Abstract
Background/Objectives: Pancreatic fluid collections (PFCs) in acute pancreatitis require drainage when symptomatic or infected. Walled-off necrosis (WON) is difficult to drain with plastic stents alone. A lumen-apposing metal stent (LAMS) offers larger calibre drainage, lower migration risk than conventional methods, and the option of direct endoscopic necrosectomy through the stent. However, the paediatric literature on LAMSs is sparse. We report our institutional experience, and summarise current evidence on the feasibility, efficacy and safety of LAMSs for PFC drainage in children. Methods: We performed a retrospective study at the National University Hospital (NUH) and a full review of the literature on LAMS use in children for endoscopic trans-gastric drainage of PFCs from April 2012 to September 2024. Results: There were, respectively, 2 (males, 10 and 17 years) and 18 children who underwent endoscopic trans-gastric LAMS insertion for drainage of PFCs in acute pancreatitis in the NUH and across the nine included studies, which were published between 2015 and 2024. The technical and clinical success was 100%. There were no complications during insertion or indwell time (28 and 50 days in the NUH and 40 days, range of 7–100 days in the systematic review, respectively). Endoscopic removal of LAMSs was uneventful. There were no recurrent PFCs over a 4-month (1,7 months) and 12-month (range, 2–44 months) follow-up, respectively. Migration of LAMSs to colon following the collapse of the WON was reported in one case. Conclusions: An transgastric LAMS (with trans-stent necrosectomy) is a technically feasible method of drainage of WON following acute pancreatitis in children with minimal complications.
1. Introduction
The incidence of acute pancreatitis in children is increasing (3.6–13/100,000 children-year) [,]. Pancreatic fluid collections (PFCs) may occur in 23% to 61% of paediatric pancreatitis [,,]. As per the North American Society for Paediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN), a significant proportion of acute PFCs in children resolve spontaneously without requiring any drainage []. However, symptomatic or infected PFCs may require therapeutic drainage (18.2% of pseudocysts and 35.7% of walled-off necrosis (WON)) [,].
In children, NASPGHAN recommends endoscopic methods as favoured over open approaches, which aligns with adult guidelines for a step-up, minimally invasive approach [,,,]. The endoscopic management includes endoscopic transluminal drainage of PFCs and insertion of plastic stents with the option of direct endoscopic necrosectomies for clearance []. One systematic review in children showed 88.7% and 92.3% success after one and two interventions, respectively, with plastic stents used in the majority [,].
Issues with plastic and biliary fully-covered self-expanding metallic stents (FCSEMS), such as blockage and migration, have led to the development and use of lumen-apposing metal stents (LAMS) since 2012 [,,,,]. Unlike traditional narrow-calibre plastic stents, LAMSs have been shown to be particularly resistant to occlusion by necrotic tissue within the pseudocyst in adults due to their larger lumen and the option of direct endoscopic necrosectomy via the stent itself [].
However, the literature on endoscopic transgastric LAMSs for PFCs in paediatric pancreatitis is limited to case reports and small series, which precludes drawing firm conclusions. We report our experience with two cases and a systematic review of the literature on the feasibility, efficacy, and safety of LAMSs in the endoscopic trans-gastric drainage of PFCs following acute pancreatitis in children.
2. Materials and Methods
This is a retrospective study of the first 2 cases at the National University Hospital, Singapore, and a review of the literature on the use of LAMSs for endoscopic trans-gastric drainage of PFCs in paediatric acute pancreatitis. This study was approved by the Central Institutional Review Board (Ref: 2019/2040).
(1) A 10-year-old boy presented with a 1-day history of abdominal pain, vomiting and fever (37.7 Celsius). Amylase and lipase were elevated to more than 3 times normal (863 U/L and 969 U/L, respectively). A computerised tomography (CT) scan on day 3 revealed an oedematous pancreas with PFC in the lesser sac (6.6 × 6.0 cm). Oral feeds could be commenced and progressed to a full diet by day 16 of admission. Vomiting recurred together with high-spiking fevers by day 18. A CT scan on day 20 revealed preserved homogenous enhancement of the pancreas and a large PFC (10.7 × 9.9 × 19.1 cm) with internal debris and well-defined walls that were abutting the stomach, causing a significant mass effect (Figure 1a). On day 21, under general anaesthesia, an endoscopic ultrasound (EUS)-guided cysto-gastrostomy was performed and a Boston Hot AXIOS LAMS (10 length × 15 mm diameter) was inserted (Figure 1b). Endoscopy was repeated one week later to perform a necrosectomy (using a grasper, Roth net, and snare) and lavage using hydrogen peroxide. A CT scan at 2 weeks after LAMS insertion revealed a reduction in the size of the PFC to 5.2 × 2.8 cm. Necrosectomy was repeated for persistent PFC on day 14 after the initial insertion of the LAMS (Figure 1c). Antibiotics were tailored to the results of the culture of the necrotic tissue obtained at endoscopy. His fevers settled, and his general condition improved with the re-commencement and establishment of full feeds by week 5 of admission. The LAMS was removed endoscopically using a snare at 5 weeks after insertion (Figure 1d). He was discharged well at 2 months after admission. Magnetic resonance cholangiopancreatography 4 months after discharge showed no pancreatic duct disruption or any pancreatic or bile duct anomaly. He remains well at 24 months with complete resolution of the PFC on follow-up ultrasound scans.
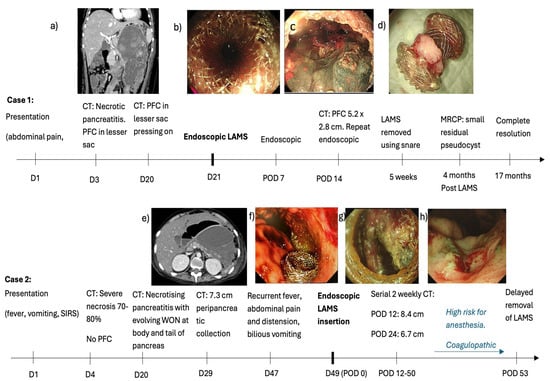
Figure 1.
Clinical course of two cases of acute pancreatitis with pancreatic fluid collections (PFCs). (a) Day 20 CT scan showing a large PFC abutting the stomach, causing a significant mass effect (b) Endoscopic ultrasound (EUS)-guided cysto-gastrostomy on Day 21 using a Boston Hot AXIOS LAMS (10 length × 15 mm diameter). (c) Necrosectomy repeated 14 days later (d) LAMS removed at 5 weeks (e) CT scan shows an enlarging PFC causing compression and anterior displacement of stomach. (f) LAMS deployed on day 49 of illness. (g) Endoscopic necrosectomy performed for persistent WON 50 days after LAMS insertion. (h) Patent healthy cystogastrostomy tract after removal of LAMS..
(2) A 17-year-old boy with relapsed pre-B cell acute lymphocytic leukaemia and Klinefelter syndrome presented with fever, nausea, and vomiting 13 days after receiving Peg-asparaginase. Amylase (890 U/L) and lipase (2084 U/L) were elevated to more than 3 times the upper limit of normal. A CT scan revealed acute pancreatitis with no obvious necrosis or PFC. This progressed to severe necrosis of 70–80% of the pancreatic parenchyma on day 4. There was no significant PFC. CT scan on day 20 for new-onset fever (>38 C) revealed necrotising pancreatitis with evolving WON in the body and tail of the pancreas. He developed thrombocytopenia in addition to his deranged INR, resulting in a spontaneous retroperitoneal hematoma on day 29. The CT scan, which was performed for the retroperitoneal haematoma, revealed similar appearances of the pancreas. He developed bilious vomiting with abdominal pain and distension by day 47. A CT scan revealed an enlarging PFC (12.2 cm from 7.3 cm), resulting in compression and anterior displacement of the stomach (Figure 1e). There was internal debris suggestive of WON. On day 49, under general anaesthesia, he underwent EUS and endoscopic cyst-gastrostomy with deployment of LAMS like case 1 as above. (Figure 1f) The aspirated cyst fluid was rich in amylase (1724 U/L vs. serum amylase 31 U/L), and cyst fluid culture yielded Stenotrophomonas and Actinomyces. He developed pancreatic endocrine and exocrine insufficiency requiring long-term insulin for hyperglycemia and transient octreotide for loose stools, respectively. He developed other co-morbidities such as mycobacterium tuberculosis pneumonia requiring a 4-drug anti-tuberculosis regimen, cytomegalovirus viraemia requiring ganciclovir, left iliac vein thrombosis, hypertension due to chronic steroid use, and BK virus haemorrhagic cystitis, which responded to ciprofloxacin and herpes simplex keratitis in his right eye. Further endoscopy for necrosectomy could not be performed because of his poor general condition, high risk for anaesthesia, and coagulopathy. The LAMS was left in situ with surveillance CT scans, which revealed a progressive decrease in maximum WON size from 12.2 cm to 6.7 cm and 6.2 cm on days 63, 75, and 93 of illness and days 12, 24, and 50 of the LAMS, respectively. In view of the improving and stable general condition with persistent residual WON, endoscopic necrosectomy was performed as per case 1 on day 93 of illness and day 50 of LAMS insertion (Figure 1g). The necrosectomy seemed complete with healthy tissue within the cavity. Hence, the LAMS was removed using a snare. At the end of the procedure, a patent healthy cystogastrostomy tract could be visualised (Figure 1h). A CT scan on the day after the LAMS removal revealed no debris and a small residual PFC. Our plan was to monitor progression with surveillance scans. Unfortunately, the family decided to bring the boy back home overseas, where he succumbed after 3 weeks despite continued management.
Literature Review
To perform as comprehensive a literature review as possible, we followed the PRISMA (Preferred Reporting Items for Systematic review and Meta-Analysis) 2020 checklist and searched the electronic databases of PubMed, Google Scholar, Science Direct, and EMBASE from April 2012 (with the first reported use of LAMSs for PFCs in the literature) until September 2024 using the following search terms: (“pancreatic fluid collection” OR “PFC” OR “walled-off necrosis” OR “WON” OR “pancreatic pseudocyst”) AND (“lumen apposing metal stent” OR “LAMS” OR “AXIOS stent”) AND (“pediatric” OR “paediatric” OR “children” OR “child” OR “adolescent” OR “infant” OR “teenager”) AND (“endoscopic drainage” OR “endoscopic ultrasound” OR “EUS-guided” OR “transgastric”) [,]. A database search was conducted from 5 September 2024 to 8 February 2025. References of identified studies were screened to identify further eligible studies. We included studies that (1) reported paediatric patients (<18 years) with (2) pancreatitis only (3) undergoing transgastric drainage using (4) LAMSs for PFCs, (5) provided sufficient clinical data, and (6) were in English. Titles and abstracts of all eligible studies retrieved were independently screened by two reviewers (TI and MV), with disagreements resolved by discussion. Full texts were assessed in detail for final inclusion in the review. Primary outcomes were technical and clinical success. Clinical success is defined as the resolution of PFCs, as verified on imaging or endoscopy. Secondary outcomes were procedure-related complications and recurrence of PFCs requiring further interventions. Studies were appraised using the Joanna Briggs Institute’s (JBI) critical appraisal checklist for cohort and case series/reports by 2 authors (TI, MV), with any discrepancies being resolved with a third author (ZJ) []. Statistical pooling was not performed due to heterogeneity and the descriptive nature of the included studies. As per PRISMA 2020 and Cochrane guidance, narrative synthesis is an acceptable approach when meta-analysis is not feasible [,].
3. Results
The National University Hospital is a tertiary academic medical centre in Singapore with adult and paediatric facilities at one site. The procedures were performed by a senior adult endoscopist with extensive expertise in paediatric interventional endoscopies. The time from presentation to insertion of LAMSs was 3 weeks and 7 weeks, respectively. There were no procedure-related or stent-related complications over a stent dwell time of 28 and 50 days, respectively. Stent removals were uneventful. There was no recurrence of PFCs in the one survivor.
Our literature search produced 231 records, of which 23 were duplicates. Through titles and abstract screening, a further 174 reports were excluded as per the following criteria: adult population (patients aged >18 years), reports of LAMS use for indications other than for drainage of PFCs, drainage conducted through the oesophagus, and reports of other methods of PFC drainage not attributed to LAMSs. The full text of the remaining 34 reports was reviewed and analysed. Only reports describing cases or case series of LAMS deployment for trans-gastric drainage of PFC (for both WON and pancreatic pseudocyst) in children ≤ 18 years were included. There were 9 studies that fulfilled the inclusion criteria after full-text review (Figure 2) (Table 1). All studies met the majority of the JBI checklist items; common limitations are in line with the nature of case reports and small case series, such as a lack of consecutive case inclusion. (Supplemental Material). There were two studies from the same author, one with single-institution and one with multi-institutional experience [,]. From a review of the two studies, we could identify five individual children who are included in this systematic review (Table 1).
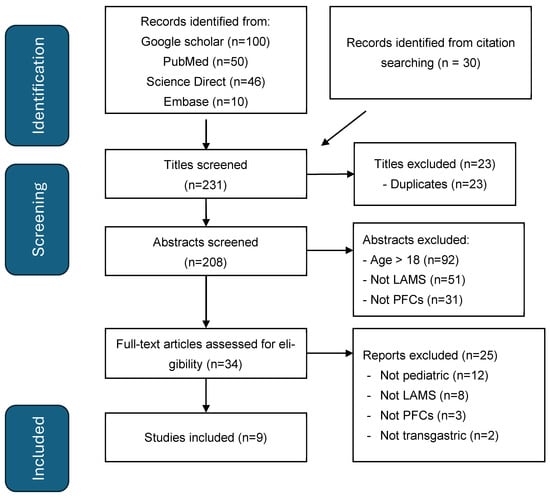
Figure 2.
PRISMA flow diagram for LAMSs in pancreatitis in children.

Table 1.
Patient characteristics from systematic review.
3.1. Patient Characteristics
There were 18 children who underwent endoscopic trans-gastric LAMS insertion for drainage of PFC in acute pancreatitis across the nine included studies, which were published between 2015 and 2024 (Table 1, Table 2 and Table 3). The mean age and follow-up were 12.3 years (range, 3 to 17 years) and 12 months (range, 2 to 44 months), respectively. There were 10 males. Chemotherapy (n = 6), idiopathic, trauma, and gallstone-pancreatitis (n = 2 each) accounted for the aetiology in the majority of the cases. (Table 1).
3.2. PFC Characteristics
A LAMS was inserted for symptomatic PFC (n = 2), WON (n = 7), pseudocysts (n = 4), and as a rescue for recurrent pseudocysts after initial endoscopic ultrasound-guided aspiration (n = 3) or percutaneous drainage (n = 1) or laparoscopic debridement (n = 1). The interval from presentation to the insertion of the LAMS was described in three cases (1, 4, and 12 weeks, respectively) [,,]. The size of the PFC at the LAMS insertion ranged from 5.5 to 22 cm. Two sizes of the LAMS were described, with 5 children using 10 × 10 mm LAMSs, and 13 children using 10 × 15 mm LAMSs, including the youngest child who was 3 years of age at the time of the LAMS insertion. The choice of LAMS size was not clearly dictated by the size or type of the PFC. Of the 18 children, 7 had one, and 4 had multiple endoscopic necrosectomies performed via the LAMS to achieve resolution of PFCs. For children requiring multiple endoscopic necrosectomies, the interval between necrosectomies was reported in three children and ranged from 4 to 14 days. The mean duration for which the LAMS was kept in situ was 41 days (range, 7 to 100 days) (Table 2).

Table 2.
LAMS details and treatment outcomes from systematic review.
Table 2.
LAMS details and treatment outcomes from systematic review.
| LAMS Details and Treatment (N = 18) | n (%) |
|---|---|
| Stent size (mm) | |
| 10 × 15 mm | 13 (72%) |
| 10 × 10 mm | 5 (28%) |
| Time to resolution, days, mean (range) | 40 (7–100) |
| Pseudocyst | 41.6 (19–100) |
| Walled-off necrosis | 40.6 (28–56) |
| Stent indwelling duration, days, mean (range) | 40 (7–100) |
| No. of cases requiring necrosectomy, n (%) | 8 (44%) |
| Interval between necrosectomies, days, mean (range) | 7 (4–14) |
| Follow-up duration, months, mean (range) | 12 (2–44) |
3.3. Outcomes
All studies reported 100% technical success with no procedural complications. All children recovered with resolution of their PFCs and without any recurrence over the duration of follow-up (12 months, range 2–44 months). Endoscopic removal of LAMSs was uneventful in all children. Migration of LAMSs into the colon (following collapse of the WON) was reported in one case (Table 3).

Table 3.
Studies on paediatric pancreatic fluid collections (PFCs) drainage with lumen-apposing metal stents (LAMSs) 2015–2024.
Table 3.
Studies on paediatric pancreatic fluid collections (PFCs) drainage with lumen-apposing metal stents (LAMSs) 2015–2024.
| Author/Year | No. | Age (Year) | M/F | Aetiology | Type of PFC | Indication for Drainage | Time to LAMS Insertion (Days) | PFC Size (cm) | Stent Size (mm) | Time to Resolution (Days) | LAMS Indwell Duration (Days) | No. of Necro-sectomy | Necrosectomy Interval (Days) | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nieto et al., 2015 [] | 3 | 3–16 | 3 M | 2 Trauma 1 Alcohol | 1 pseudocyst 2 WON (3 first-line) | Unknown | Unknown | 5.5–14 | 10 × 15 | 30 | 30 | 1 1 0 | Unknown | 2–9 |
| 1 | 7 | F | Familial | Pseudocyst (Rescue, EUS aspiration) | Pressure | Unknown (third recurrence) | 7 | 10 × 10 | 30 | 30 | 0 | NA | 4 | |
| 1 | 6 | F | Metastatic neuro- blastoma | Pseudocyst (Rescue, EUS aspiration) | Pressure | Unknown (second recurrence) | 7.6 | 10 × 10 | 30 | 30 | 0 | NA | 4 | |
| Trindade et al., 2016 [] | 1 | 14 | M | Psychiatric Medication | WON (First-line) | Sepsis | Unknown | 10 | 10 × 10 | 56 | 56 | 1 | 7 | 2 |
| Giefer et al., 2016 [] | 1 | 11 | F | Gallstone | Symptomatic PFC (First-line) | Pressure | 28 | 20 | 10 × 15 | 30 | 14 | 3 | Unknown | 2 |
| Bang et al., 2016 [] | 1 | 17 | F | Idiopathic | WON (First-line) | Unknown | Unknown | 6 | 10 × 15 | Un-known | 56 | 0 | NA | 26 (median) |
| Kim C et al., 2019 [] | 2 | 17 12 | M M | Gallstone Idiopathic | Pseudocyst WON | Abdominal pain Abdominal pain | Unknown Unknown | 11 10 | 10 × 10 10 × 15 | 21 Unknown | 21 Unknown | 0 2 | NA Unknown | Unknown |
| Costa et al., 2020 [] | 1 | 12 | F | Chemo- therapy | Pseudocyst (First-line) | Pressure | Unknown | Unknown | 10 × 15 | 100 | 100 | 0 | NA | Unknown |
| 1 | 17 | M | Chemo- therapy | Pseudocyst (Rescue, percutaneous drainage) | Pressure | Unknown (First recurrence) | Unknown | 10 × 15 | 34 | 34 | 0 | NA | Unknown | |
| 1 | 11 | M | Chemo- therapy | Pseudocyst (First-line) | Pressure | Unknown | Unknown | 10 × 15 | 82 | 82 | 2 (D37, D41) | 4 | Unknown | |
| Li et al., 2023 [] | 1 | 15 | F | COVID-19 | WON (First-line) | Sepsis | Unknown | 9 | 10 × 15 | 42 | 42 | 4 | 7–14 | 18 |
| Fiumana et al., 2023 [] | 1 | 11 | F | Chemo- therapy | Symptomatic PFC (First-line) | Pressure | 19 | 13 | 10 × 10 | 7 | 7 | 1 | NA | 8 |
| Pasqualetto et al., 2024 [] | 1 | 15 | F | Pancreatic leak post surgery | Pseudocyst (Rescue, laparoscopic debridement) | Pressure | 7 | 10 | 10 × 15 | 19 | 21 | 0 | NA | 44 |
| 1 | 15 | M | Chemo- therapy (B ALL) | Pseudocyst (Rescue, EUS aspiration) | Sepsis (fever) | Unknown (second recurrence) | 12 | 10 × 15 | 28 | 28 | 0 | NA | 15 | |
| 1 | 10 | M | Chemo- therapy (T LL) | WON (First-line) | Pressure | Unknown | 22 | 10 × 15 | 48 | 48 | 0 | NA | 12 | |
| Our experience | 1 | 10 | M | Steroids | WON (First-line) | Sepsis | 21 | 19.1 | 10 × 15 | 28 | 28 | 3 | 7 | 7 |
| 1 | 17 | M | PEG-asparaginase | WON (First-line) | Pressure | 49 | 12.2 | 10 × 15 | 50 | 50 | 2 | 50 | 1 |
4. Discussion
We report our initial experience with two cases and the evidence from our literature review on endoscopic trans-gastric drainage of PFCs using LAMSs in paediatric acute pancreatitis. In our limited experience with two cases performed by a senior adult interventional endoscopist with paediatric expertise, LAMSs were indicated for PFCs causing extrinsic pressure effects and/or sepsis. In expert hands, LAMS insertion was a technical success and achieved drainage of the PFCs. There were no complications related to the insertion or during the stent dwell period of 28 and 50 days, respectively.
Furthermore, we narrate the synthesised evidence from a review of 18 children who underwent endoscopic transgastric LAMS insertion for the drainage of PFCs due to acute pancreatitis from nine studies published between 2015 and 2024, with the initial paediatric report in the literature in 2015 [,].
The endoscopic management of PFCs in acute pancreatitis evolved from plastic stents to FCSEMS and, recently, to the use of LAMSs.The most common indications for LAMSs in paediatric pancreatitis include first-line treatment for WON and drainage of recurrent pseudocyst after prior intervention [,,,,,,,].
EUS-guided transgastric LAMSs achieved 100 % technical and clinical success in all children. The prerequisites for the technical success of FCSEMS in children were the maturity of the PFCs to 4 weeks or longer, proximity to the stomach wall (<1.5 cm), and a body weight in excess of 15 kg []. Similarly, the criteria for LAMS success in children were deemed to be (a) maturity of the PFCs with a well-defined wall thicker than 1 mm, and (b) a < 1 cm gap between the adjacent walls of the PFCs and the stomach, with the body weight not finding mention as a criterion in our systematic review []. NASPGHAN recommends a minimum interval of 4 weeks to intervention for necrosis in paediatric pancreatitis []. The interval between initial presentation and insertion of LAMSs in our systematic review ranged from 1 to 19 weeks, with the shortest interval of 1 week being for postoperative PFCs following pancreatectomy, which recurred despite laparoscopic drainage. Pre-LAMS Magnetic Resonance Imaging had characterised the wall of the PFC, which was abutting the stomach []. In our first case, LAMS insertion at 3 weeks was a technical and clinical success because of progressive thickening of the wall of the PFC, which was adjacent to the stomach. Size is a criterion in adult guidelines for LAMS drainage of PFCs []. However, based on our review, size or age alone may not influence the success of LAMSs since the youngest child was 3 years old and the largest PFC was 22 cm in size [,].
As a rescue measure, LAMS insertion was successful for drainage of PFCs in five children following either endoscopic ultrasound-guided aspiration (n = 3), percutaneous drainage (n = 1), or laparoscopic drainage (n = 1) [,,].
The excellent success of LAMSs may be due to its unique configuration, which enables ease of technical insertion by a single operator with reduced exchanges. EUS maps a passage between the stomach wall and the PFC, thereby obviating the need for either a visible bulge in the gastric lumen or a guidewire or fluoroscopy as a guide to the PFC, and ensures Doppler identification and avoidance of any sizeable blood vessels during deployment. The electrocautery-based catheter delivery system reduces the risk of intra-procedural bleeding []. The saddle shape with the flanges at the two ends ensures close apposition between the walls of the PFC and the stomach, thereby preventing dislocation or migration. Reportedly, the LAMS is amenable to endoscopic repositioning in case of mis-deployment at the initial procedure, which was not required in any of our cases or in this systematic review []. The effective lumen obviates the need for dilatation, ensures efficient drainage and provides access for endoscopic necrosectomy through the stent. Direct endoscopic necrosectomy, either at the time of insertion of the LAMS or subsequently, was indicated for clinically symptomatic collections with debris. Although it is conceivable that food particles may enter and occlude the LAMS, this has not been observed in our two cases and in this systematic review. Therefore, patency measures such as multiple pigtail stents within the lumen of the LAMS are not necessary.
Paediatric guidelines recommend LAMS removal within 4 weeks for pseudocysts and 6 weeks for WON []. Our literature review revealed that the mean dwell time for LAMS was up to 6 weeks (41 days). Five children tolerated a stent in dwell time beyond 6 weeks without any complications over a mean follow-up of 13.3 months (range, 2–26 months). The reported maximum duration of the stent dwell was 100 days for persistence of pseudocyst due to a disrupted pancreatic duct that required a pancreatic stent 1 month after LAMS insertion []. LAMS removal could be performed with basic endoscopic techniques. Our review and institutional experience demonstrated no recurrence after LAMS removal without insertion of prophylactic plastic stents at LAMS removal. Therefore, the LAMS was effective in reducing the need for additional interventions for PFC in children, which agrees with published literature in the adult population [].
Multi-centre adult studies report the risk of perforation (5%) and bleeding (14%) with direct endoscopic necrosectomy, and stent burial with prolonged indwelling from tissue in-growth, pseudoaneurysm, and bleeding from mucosal erosion [,,]. These were not observed in our two cases, and in this systematic review which included a maximum stent dwell of 50 days and 100 days, respectively. We speculate that increasing application of LAMS may further reveal its safety profile in the paediatric population in comparison to published large-scale adult studies [].
Our study is limited by short follow-up time (2–44 months). In addition, the literature on this emerging field in children consists largely of case reports and small case series. Due to the limited numbers, comparisons with plastic stents and adult literature, including meta-analyses, were not performed because of the possible skewed data for paediatric LAMSs.
To the best of our knowledge, guidelines for endoscopic LAMSs for PFCs, especially WON, in paediatric pancreatitis are forthcoming. Further to the initial success with our first two cases, we are inclined to cautiously adopt endoscopic transgastric LAMSs as our initial approach for the drainage of WON in paediatric pancreatitis, as guided by CT characterisation of maturity and proximity to the stomach.
5. Conclusions
In conclusion, endoscopic transgastric LAMSs, together with endoscopic necrosectomy when indicated, may be a technically feasible method of drainage of WON in acute pancreatitis in children with minimal complications. Centres with expertise in advanced therapeutic endoscopy may consider LAMSs as the first-line in a step-up approach for the drainage of pancreatic WON in conjunction with a multidisciplinary team for the management of complicated pancreatitis in children. Large, multi-centre paediatric studies may further characterise the efficacy and safety of LAMSs in comparison to other stents and drainage procedures in the adult population.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/children12080965/s1, Evaluation of studies using JBI.
Author Contributions
Conceptualization, I.W.H.T. and V.P.M.; Methodology, I.W.H.T. and V.P.M.; Validation, I.W.H.T. and V.P.M.; Formal analysis, I.W.H.T.; Resources, Z.J.K., K.-Y.H., S.V.K. and V.P.M.; Data curation, I.W.H.T., Z.J.K. and K.-Y.H.; Writing—original draft, I.W.H.T.; Writing—review & editing, I.W.H.T., Z.J.K. and V.P.M.; Supervision, Z.J.K., K.-Y.H., S.V.K. and V.P.M.; Project administration, I.W.H.T.; Funding acquisition, V.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Institutional Review Board Statement
Our study is exempted according to SingHealth Centralized Institutional Review Board (CIRB reference number: 2019/2040) waiver of consent for retrospective studies.
Informed Consent Statement
Patient consent was waived as per ethics approval.
Data Availability Statement
Data sharing is not applicable as no separate datasets were generated and/or analysed for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PFC | Pancreatic fluid collections |
| WON | Walled-off necrosis |
| LAMS | Lumen-apposing metal stent |
| NUH | National University Hospital |
| NASPGHAN | North American Society for Paediatric Gastroenterology, Hepatology and Nutrition |
| FCSEMS | Biliary fully covered self-expanding metallic stents |
| CT | Computerised tomography scan |
| EUS | Endoscopic ultrasound |
| INR | International Normalized Ratio (coagulation) |
References
- Lal, S.B.; Venkatesh, V.; Rana, S.S.; Anushree, N.; Bhatia, A.; Saxena, A. Paediatric Acute Pancreatitis: Clinical Profile and Natural History Of Collections Short Title: Outcome of Collections in Paediatric Acute Pancreatitis. Pancreatology 2020, 20, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Majbar, A.A.; Cusick, E.; Johnson, P.; Lynn, R.M.; Hunt, L.P.; Shield, J.P. Incidence and Clinical Associations of Childhood Acute Pancreatitis. Pediatrics 2016, 138, e20161198. [Google Scholar] [CrossRef] [PubMed]
- Bolia, R.; Srivastava, A.; Yachha, S.K.; Poddar, U.; Kumar, S. Prevalence, Natural History, and Outcome of Acute Fluid Collection and Pseudocyst in Children with Acute Pancreatitis. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.; Lee, H.; Huang, F.; Ho, M.; Kao, H.; Liang, D.; Hsu, C.; Hung, H.; Chang, P.; Sheu, J. Pancreatitis in Children —Experience with 43 Cases. Eur. J. Pediatr. 1996, 155, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Abu-El-Haija, M.; Kumar, S.; Quiros, J.A.; Balakrishnan, K.; Barth, B.; Bitton, S.; Eisses, J.F.; Foglio, E.J.; Fox, V.; Francis, D. Management of Acute Pancreatitis in the Pediatric Population: A Clinical Report from the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Pancreas Committee. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 159–176. [Google Scholar] [CrossRef] [PubMed]
- van Brunschot, S.; van Grinsven, J.; van Santvoort, H.C.; Bakker, O.J.; Besselink, M.G.; Boermeester, M.A.; Bollen, T.L.; Bosscha, K.; Bouwense, S.A.; Bruno, M.J. Endoscopic or Surgical Step-up Approach for Infected Necrotising Pancreatitis: A Multicentre Randomised Trial. Lancet 2018, 391, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Tenner, S.; Vege, S.S.; Sheth, S.G.; Sauer, B.; Yang, A.; Conwell, D.L.; Yadlapati, R.H.; Gardner, T.B. American College of Gastroenterology Guidelines: Management of Acute Pancreatitis. Off. J. Am. Coll. Gastroenterol. ACG 2024, 119, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, Y.; Okata, Y.; Toyama, H.; Yoshimura, S.; Uemura, S.; Hatakeyama, T.; Bitoh, Y. Laparoscopic Necrosectomy for Walled-off Necrosis Following Necrotizing Pancreatitis. Pediatr. Int. 2023, 65, e15569. [Google Scholar] [CrossRef] [PubMed]
- Boston Scientific. AXIOS Stent and Electrocautery Enhanceddelivery System. Available online: https://www.bostonscientific.com/en-US/products/stents--gastrointestinal/axios-stent-and-electrocautery-enhanced-delivery-system.html (accessed on 9 January 2025).
- Itoi, T.; Binmoeller, K.F.; Shah, J.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N. Clinical Evaluation of a Novel Lumen-Apposing Metal Stent for Endosonography-Guided Pancreatic Pseudocyst and Gallbladder Drainage (with Videos). Gastrointest. Endosc. 2012, 75, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Talreja, J.P.; Shami, V.M.; Ku, J.; Morris, T.D.; Ellen, K.; Kahaleh, M. Transenteric Drainage of Pancreatic-Fluid Collections with Fully Covered Self-Expanding Metallic Stents (with Video)—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0016510708020841 (accessed on 27 January 2025).
- Penn, D.E.; Draganov, P.V.; Wagh, M.S.; Forsmark, C.E.; Gupte, A.R.; Chauhan, S.S. Prospective Evaluation of the Use of Fully Covered Self-Expanding Metal Stents for EUS-Guided Transmural Drainage of Pancreatic Pseudocysts. Gastrointest. Endosc. 2012, 76, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, C.; Luigiano, C.; Cennamo, V.; Polifemo, A.M.; Barresi, L.; Jovine, E.; Traina, M.; D’Imperio, N.; Tarantino, I. Endoscopic Ultrasound-Guided Transmural Drainage of Infected Pancreatic Fluid Collections with Placement of Covered Self-Expanding Metal Stents: A Case Series. Endoscopy 2012, 44, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Kumta, N.A.; Tyberg, A.; Bhagat, V.H.; Siddiqui, A.A.; Kowalski, T.E.; Loren, D.E.; Desai, A.P.; Sarkisian, A.M.; Brown, E.G.; Karia, K. EUS-Guided Drainage of Pancreatic Fluid Collections Using Lumen Apposing Metal Stents: An International, Multicenter Experience. Dig. Liver Dis. 2019, 51, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetc, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2024. [Google Scholar]
- Cochrane Handbook for Systematic Reviews of Interventions|Cochrane. Available online: https://www.cochrane.org/authors/handbooks-and-manuals/handbook (accessed on 6 July 2025).
- Nieto, J.; Hathaway, K. Endoscopic Ultrasound-Guided Pseudocyst Drainage Using a Novel Lumen-Apposing Metal Stent (AXIOS) in Two Pediatric Patients: 2018. Off. J. Am. Coll. Gastroenterol. ACG 2014, 109, S597. [Google Scholar] [CrossRef]
- Nieto, J.; Koul, A.; Willingham, F.F. Sa1658 EUS-Guided Drainage of Pancreatic Fluid Collections in Pediatric Patients Using a Novel Fully Covered Lumen-Apposing Self Expanding Metal Stent: A Multicenter Experience. Gastrointest. Endosc. 2015, 81, AB298. [Google Scholar] [CrossRef]
- Pasqualetto, A.F.; Boroni, G.; Moneghini, D.; Parolini, F.; Orizio, P.; Bulotta, A.L.; Missale, G.; Alberti, D. Single Center Experience of Eus-Guided Cystogastrostomy and Lumen-Apposing Metal Stent (LAMS) Positioning in Children with Pancreatic Fluid Collections: A Case Series. Children 2024, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Trindade, A.J.; Inamdar, S.; Bitton, S. Pediatric Application of a Lumen-Apposing Metal Stent for Transgastric Pancreatic Abscess Drainage and Subsequent Necrosectomy. Endoscopy 2016, 48, E204–E205. [Google Scholar] [CrossRef] [PubMed]
- Giefer, M.J.; Balmadrid, B.L. Pediatric Application of the Lumen-Apposing Metal Stent for Pancreatic Fluid Collections. Gastrointest. Endosc. 2016, 84, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Varadarajulu, S. Endoscopic Treatment of Walled-off Necrosis in Children: Clinical Experience and Treatment Outcomes. J. Pediatr. Gastroenterol. Nutr. 2016, 63, e31–e35. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Trevino, J. Sa2060 PEDIATRIC APPLICATIONS OF A LUMEN-APPOSING METAL STENT (LAMS): A CASE SERIES. Gastrointest. Endosc. 2019, 89, AB290. [Google Scholar] [CrossRef]
- Costa, P.A.; Ho, S.; Manvar, A.; Rivas, Y.; Novak, I. Use of Lumen-Apposing Metal Stents for Endoscopic Drainage of Intra-Abdominal Fluid Collections in Pediatric Patients. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mostafavi, M.; Miller, J.W., Jr.; Hirsch, B.; O’Connor, S.; Sayej, W.N. Severe Necrotizing Pancreatitis in a Pediatric Patient with COVID-19: A Case Report. JPGN Rep. 2023, 4, e307. [Google Scholar] [CrossRef] [PubMed]
- Fiumana, G.; Pancaldi, A.; Bertani, H.; Boarino, V.; Cellini, M.; Iughetti, L. Asparaginase-Associated Pancreatitis Complicated by Pancreatic Fluid Collection Treated with Endoscopic Cistogastrostomy in Pediatric Acute Lymphoblastic Leukemia: A Case Report and Systematic Review of the Literature. Clin. Hematol. Int. 2023, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Kowalski, T.E.; Loren, D.E.; Khalid, A.; Soomro, A.; Mazhar, S.M.; Isby, L.; Kahaleh, M.; Karia, K.; Yoo, J.; et al. Fully Covered Self-Expanding Metal Stents versus Lumen-Apposing Fully Covered Self-Expanding Metal Stent versus Plastic Stents for Endoscopic Drainage of Pancreatic Walled-off Necrosis: Clinical Outcomes and Success. Gastrointest. Endosc. 2017, 85, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Armellini, E.; Metelli, F.; Anderloni, A.; Cominardi, A.; Aragona, G.; Marini, M.; Pace, F. Lumen-Apposing-Metal Stent Misdeployment in Endoscopic Ultrasound-Guided Drainages: A Systematic Review Focusing on Issues and Rescue Management. World J. Gastroenterol. 2023, 29, 3341–3361. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.; Biermer, M.; Schmitt, W.; Jurgensen, C.; Will, U.; Gerlach, R.; Kreitmair, C.; Meining, A.; Wehrmann, T.; Rosch, T. Transluminal Endoscopic Necrosectomy after Acute Pancreatitis: A Multicentre Study with Long-Term Follow-up (the GEPARD Study). Gut 2009, 58, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Perbtani, Y.B.; Mramba, L.K.; Kerdsirichairat, T.; Prabhu, A.; Manvar, A.; Ho, S.; Pannu, D.; Keswani, R.N.; Strand, D.S. Safety and Rate of Delayed Adverse Events with Lumen-Apposing Metal Stents (LAMS) for Pancreatic Fluid Collections: A Multicenter Study. Endosc. Int. Open 2018, 6, E1267–E1275. [Google Scholar] [CrossRef] [PubMed]
- Bazaga, S.; García-Alonso, F.J.; Tormo, J.R.A.; Moreno, B.M.; Sanchiz, V.; Suria, C.; Garcia-Sumalla, A.; Gornals, J.B.; Chavarría, C.; Loras, C.; et al. Endoscopic Removal of Lumen-Apposing Metal Stents—Risk Factors for Stent Embedment, Complex Removals, and Adverse Events: Analysis from a Multicenter Prospective Case Series. Endoscopy 2023, 55, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Adler, D.G.; Nieto, J.; Shah, J.N.; Binmoeller, K.F.; Kane, S.; Yan, L.; Laique, S.N.; Kowalski, T.; Loren, D.E. EUS-Guided Drainage of Peripancreatic Fluid Collections and Necrosis by Using a Novel Lumen-Apposing Stent: A Large Retrospective, Multicenter US Experience (with Videos). Gastrointest. Endosc. 2016, 83, 699–707. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).