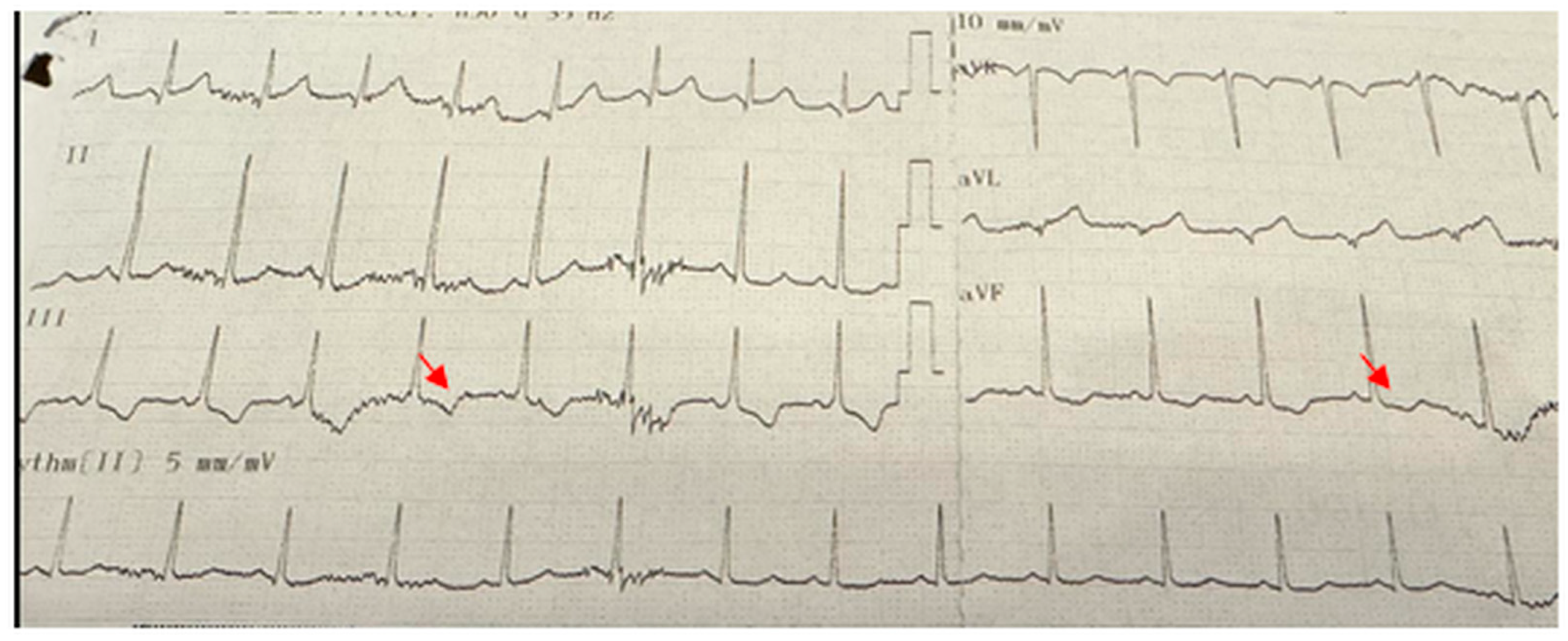
Cardiological assessments revealed important findings. The most frequently observed ECG abnormality was pathological Q waves (24%), consistent with literature reports indicating a 30–40% prevalence in DMD patients. This finding is considered an early marker of silent myocardial fibrosis and is associated with cardiomyopathy [
14]. Another study linked frequent pathological Q waves in DMD patients with sudden cardiac death (SCD) [
15].
Cardiac complications in DMD are known to commence early and become more pronounced with age [
16]. Myocardial fibrosis is typically detected by cardiac MRI around the early teenage years (median age approximately 13.8 years; range 7–17 years) [
17], and initial left ventricular systolic dysfunction often becomes apparent by echocardiography during mid-adolescence [
18]. In our cohort, the mean age of patients with echocardiographic or electrocardiographic abnormalities was 12.1 years, reinforcing that clinically significant cardiac involvement may already be present at this stage. Pathological Q waves were identified at mean age of 12 (range 6–18 years), indicating that this abnormality may appear as early as the first decade of life and persist into late adolescence. T-wave inversion was noted at 11 years of age. Systolic left ventricular dysfunction was observed at 14 and 17 years, while dilated cardiomyopathy was diagnosed at 18 years, underscoring that severe cardiac involvement can manifest relatively early in the disease course. However, no statistically significant age difference was detected between the groups with and without cardiac pathology in our study.
The detection of severe cardiac abnormalities such as dilated cardiomyopathy on echocardiography underscores the necessity of continuous cardiological monitoring. In our cohort, echocardiographic evaluation revealed left ventricular dysfunction or structural anomalies in 16% (4/25) of patients. The weak correlation observed between echocardiographic abnormalities and ECG findings further emphasizes the limited diagnostic value of ECG as a standalone tool in DMD [
19]. Moreover, cardiac MRI is recognized as the gold standard imaging modality for the early detection of cardiovascular involvement in DMD, particularly for identifying myocardial fibrosis and subclinical dysfunction [
20]. In our center, cardiac MRI is available and performed in some patients; however, due to the retrospective design and lack of standardized MRI data across the cohort, these results were not included in the present analysis. Nevertheless, it should be acknowledged that while echocardiography remains the most accessible technique in routine practice, cardiac MRI provides superior sensitivity for detecting early cardiac pathology.
Beyond conventional monitoring, a potential strategy to reduce cardiovascular comorbidities, mitigate the progressive impact of DMD, and improve quality of life is to ensure normal pubertal development, which is frequently delayed due to chronic corticosteroid therapy. Although no official guidelines currently exist regarding puberty induction protocols, a recent scoping review has synthesized the available evidence, highlighting that timely pubertal induction may enhance quality of life and, indirectly, contribute to a reduction in cardiovascular risk [
21].
Cardiac treatments employed included ACE inhibitors, beta blockers, and digoxin. These therapies are vital for managing cardiomyopathy and other cardiac complications, highlighting the critical role of cardiac monitoring and treatment in DMD patients [
22]. The use of cardiac therapy was confined to patients with ECG or echocardiographic abnormalities, consistent with current recommendations. Nevertheless, it should also be acknowledged that some guidelines and workshop reports recommend the prophylactic initiation of ACE inhibitors by the age of 10, even in the absence of echocardiographic evidence of disease [
23]. Specifically, one patient with left ventricular systolic dysfunction and pathological Q waves was treated with an ACE inhibitor in combination with a beta blocker, another patient with dilated cardiomyopathy, concomitant left ventricular systolic dysfunction, and pathological Q waves received digoxin, an ACE inhibitor+ beta blocker, and a patient who presented with supraventricular tachycardia (SVT) was managed with a beta blocker. No significant relationship was found between clinical or biochemical parameters and cardiac treatment. These findings indicate that cardiac complications should be closely monitored through ECG and echocardiography, and that a multidisciplinary approach is necessary in managing these patients [
17].
When comparing hematological parameters, hemoglobin levels were significantly higher in the patient group (
p = 0.006). Although elevated hemoglobin is uncommon in DMD, some studies report increases secondary to corticosteroid therapy [
14]. However, aside from this finding, no statistically significant differences were observed between groups for the other hematological parameters.
Yükcü and Arslan demonstrated that MLR, SIRI, and PIV have acceptable diagnostic value for detecting ascending aortic dilation in children with bicuspid aortic valve [
10]. In contrast, in our patients no significant differences were observed between the patient and control groups with respect to inflammatory markers, suggesting a limited role for systemic inflammatory biomarkers in the routine monitoring of DMD [
24]. Nevertheless, it should be acknowledged that corticosteroid therapy, which is routinely administered in DMD, has been shown to delay the progression of cardiomyopathy, and this beneficial effect may be at least partly mediated by modulation of inflammatory pathways. Therefore, while our findings suggest a limited direct role of systemic inflammatory indices, the contribution of inflammation cannot be fully excluded.
A notable finding in our study is the observed positive correlation between Pro-BNP and PLR. However, this result should be interpreted with caution, as only a limited number of patients in our cohort had elevated NT-proBNP and ventricular dysfunction. While the association may suggest a potential link between increased cardiovascular burden and inflammatory response, the small sample size precludes definitive conclusions. Although several studies have examined the prognostic value of PLR in cardiovascular diseases, it is not considered an independent predictor of mortality. For example, a 2019 cohort study reported that higher PLR levels in patients with acute heart failure (AHF) were associated with increased mortality rates [
25]. Studies directly evaluating the relationship between Pro-BNP and PLR remain limited; however, both biomarkers have been shown to be important in assessing heart failure and acute coronary syndromes. For example, a study on chronic heart failure patients found a positive correlation between NT-proBNP levels and PLR [
26]. To our knowledge, no previous studies have investigated this correlation specifically in the DMD population, and further research with larger cohorts is warranted to clarify its clinical significance. However, it should be noted that PLR values in our cohort demonstrated a large standard deviation, and only a small subset of patients exhibited elevated NT-proBNP levels. Therefore, the observed correlation between PLR and NT-proBNP should be interpreted with caution. Moreover, NT-proBNP was evaluated only in the patient group, and not in the controls, which further limits the generalizability of this finding. Therefore, the significant correlation observed between PLR and NT-proBNP should be interpreted with caution, as it may represent a type I error rather than a definitive biological association. Future studies with larger cohorts and appropriate statistical corrections are required to validate this result.
The ROC analysis revealed that none of the inflammatory parameters achieved sufficient diagnostic accuracy, limiting their utility for routine screening in DMD. Furthermore, the impact of routine steroid use on inflammatory indices remains unclear and warrants further investigation. Although corticosteroids are known to impact hematological parameters—most notably by causing neutrophilia and lymphopenia, the magnitude of these changes is typically modest. Moreover, some clinical studies indicate that systemic inflammatory indices such as NLR and PLR may not be significantly altered in every context. For example, in children with steroid-sensitive nephrotic syndrome, no significant differences in PLR were observed before and after corticosteroid therapy (
p > 0.05), suggesting that PLR may remain a stable inflammatory marker independent of steroid exposure [
27,
28]. Similarly, increases in NLR in pediatric asthma have been attributed primarily to disease severity rather than corticosteroid use [
29]. In contrast, the hematologic alterations observed in Duchenne Muscular Dystrophy (DMD) are more likely to reflect the underlying disease burden rather than medication effects alone. In DMD, corticosteroids are generally administered at a maintenance dose of approximately 0.75 mg/kg/day, which has been shown to slow—but not halt—disease progression. While the adverse effects of steroids are dose-dependent, their benefits in reducing muscle degeneration outweigh these risks. This benefit–risk balance may also explain why systemic inflammatory biomarkers (e.g., NLR, PLR) did not demonstrate marked abnormalities in our cohort, despite chronic steroid therapy, and should be acknowledged as a potential confounder. In addition, all patients in the study group received steroid therapy according to the same protocol, and the within-group comparisons were conducted in a clinically homogeneous patient population. Prospective studies are warranted to clarify these findings.