A Novel Approach to the Management of Children with Primary Nocturnal Enuresis
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
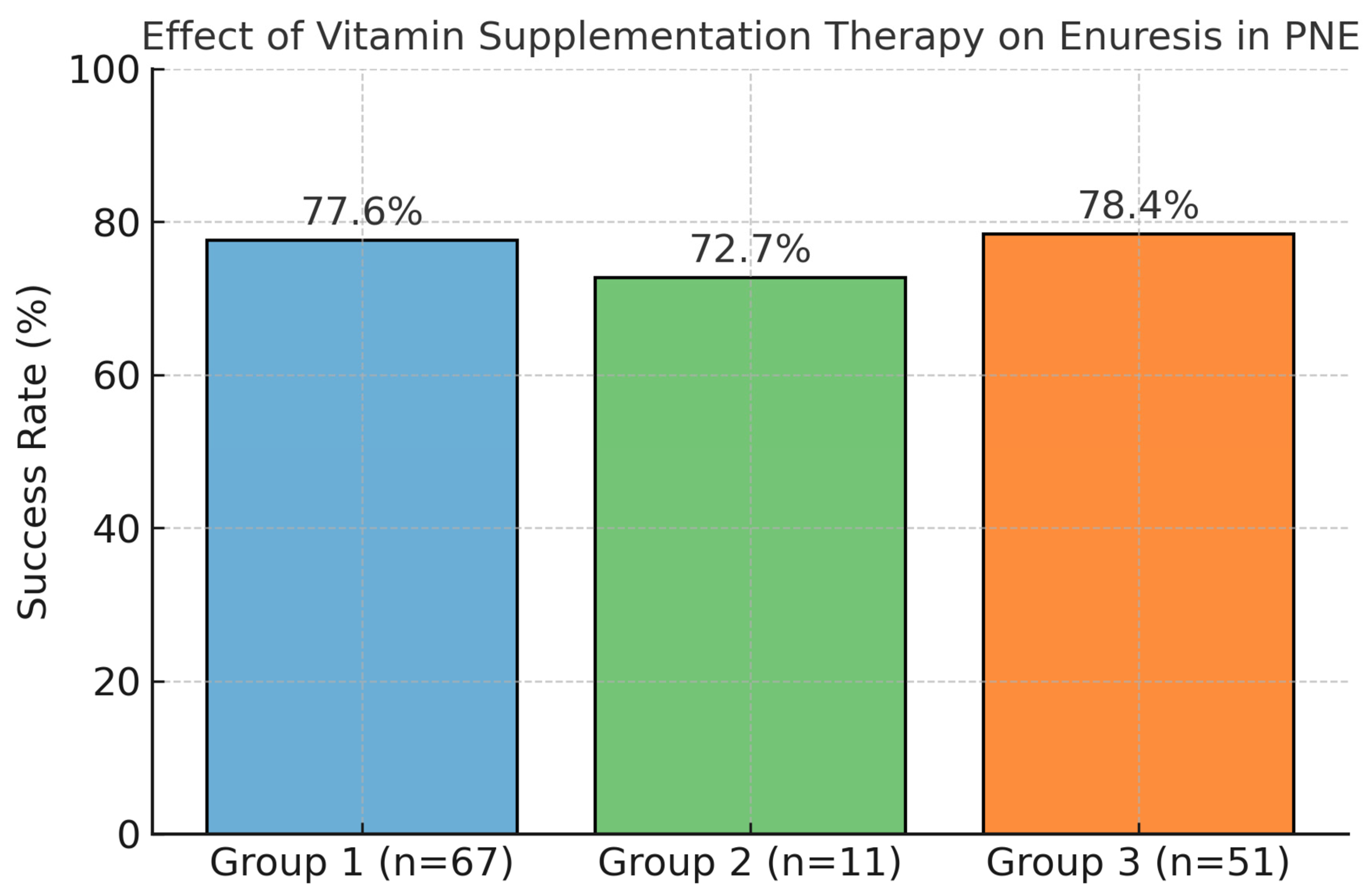
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rittig, S. Enuresis. In Pediatric Nephrology, 8th ed.; Emma, F., Ed.; Springer Nature: Cham, Switzerland, 2022; pp. 1412–1419. [Google Scholar]
- Yeung, C.K.; Sreedhar, B.; Sihoe, J.D.Y.; Sit, F.K.Y.; Lau, J.T.F. Differences in characteristics of nocturnal enuresis between children and adolescents: A critical appraisal from a large epidemiological study. BJU Int. 2006, 97, 1069–1073. [Google Scholar] [CrossRef]
- Krantz, I.; Jylkäs, E.; Ahlberg, B.M.; Wedel, H. On the epidemiology of nocturnal enuresis: A critical review of methods used in descriptive epidemiological studies on nocturnal enuresis. Scand. J. Urol. Nephrol. Suppl. 1994, 163, 75–82. [Google Scholar]
- Baird, D.C.; Seehusen, D.A.; Bode, D.V. Enuresis in children: A case-based approach. Am. Fam. Physician 2014, 90, 560–568. [Google Scholar]
- Altunoluk, B.; Davutoglu, M.; Garipardic, M.; Seydanoglu, A. Decreased vitamin B12 levels in children with nocturnal enuresis. ISRN Urol. 2012, 2012, 789706. [Google Scholar]
- Serin, H.M.; Arslan, E.A. Neurological symptoms of vitamin B12 deficiency: Analysis of pediatric patients. Acta Clin. Croat. 2019, 58, 295–302. [Google Scholar] [CrossRef]
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci. Ther. 2020, 26, 5–13. [Google Scholar] [CrossRef]
- Siroosbakht, S. Are vitamin D levels linked to primary monosymptomatic nocturnal enuresis in children? Six years of experience about a controversy in medicine. A case-control study. Iran. J. Pediatr. 2023, 33, e133755. [Google Scholar] [CrossRef]
- Tokonami, N.; Cheval, L.; Monnay, I.; Ranieri, M.; Can, A.; Pierrat, F.; Chambrey, R.; Eladari, D. Endothelin-1 mediates natriuresis but not polyuria during vitamin D-induced acute hypercalcaemia. J. Physiol. 2017, 595, 2535–2550. [Google Scholar] [CrossRef]
- Baer, R.; Tene, L.; Weintraub, A.Y.; Yohai, D.; Yohay, Z.; Reuveni Salzman, A. The effect of vitamin D deficiency and supplementation on urinary incontinence: Scoping review. Int. Urogynecology J. 2022, 33, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Daley, S.F.; Gomez Rincon, M.; Leslie, S.W. Enuresis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK558964/ (accessed on 13 June 2025).
- Gominak, S.C.; Stumpf, W.E. The world epidemic of sleep disorders is linked to vitamin D deficiency. Med. Hypotheses 2012, 79, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global consensus recommendations on prevention and management of nutritional rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef] [PubMed]
- Arıcan, P.; Bozkurt, O.; Cavusoglu, D.; Gencpınar, P.; Haspolat, S.; Duman, O.; Olgac Dundar, N. Various neurological symptoms with vitamin B12 deficiency and posttreatment evaluation. J. Pediatr. Neurosci. 2020, 15, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Zeybek, C. Vitamin B12 and folate levels in children with primary nocturnal enuresis. Gulhane Med. J. 2024, 66, 30–35. [Google Scholar] [CrossRef]
- Khan, K.M.; Jialal, I. Folic acid deficiency. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537286/ (accessed on 13 June 2025).
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- Austin, P.F.; Bauer, S.B.; Bower, W.; Chase, J.; Franco, I.; Hoebeke, P.; Rittig, S.; Walle, J.V.; Yang, S.S.; Nevéus, T.; et al. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the Standardization Committee of the International Children’s Continence Society. Neurourol. Urodyn. 2016, 35, 471–481. [Google Scholar] [CrossRef]
- Turkish Society of Hematology. National Treatment Guide: Vitamin B12 Deficiency. Available online: https://www.thd.org.tr (accessed on 24 April 2018).
- Jørgensen, C.S.; Kamperis, K.; Walle, J.V.; Rittig, S.; Raes, A.; Dossche, L. The efficacy of standard urotherapy in the treatment of nocturnal enuresis in children: A systematic review. J. Pediatr. Urol. 2023, 19, 163–172. [Google Scholar] [CrossRef]
- Borgström, M.; Bergsten, A.; Tunebjer, M.; Hedin Skogman, B.; Nevéus, T. Daytime urotherapy in nocturnal enuresis: A randomised, controlled trial. Arch. Dis. Child. 2022, 107, 570–574. [Google Scholar] [CrossRef]
- Hägglöf, B.; Andrén, O.; Bergström, E.; Marklund, L.; Wendelius, M. Self-esteem in children with nocturnal enuresis and urinary incontinence: Improvement of self-esteem after treatment. Eur. Urol. 1998, 33 (Suppl. 3), 16–19. [Google Scholar]
- Keleş, A.; Yıldız, N.; Kaya, E.; Keleş, S.; Genç, G. The roles of vitamin B12, 25(OH) D, and folate in primary nocturnal enuresis: A single center experience in an immigration area. Eur. Urol. Suppl. 2022, 21 (Suppl. 1), S515. [Google Scholar] [CrossRef]
- Kara, İ.S.; Peker, N.A.; Dolğun, İ.; Mertoğlu, C. Vitamin B12 level in children. J. Curr. Pediatr. 2023, 21, 127–134. [Google Scholar]
- Karabayir, N.; Teber, B.G.; Dursun, H.K.; Pehlivan, L.S. Is there an association between vitamin B12 level and vitamin D status in children? J. Pediatr. Hematol. Oncol. 2022, 44, e677–e681. [Google Scholar] [CrossRef]
- Ibrahim, H.A.A.; Menshawy, S.S.; Hassan, F.E.; El-Makawi, S.M.; Amn, O.R.; Bastawy, N.; Saad, S.; Hussein, S.M.; Mahmoud, D.; ElKhashab, K.M.A. Vitamin D and vitamin B12 profiles in children with primary nocturnal enuresis: An analytical cross-sectional study. Ann. Med. 2024, 56, 2352030. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr. Bull. 2008, 29 (Suppl. 2), S126–S131. [Google Scholar] [CrossRef]
- El-Baz, F.M.; Abdelsayed, M.G.R.; Abdel-Hafeez, A.S.; Saad, M.M. Decreased vitamin D levels in children with primary monosymptomatic nocturnal enuresis. Egypt. J. Med Hum. Genet. 2021, 22, 71. [Google Scholar] [CrossRef]
- Rahmani, E.; Eftekhari, M.H.; Fallahzadeh, M.H.; Fararouei, M.; Massoumi, S.J. Effect of vitamin D and omega-3 on nocturnal enuresis of 7–15-year-old children. J. Pediatr. Urol. 2018, 14, 257.e1–257.e6. [Google Scholar] [CrossRef]
- Kompani, F.; Barati, L.; Mehrjerdian, M.; Vakili, M.; Nodehsharifi, A. Folic acid and vitamin B12 deficiency, two new findings in pediatric enuresis. Iran. J. Pediatr. 2023, 33, e129308. [Google Scholar] [CrossRef]
- Li, L.; Zhou, H.; Yang, X.; Zhao, L.; Yu, X. Relationships between 25-hydroxyvitamin D and nocturnal enuresis in five- to seven-year-old children. PLoS ONE 2014, 9, e99316. [Google Scholar] [CrossRef]
- Albayrak, S.; Zengin, K.; Tanik, S.; Daar, G.; Ozdamar, M.Y.; Bakirtas, H.; Imamoglu, M.A.; Gurdal, M. Vitamin B12, folate and iron levels in primary nocturnal enuresis. Pak. J. Med. Sci. 2015, 31, 87–90. [Google Scholar] [CrossRef]
- Mostafa, D.; Shaker, H.; Badr, A.; Abuelnaga, M. A comparative study of vitamin D serum levels in monosymptomatic enuretic children and non-enuretic children. Neurourol. Urodyn. 2025, 44, 212–219. [Google Scholar] [CrossRef]
| Characteristic | Value |
|---|---|
| Age (years), mean ± SD (range) | 8.78 ± 2.88 |
| Sex, n (%) | Male: 85 (56.7) Female: 65 (43.3) |
| Male-to-female ratio | 1.3 |
| Number of wet nights per week, mean ± SD | 6.39 ± 1.20 |
| Body weight (kg), mean ± SD | 34.97 ± 19.81 |
| Height (cm), mean ± SD | 134.17 ± 16.62 |
| Family history, n (%) | 74 (49.3) [Mother: 24 (16.0) Father: 24 (16.0) Others: 26 (17.3)] |
| Severity of enuresis, n (%) | Mild: 2 (1.3) Moderate: 12 (8.0) Severe: 136 (90.7) |
| Vitamin deficiencies, n (%) | None: 21 (14.0) Low B12: 118 (78.6) Low 25OHD: 62 (41.3) Low B12 + 25OHD: 51 (34.0) |
| Parameter | n (%) | Mean ± SD | Range |
|---|---|---|---|
| Vitamin B12 (pg/mL) | 242.51 ± 120.38 | 63–1334 | |
| Normal (>300) | 32 (21.4%) | ||
| Low (<300) | 118 (78.6%) | ||
| Deficient (<200) | 66 (44.0%) | ||
| 25-Hydroxyvitamin D (ng/mL) | 22.04 ± 9.01 | 4–58 | |
| Normal (>20) | 88 (58.7%) | ||
| Low (<20, total) | 62 (41.3%) | ||
| Deficient (<12) | 19 (12.6%) | ||
| Ferritin (ng/mL) | 22.56 ± 17.98 | 3–145 | |
| Normal (≥10) | 124 (82.7%) | ||
| Low (<10) | 26 (17.3%) | ||
| Folate (ng/mL) | 10.11 ± 3.65 | 5–24.9 | |
| Normal (>4) | 150 (100.0%) | ||
| Low (2–4) | 0 (0.0%) | ||
| Deficient (<2) | 0 (0.0%) |
| Authors | Year | Objective | Study Design | Sample Size (Patient/Control) | Key Findings |
|---|---|---|---|---|---|
| Altunoluk et al. [5] | 2012 | To evaluate the relationship between vitamin B12/folate levels and neurogenic maturation in children with PEN | Cross-sectional study | 30/31 | Significantly lower B12 and folate levels in PEN group |
| Siroosbakht et al. [8] | 2023 | To investigate the association between vitamin D levels and the development/severity of PEN | 6-year case–control study | 267/267 | Vitamin D levels significantly lower in PEN group |
| Zeybek et al. [15] | 2024 | To assess serum B12, folate, and ferritin levels in children with PEN | Retrospective, single-center | 86/90 | B12 and folate levels significantly lower; no difference in ferritin |
| Keleş et al. [23] | 2024 | To evaluate vitamin levels in PEN | Retrospective cohort study | 146/102 | Significantly lower B12, folate, and vitamin D levels in PEN group |
| Ibrahim et al. [26] | 2024 | To assess the relationship between vitamin D/B12 deficiency and PEN severity | Cross-sectional study | 288/— | Vitamin D deficiency common and associated with PEN severity |
| El-Baz et al. [28] | 2021 | To compare vitamin D levels in children with and without PEN | Case–control study | 50/50 | Vitamin D levels significantly lower in PEN group |
| Rahmani et al. [29] | 2018 | To assess the effect of vitamin D and omega-3 on enuresis | Observational study | 162/— | Vitamin D found beneficial for PEN |
| Kompani et al. [30] | 2023 | To evaluate serum B12 and folic acid levels in children with PEN | Case–control study | 43/99 | Significantly lower B12 and folic acid levels in PEN group |
| Li et al. [31] | 2014 | To examine the association between 25OHD levels and enuresis | Observational study | 43 PEN cases/247 total | Higher prevalence of PEN among children with low vitamin D |
| Albayrak et al. [32] | 2015 | To compare levels of B12, folate, and iron in PEN | Comparative study | 40/30 | B12 and folate lower; iron levels higher in PEN group |
| Mostafa et al. [33] | 2024 | To evaluate the association between vitamin D deficiency and PEN | Case–control study | 60/60 | Significant difference in vitamin D status between groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agar, B.E.; Gurgoze, M.K.; Kara, A. A Novel Approach to the Management of Children with Primary Nocturnal Enuresis. Children 2025, 12, 1128. https://doi.org/10.3390/children12091128
Agar BE, Gurgoze MK, Kara A. A Novel Approach to the Management of Children with Primary Nocturnal Enuresis. Children. 2025; 12(9):1128. https://doi.org/10.3390/children12091128
Chicago/Turabian StyleAgar, Buket Esen, Metin Kaya Gurgoze, and Aslihan Kara. 2025. "A Novel Approach to the Management of Children with Primary Nocturnal Enuresis" Children 12, no. 9: 1128. https://doi.org/10.3390/children12091128
APA StyleAgar, B. E., Gurgoze, M. K., & Kara, A. (2025). A Novel Approach to the Management of Children with Primary Nocturnal Enuresis. Children, 12(9), 1128. https://doi.org/10.3390/children12091128







