Biological, Psychosocial, and Microbial Determinants of Childhood-Onset Obsessive–Compulsive Disorder: A Narrative Review
Abstract
1. Introduction
2. Method
3. Biological Determinants in the Etiology of OCD
3.1. Genetic Factors
3.2. Epigenetic Factors
3.3. Neurobiological Factors
3.3.1. Structural Abnormalities
3.3.2. Dysregulation of Specific Neurotransmitters
3.4. Immune Factors
4. Psychosocial Determinants in the Etiology of OCD
4.1. Psychological Mechanisms
4.1.1. Behavioral and Cognitive Processes
4.1.2. Metacognitive and Inferential Biases
4.1.3. Emotional Dynamics
4.2. Impact of Early-Life Adversity
4.3. Other Major Psychosocial Influences
4.3.1. Internalized Stigma
4.3.2. Family Environment and Parenting
4.3.3. Dermatological Health and Psychosocial Interactions
4.3.4. Lifestyle Patterns: Physical Activity and Diet
4.3.5. Functional Impact and Psychosocial Burden
5. The Gut Microbiome in OCD
5.1. GM Dysbiosis and OCD
5.2. GM Dysbiosis and Neurotransmitters
5.3. GM Dysbiosis and Environmental Factors
5.4. Comparative GM Dysbiosis in OCD and Related Conditions
6. Psychobiotics as Therapeutic Tools for OCD
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OCD | Obsessive–compulsive disorder |
| OCS | Obsessive–compulsive symptoms |
| GM | Gut microbiome |
| GBA | Gut–brain axis |
| GAD | Generalized anxiety disorder |
| MDD | Major depressive disorder |
| ADHD | Attention-deficit/hyperactivity disorder |
| ELS | Early-life stress |
| CSTC | Cortico-striato-thalamo-cortical |
| GWAS | Genome-wide association studies |
| MAF | Minor allele frequency |
| CNV | Copy number variant |
| WES | Whole exome sequencing |
| DNAm | DNA methylation |
| miRNA | microRNA |
| EWAS | Epigenome-wide association studies |
| fMRI | Functional magnetic resonance imaging |
| SRIs | Serotonin reuptake inhibitors |
| SSRIs | Selective serotonin reuptake inhibitors |
| PANDAS | Pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection |
| PANS | Pediatric acute-onset neuropsychiatric syndrome |
| TAF | Thought–action fusion |
| NP | Negative priming |
| IBA | Inverse Bayesian account |
| SLEs | Stressful life events |
| ACEs | Adverse childhood experiences |
| SCFA | Short-chain fatty acid |
| CNS | Central nervous system |
| MR | Mendelian randomization |
| BBB | Blood–brain barrier |
| ASD | Autism spectrum disorder |
| GABA | γ-aminobutyric acid |
| CBT | Cognitive–behavioral therapy |
| GOS | Galactooligosaccharides |
| FOS | Fructooligosaccharides |
| NJREs | Not just right experiences |
| FMT | Fecal microbiota transplantation |
References
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Revision (DSM-5-TR); American Psychiatric Association: Arlington, VA, USA, 2022. [Google Scholar]
- Stein, D.J.; Costa, D.L.C.; Lochner, C.; Miguel, E.C.; Reddy, Y.C.J.; Shavitt, R.G.; van den Heuvel, O.A.; Simpson, H.B. Obsessive-compulsive disorder. Nat. Rev. Dis. Primers 2019, 5, 52. [Google Scholar] [CrossRef]
- Cervin, M. Obsessive-Compulsive Disorder: Diagnosis, Clinical Features, Nosology, and Epidemiology. Psychiatr. Clin. N. Am. 2023, 46, 1–16. [Google Scholar] [CrossRef]
- Hezel, D.M.; McNally, R.J. A theoretical review of cognitive biases and deficits in obsessive-compulsive disorder. Biol. Psychol. 2016, 121, 221–232. [Google Scholar] [CrossRef]
- García-Soriano, G.; Belloch, A. Symptom dimensions in obsessive-compulsive disorder: Differences in distress, interference, appraisals and neutralizing strategies. J. Behav. Ther. Exp. Psychiatry 2013, 44, 441–448. [Google Scholar] [CrossRef]
- Ferreira, S.; Pêgo, J.M.; Morgado, P. A Systematic Review of Behavioral, Physiological, and Neurobiological Cognitive Regulation Alterations in Obsessive-Compulsive Disorder. Brain Sci. 2020, 10, 797. [Google Scholar] [CrossRef]
- Vivan, A.D.S.; Rodrigues, L.; Wendt, G.; Bicca, M.G.; Braga, D.T.; Cordioli, A.V. Obsessive-compulsive symptoms and obsessive-compulsive disorder in adolescents: A population-based study. Braz. J. Psychiatry 2014, 36, 111–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Geller, D.A. Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiatr. Clin. N. Am. 2006, 29, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Stiede, J.T.; Spencer, S.D.; Onyeka, O.; Mangen, K.H.; Church, M.J.; Goodman, W.K.; Storch, E.A. Obsessive-Compulsive Disorder in Children and Adolescents. Annu. Rev. Clin. Psychol. 2024, 20, 355–380. [Google Scholar] [CrossRef]
- Ruscio, A.M.; Stein, D.J.; Chiu, W.T.; Kessler, R.C. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol. Psychiatry 2010, 15, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Albert, U.; Manchia, M.; Tortorella, A.; Volpe, U.; Rosso, G.; Carpiniello, B.; Maina, G. Admixture analysis of age at symptom onset and age at disorder onset in a large sample of patients with obsessive-compulsive disorder. J. Affect. Disord. 2015, 187, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Selles, R.R.; Storch, E.A.; Lewin, A.B. Variations in symptom prevalence and clinical correlates in younger versus older youth with obsessive-compulsive disorder. Child Psychiatry Hum. Dev. 2014, 45, 666–674. [Google Scholar] [CrossRef]
- Farrell, L.; Barrett, P.; Piacentini, J. Obsessive-Compulsive Disorder Across the Developmental Trajectory: Clinical Correlates in Children, Adolescents and Adults. Behav. Change 2006, 23, 103–120. [Google Scholar] [CrossRef]
- Mathes, B.M.; Morabito, D.M.; Schmidt, N.B. Epidemiological and Clinical Gender Differences in OCD. Curr. Psychiatry Rep. 2019, 21, 36. [Google Scholar] [CrossRef]
- Sharma, E.; Sharma, L.P.; Balachander, S.; Lin, B.; Manohar, H.; Khanna, P.; Lu, C.; Garg, K.; Thomas, T.L.; Au, A.C.L.; et al. Comorbidities in Obsessive-Compulsive Disorder Across the Lifespan: A Systematic Review and Meta-Analysis. Front. Psychiatry 2021, 12, 703701. [Google Scholar] [CrossRef]
- Storch, E.A.; Merlo, L.J.; Larson, M.J.; Geffken, G.R.; Lehmkuhl, H.D.; Jacob, M.L.; Murphy, T.K.; Goodman, W.K. Impact of comorbidity on cognitive-behavioral therapy response in pediatric obsessive-compulsive disorder. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 583–592. [Google Scholar] [CrossRef]
- Ujjwal, P.; Sanjita, D.; Kumar, F.N. A Comprehensive Review on Obsessive-Compulsive Disorder: An Update. Pharmacophore 2024, 15, 54–62. [Google Scholar] [CrossRef]
- Blanco-Vieira, T.; Radua, J.; Marcelino, L.; Bloch, M.; Mataix-Cols, D.; do Rosário, M.C. The genetic epidemiology of obsessive-compulsive disorder: A systematic review and meta-analysis. Transl. Psychiatry 2023, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Mahjani, B.; Bey, K.; Boberg, J.; Burton, C. Genetics of obsessive-compulsive disorder. Psychol. Med. 2021, 51, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Kracker Imthon, A.; Antônio Caldart, C.; do Rosário, M.C.; Fontenelle, L.F.; Constantino Miguel, E.; Arzeno Ferrão, Y. Stressful Life Events and the Clinical Expression of Obsessive-Compulsive Disorder (OCD): An Exploratory Study. J. Clin. Med. 2020, 9, 3371. [Google Scholar] [CrossRef]
- Mataix-Cols, D.; Boman, M.; Monzani, B.; Rück, C.; Serlachius, E.; Långström, N.; Lichtenstein, P. Population-based, multigenerational family clustering study of obsessive-compulsive disorder. JAMA Psychiatry 2013, 70, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Batistuzzo, M.C.; Sottili, B.A.; Shavitt, R.G.; Lopes, A.C.; Cappi, C.; de Mathis, M.A.; Pastorello, B.; Diniz, J.B.; Silva, R.M.F.; Miguel, E.C.; et al. Lower Ventromedial Prefrontal Cortex Glutamate Levels in Patients with Obsessive-Compulsive Disorder. Front. Psychiatry 2021, 12, 668304. [Google Scholar] [CrossRef] [PubMed]
- Jalal, B.; Chamberlain, S.R.; Sahakian, B.J. Obsessive-compulsive disorder: Etiology, neuropathology, and cognitive dysfunction. Brain Behav. 2023, 13, e3000. [Google Scholar] [CrossRef]
- Robbins, T.W.; Vaghi, M.M.; Banca, P. Obsessive-Compulsive Disorder: Puzzles and Prospects. Neuron 2019, 102, 27–47. [Google Scholar] [CrossRef]
- Karagüzel, E.Ö.; Arslan, F.C.; Uysal, E.K.; Demir, S.; Aykut, D.S.; Tat, M.; Karahan, S.C. Blood levels of interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha and cognitive functions in patients with obsessive compulsive disorder. Compr. Psychiatry 2019, 89, 61–66. [Google Scholar] [CrossRef]
- Taylor, S.; Asmundson, G.J.; Jang, K.L. Etiology of obsessive-compulsive symptoms and obsessive-compulsive personality traits: Common genes, mostly different environments. Depress. Anxiety 2011, 28, 863–869. [Google Scholar] [CrossRef]
- Hühne, V.; Dos Santos-Ribeiro, S.; Moreira-de-Oliveira, M.E.; de Menezes, G.B.; Fontenelle, L.F. Towards the Correlates of Stressful Life Events as Precipitants of Obsessive-Compulsive Disorder: A Systematic Review and Metanalysis. CNS Spectr. 2024, 29, 252–260. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. An updated overview on the relationship between human gut microbiome dysbiosis and psychiatric and psychological disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 128, 110861. [Google Scholar] [CrossRef]
- Turna, J.; Grosman Kaplan, K.; Anglin, R.; Van Ameringen, M. “What’s Bugging the Gut in OCD?” A Review of the Gut Microbiome in Obsessive-Compulsive Disorder. Depress. Anxiety 2016, 33, 171–178. [Google Scholar] [CrossRef]
- Turna, J.; Patterson, B.; Van Ameringen, M. An Update on the Relationship Between the Gut Microbiome and Obsessive-Compulsive Disorder. Psychiatr. Ann. 2017, 47, 542–551. [Google Scholar] [CrossRef][Green Version]
- Rees, J.C. Obsessive-compulsive disorder and gut microbiota dysregulation. Med. Hypotheses 2014, 82, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Kantak, P.A.; Bobrow, D.N.; Nyby, J.G. Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behav. Pharmacol. 2014, 25, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Nishino, R.; Mikami, K.; Takahashi, H.; Tomonaga, S.; Furuse, M.; Hiramoto, T.; Aiba, Y.; Koga, Y.; Sudo, N. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol. Motil. 2013, 25, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Sanikhani, N.S.; Modarressi, M.H.; Jafari, P.; Vousooghi, N.; Shafei, S.; Akbariqomi, M.; Heidari, R.; Lavasani, P.S.; Yazarlou, F.; Motevaseli, E.; et al. The Effect of Lactobacillus casei Consumption in Improvement of Obsessive-Compulsive Disorder: An Animal Study. Probiotics Antimicrob. Proteins 2020, 12, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Sukhera, J. Narrative Reviews: Flexible, Rigorous, and Practical. J. Grad. Med. Educ. 2022, 14, 414–417. [Google Scholar] [CrossRef]
- Browne, H.A.; Gair, S.L.; Scharf, J.M.; Grice, D.E. Genetics of Obsessive-Compulsive Disorder and Related Disorders. Psychiatr. Clin. N. Am. 2014, 37, 319–335. [Google Scholar] [CrossRef]
- Iervolino, A.C.; Rijsdijk, F.V.; Cherkas, L.; Fullana, M.A.; Mataix-Cols, D. A multivariate twin study of obsessive-compulsive symptom dimensions. Arch. Gen. Psychiatry 2011, 68, 637–644. [Google Scholar] [CrossRef]
- den Braber, A.; Rodrigues Zilhao Nogueira, N.; Fedko, I.O.; Hottenga, J.J.; Pool, R.; Smit, D.J.A.; Cath, D.C.; Boomsma, D.I. Obsessive-compulsive symptoms in a large population-based twin-family sample are predicted by clinically based polygenic scores and by genome-wide SNPs. Transl. Psychiatry 2016, 6, e731. [Google Scholar] [CrossRef]
- Stewart, S.E.; Yu, D.; Scharf, J.M.; Neale, B.M.; Fagerness, J.A.; Mathews, C.A.; Arnold, P.D.; Evans, P.D.; Gamazon, E.R.; Davis, L.K.; et al. Genome-wide association study of obsessive-compulsive disorder. Mol. Psychiatry 2013, 18, 788–798. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-wide association studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Strom, N.I.; Halvorsen, M.W.; Tian, C.; Rück, C.; Kvale, G.; Hansen, B.; Bybjerg-Grauholm, J.; Grove, J.; Boberg, J.; Nissen, J.B.; et al. Genome-wide association study identifies new loci associated with OCD. medRxiv 2024. [Google Scholar] [CrossRef]
- Dickson, S.P.; Wang, K.; Krantz, I.; Hakonarson, H.; Goldstein, D.B. Rare Variants Create Synthetic Genome-Wide Associations. PLoS Biol. 2010, 8, e1000294. [Google Scholar] [CrossRef] [PubMed]
- McGrath, L.M.; Yu, D.; Marshall, C.; Davis, L.K.; Thiruvahindrapuram, B.; Li, B.; Cappi, C.; Gerber, G.; Wolf, A.; Schroeder, F.A.; et al. Copy number variation in obsessive-compulsive disorder and Tourette syndrome: A cross-disorder study. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 910–919. [Google Scholar] [CrossRef]
- Mahjani, B.; Birnbaum, R.; Buxbaum Grice, A.; Cappi, C.; Jung, S.; Avila, M.N.; Reichenberg, A.; Sandin, S.; Hultman, C.M.; Buxbaum, J.D.; et al. Phenotypic Impact of Rare Potentially Damaging Copy Number Variation in Obsessive-Compulsive Disorder and Chronic Tic Disorders. Genes 2022, 13, 1796. [Google Scholar] [CrossRef]
- Crowley, J.J. Genomics of Obsessive-Compulsive Disorder and Related Disorders: What the Clinician Needs to Know. Psychiatr. Clin. N. Am. 2023, 46, 39–51. [Google Scholar] [CrossRef]
- Seaby, E.G.; Pengelly, R.J.; Ennis, S. Exome sequencing explained: A practical guide to its clinical application. Brief. Funct. Genom. 2016, 15, 374–384. [Google Scholar] [CrossRef]
- Cappi, C.; Oliphant, M.E.; Péter, Z.; Zai, G.; Conceição do Rosário, M.; Sullivan, C.A.W.; Gupta, A.R.; Hoffman, E.J.; Virdee, M.; Olfson, E.; et al. De Novo Damaging DNA Coding Mutations Are Associated with Obsessive-Compulsive Disorder and Overlap with Tourette’s Disorder and Autism. Biol. Psychiatry 2020, 87, 1035–1044. [Google Scholar] [CrossRef]
- Halvorsen, M.; Samuels, J.; Wang, Y.; Greenberg, B.D.; Fyer, A.J.; McCracken, J.T.; Geller, D.A.; Knowles, J.A.; Zoghbi, A.W.; Pottinger, T.D.; et al. Exome Sequencing in Obsessive-Compulsive Disorder Reveals a Burden of Rare Damaging Coding Variants. Nat. Neurosci. 2021, 24, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.; Martinez-Pinteño, A.; Blázquez, A.; Ortiz, A.E.; Moreno, E.; Gassó, P.; Lafuente, A.; Lazaro, L.; Mas, S. Integrative DNA Methylation and Gene Expression Analysis of Cognitive Behavioral Therapy Response in Children and Adolescents with Obsessive-Compulsive Disorder; a Pilot Study. Pharmacogenomics Pers. Med. 2021, 14, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Destrée, L.; Brierley, M.E.E.; Albertella, L.; Jobson, L.; Fontenelle, L.F. The effect of childhood trauma on the severity of obsessive-compulsive symptoms: A systematic review. J. Psychiatr. Res. 2021, 142, 345–360. [Google Scholar] [CrossRef]
- Campos-Martin, R.; Bey, K.; Elsner, B.; Reuter, B.; Klawohn, J.; Philipsen, A.; Kathmann, N.; Wagner, M.; Ramirez, A. Epigenome-wide analysis identifies methylome profiles linked to obsessive-compulsive disorder, disease severity, and treatment response. Mol. Psychiatry 2023, 28, 4321–4330. [Google Scholar] [CrossRef]
- Bonder, M.J.; Luijk, R.; Zhernakova, D.V.; Moed, M.; Deelen, P.; Vermaat, M.; van Iterson, M.; van Dijk, F.; van Galen, M.; Bot, J.; et al. Disease variants alter transcription factor levels and methylation of their binding sites. Nat. Genet. 2017, 49, 131–138. [Google Scholar] [CrossRef]
- Mehta, D.; Klengel, T.; Conneely, K.N.; Smith, A.K.; Altmann, A.; Pace, T.W.; Rex-Haffner, M.; Loeschner, A.; Gonik, M.; Mercer, K.B.; et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc. Natl. Acad. Sci. USA 2013, 110, 8302–8307. [Google Scholar] [CrossRef]
- Thumfart, K.M.; Jawaid, A.; Bright, K.; Flachsmann, M.; Mansuy, I.M. Epigenetics of childhood trauma: Long term sequelae and potential for treatment. Neurosci. Biobehav. Rev. 2022, 132, 1049–1066. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.H.; Karimian, M.; Mirzaei, H.; Milajerdi, A. Epigenetic modifications and obsessive-compulsive disorder: What do we know? Brain Struct. Funct. 2023, 228, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Jovanova, O.S.; Nedeljkovic, I.; Spieler, D.; Walker, R.M.; Liu, C.; Luciano, M.; Bressler, J.; Brody, J.; Drake, A.J.; Evans, K.L.; et al. DNA Methylation Signatures of Depressive Symptoms in Middle-Aged and Elderly Persons: Meta-Analysis of Multiethnic Epigenome-Wide Studies. JAMA Psychiatry 2018, 75, 949–959. [Google Scholar] [CrossRef] [PubMed]
- De Jager, P.L.D.; Srivastava, G.; Lunnon, K.; Burgess, J.; Schalkwyk, L.C.; Yu, L.; Eaton, M.L.; Keenan, B.T.; Ernst, J.; McCabe, C.; et al. Alzheimer’s disease: Early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat. Neurosci. 2014, 17, 1156–1163. [Google Scholar] [CrossRef]
- Lardenoije, R.; Roubroeks, J.A.Y.; Pishva, E.; Leber, M.; Wagner, H.; Iatrou, A.; Smith, A.R.; Smith, R.G.; Eijssen, L.M.T.; Kleineidam, L.; et al. Alzheimer’s disease-associated (hydroxy)methylomic changes in the brain and blood. Clin. Epigenet. 2019, 11, 164. [Google Scholar] [CrossRef]
- Hannon, E.; Dempster, E.; Viana, J.; Burrage, J.; Smith, A.R.; Macdonald, R.; St Clair, D.; Mustard, C.; Breen, G.; Therman, S.; et al. An integrated genetic-epigenetic analysis of schizophrenia: Evidence for co-localization of genetic associations and differential DNA methylation. Genome Biol. 2016, 17, 176. [Google Scholar] [CrossRef]
- Freytag, V.; Vukojevic, V.; Wagner-Thelen, H.; Milnik, A.; Vogler, C.; Leber, M.; Weinhold, L.; Böhmer, A.C.; Riedel-Heller, S.; Maier, W.; et al. Genetic estimators of DNA methylation provide insights into the molecular basis of polygenic traits. Transl. Psychiatry 2018, 8, 31. [Google Scholar] [CrossRef]
- Bellia, F.; Vismara, M.; Annunzi, E.; Cifani, C.; Benatti, B.; Dell’Osso, B.; D’Addario, C. Genetic and epigenetic architecture of Obsessive-Compulsive Disorder: In search of possible diagnostic and prognostic biomarkers. J. Psychiatr. Res. 2021, 137, 554–571. [Google Scholar] [CrossRef]
- Grünblatt, E.; Marinova, Z.; Roth, A.; Gardini, E.; Ball, J.; Geissler, J.; Wojdacz, T.K.; Romanos, M.; Walitza, S. Combining genetic and epigenetic parameters of the serotonin transporter gene in obsessive-compulsive disorder. J. Psychiatr. Res. 2018, 96, 209–217. [Google Scholar] [CrossRef]
- Yue, J.; Zhang, B.; Wang, H.; Hou, X.; Chen, X.; Cheng, M.; Wen, S. Dysregulated plasma levels of miRNA-132 and miRNA-134 in patients with obsessive-compulsive disorder. Ann. Transl. Med. 2020, 8, 996. [Google Scholar] [CrossRef]
- Kandemir, H.; Erdal, M.E.; Selek, S.; Ay, Ö.I.; Karababa, I.F.; Ay, M.E.; Kandemir, S.B.; Bayazıt, H.; Taşdelen, B.; Ekinci, S.; et al. Microribonucleic acid dysregulations in children and adolescents with obsessive-compulsive disorder. Neuropsychiatr. Dis. Treat. 2015, 11, 1695–1701. [Google Scholar] [CrossRef]
- Muiños-Gimeno, M.; Guidi, M.; Kagerbauer, B.; Martín-Santos, R.; Navinés, R.; Alonso, P.; Menchón, J.M.; Gratacòs, M.; Estivill, X.; Espinosa-Parrilla, Y. Allele variants in functional MicroRNA target sites of the neurotrophin-3 receptor gene (NTRK3) as susceptibility factors for anxiety disorders. Hum. Mutat. 2009, 30, 1062–1071. [Google Scholar] [CrossRef]
- Pauls, D.L.; Abramovitch, A.; Rauch, S.L.; Geller, D.A. Obsessive-compulsive disorder: An integrative genetic and neurobiological perspective. Nat. Rev. Neurosci. 2014, 15, 410–424. [Google Scholar] [CrossRef]
- Haber, S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016, 18, 7–21. [Google Scholar] [CrossRef]
- Guzick, A.; Hunt, P.J.; Bijanki, K.R.; Schneider, S.C.; Sheth, S.A.; Goodman, W.K.; Storch, E.A. Improving long term patient outcomes from deep brain stimulation for treatment -refractory obsessive-compulsive disorder. Expert Rev. Neurother. 2020, 20, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Karas, P.J.; Lee, S.; Jimenez-Shahed, J.; Goodman, W.K.; Viswanathan, A.; Sheth, S.A. Deep Brain Stimulation for Obsessive Compulsive Disorder: Evolution of Surgical Stimulation Target Parallels Changing Model of Dysfunctional Brain Circuits. Front. Neurosci. 2019, 8, 998. [Google Scholar] [CrossRef]
- McGovern, R.A.; Sheth, S.A. Role of the dorsal anterior cingulate cortex in obsessive-compulsive disorder: Converging evidence from cognitive neuroscience and psychiatric neurosurgery. J. Neurosurg. 2017, 126, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Lack, C.W.; Crowley, J.J. The etiology of Obsessive-Compulsive Disorder. In Obsessive-Compulsive Disorder: Etiology, Phenomenology, and Treatment, 2nd ed.; Lack, C.W., Ed.; Onus Books: Abingdon, UK, 2023. [Google Scholar]
- Nakao, T.; Okada, K.; Kanba, S. Neurobiological model of obsessive-compulsive disorder: Evidence from recent neuropsychological and neuroimaging findings. Psychiatry Clin. Neurosci. 2014, 68, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Sarkar, S. Neuroimaging Studies in Obsessive Compulsive Disorder: A Narrative Review. Indian J. Psychol. Med. 2016, 38, 386–394. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, H.S.; Yoo, S.Y.; Ha, T.H.; Chang, J.H.; Kim, Y.Y.; Shin, Y.W.; Kwon, J.S. Morphometric alterations of anterior superior temporal cortex in obsessive-compulsive disorder. Depress. Anxiety 2006, 23, 290–296. [Google Scholar] [CrossRef]
- Boedhoe, P.S.W.; Schmaal, L.; Abe, Y.; Ameis, S.H.; Arnold, P.D.; Batistuzzo, M.C.; Benedetti, F.; Beucke, J.C.; Bollettini, I.; Bose, A.; et al. Distinct Subcortical Volume Alterations in Pediatric and Adult OCD: A Worldwide Meta-and Mega-Analysis. Am. J. Psychiatry 2017, 174, 60–69. [Google Scholar] [CrossRef]
- Gonçalves, O.F.; Sousa, S.; Carvalho, S.; Leite, J.; Ganho, A.; Fernandes-Gonçalves, A.; Pocinho, F.; Carracedo, A.; Sampaio, A. Alterations of gray and white matter morphology in obsessive compulsive disorder. Psicothema 2017, 29, 35–42. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Blackwell, A.D.; Fineberg, N.A.; Robbins, T.W.; Sahakian, B.J. The neuropsychology of obsessive compulsive disorder: The importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci. Biobehav. Rev. 2005, 29, 399–419. [Google Scholar] [CrossRef]
- Menzies, L.; Chamberlain, S.R.; Laird, A.R.; Thelen, S.M.; Sahakian, B.J.; Bullmore, E.T. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofronto-striatalmodel revisited. Neurosci. Biobehav. Rev. 2008, 32, 525–549. [Google Scholar] [CrossRef]
- Milad, M.R.; Rauch, S.L. Obsessive-compulsive disorder: Beyond segregated cortico-striatal pathways. Trends Cogn. Sci. 2012, 16, 43–51. [Google Scholar] [CrossRef]
- van Den Heuvel, O.A.; van Wingen, G.; Soriano-Mas, C.; Alonso, P.; Chamberlain, S.R.; Nakamae, T.; Denys, D.; Goudriaan, A.E.; Veltman, D.J. Brain circuitry of compulsivity. Eur. Neuropsychopharmacol. 2016, 26, 810–827. [Google Scholar] [CrossRef]
- Fineberg, N.A.; Apergis-Schoute, A.M.; Vaghi, M.M.; Banca, P.; Gillan, C.M.; Voon, V.; Chamberlain, S.R.; Cinosi, E.; Reid, J.; Shahper, S.; et al. Mapping Compulsivity in the DSM-5 Obsessive Compulsive and Related Disorders: Cognitive Domains, Neural Circuitry, and Treatment. Int. J. Neuropsychopharmacol. 2018, 21, 42–58. [Google Scholar] [CrossRef]
- Robbins, T.W.; Gillan, C.M.; Smith, D.G.; de Wit, S.; Ersche, K.D. Neurocognitive endophenotypes of impulsivity and compulsivity: Towards dimensional psychiatry. Trends Cogn. Sci. 2012, 16, 81–91. [Google Scholar] [CrossRef]
- Cho, Y.T.; Ernst, M.; Fudge, J.L. Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. J. Neurosci. 2013, 33, 14017–14030. [Google Scholar] [CrossRef]
- Cao, L.; Li, H.; Hu, X.; Liu, J.; Gao, Y.; Liang, K.; Zhang, L.; Hu, X.; Bu, X.; Lu, L.; et al. Distinct alterations of amygdala subregional functional connectivity in early-and late-onset obsessive-compulsive disorder. J. Affect. Disord. 2021, 298, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, N.A.; Brown, A.; Reghunandanan, S.; Pampaloni, I. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int. J. Neuropsychopharmacol. 2012, 15, 1173–1191. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Baldwin, D.; Abelli, M.; Bolea-Alamanac, B.; Bourin, M.; Chamberlain, S.R.; Cinosi, E.; Davies, S.; Domschke, K.; Fineberg, N.; et al. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World J. Biol. Psychiatry 2017, 18, 162–214. [Google Scholar] [CrossRef]
- Taylor, S. Molecular genetics of obsessive–compulsive disorder: A comprehensive meta-analysis of genetic association studies. Mol. Psychiatry 2013, 18, 799–805. [Google Scholar] [CrossRef]
- Taylor, S. Disorder-specific genetic factors in obsessive-compulsive disorder: A comprehensive meta-analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171B, 325–332. [Google Scholar] [CrossRef]
- Kim, E.; Howes, O.D.; Park, J.W.; Kim, S.N.; Shin, S.A.; Kim, B.H.; Turkheimer, F.E.; Lee, Y.S.; Kwon, J.S. Altered serotonin transporter binding potential in patients with obsessive-compulsive disorder under escitalopram treatment: [11C]DASB PET study. Psychol. Med. 2016, 46, 357–366. [Google Scholar] [CrossRef]
- Sinopoli, V.M.; Burton, C.L.; Kronenberg, S.; Arnold, P.D. A review of the role of serotonin system genes in obsessive-compulsive disorder. Neurosci. Biobehav. Rev. 2017, 80, 372–381. [Google Scholar] [CrossRef]
- Péter, Z.; Oliphant, M.E.; Fernandez, T.V. Motor Stereotypies: A Pathophysiological Review. Front. Neurosci. 2017, 11, 171. [Google Scholar] [CrossRef]
- Xue, J.; Qian, D.; Zhang, B.; Yang, J.; Li, W.; Bao, Y.; Qiu, S.; Fu, Y.; Wang, S.; Yuan, T.F.; et al. Midbrain dopamine neurons arbiter OCD-like behavior. Proc. Natl. Acad. Sci. USA 2022, 119, e2207545119. [Google Scholar] [CrossRef]
- Dong, M.X.; Chen, G.H.; Hu, L. Dopaminergic System Alteration in Anxiety and Compulsive Disorders: A Systematic Review of Neuroimaging Studies. Front. Neurosci. 2020, 14, 608520. [Google Scholar] [CrossRef]
- Grant, J.E.; Hook, R.; Valle, S.; Chesisvoir, E.; Chamberlain, S.R. Tolcapone treatment in obsessive compulsive disorder: A randomized double-blind placebo-controlled crossover trial. Int. Clin. Psychopharmacol. 2021, 36, 225–229. [Google Scholar] [CrossRef]
- Gillan, C.M.; Robbins, T.W.; Sahakian, B.J.; van den Heuvel, O.A.; van Wingen, G. The role of habit in compulsivity. Eur. Neuropsychopharmacol. 2016, 26, 828–840. [Google Scholar] [CrossRef]
- Marinova, Z.; Chuang, D.M.; Fineberg, N. Glutamate-Modulating Drugs as a Potential Therapeutic Strategy in Obsessive-Compulsive Disorder. Curr. Neuropharmacol. 2017, 15, 977–995. [Google Scholar] [CrossRef]
- Karthik, S.; Sharma, L.P.; Narayanaswamy, J.C. Investigating the Role of Glutamate in Obsessive-Compulsive Disorder: Current Perspectives. Neuropsychiatr. Dis. Treat. 2020, 16, 1003–1013. [Google Scholar] [CrossRef]
- Kohlrausch, F.B.; Giori, I.G.; Melo-Felippe, F.B.; Vieira-Fonseca, T.; Velarde, L.G.; de Salles Andrade, J.B.; Fontenelle, L.F. Association of GRIN2B gene polymorphism and Obsessive Compulsive disorder and symptom dimensions: A pilot study. Psychiatry Res. 2016, 243, 152–155. [Google Scholar] [CrossRef]
- Stewart, S.E.; Mayerfeld, C.; Arnold, P.D.; Crane, J.R.; O’Dushlaine, C.; Fagerness, J.A.; Yu, D.; Scharf, J.M.; Chan, E.; Kassam, F.; et al. Meta-analysis of association between obsessive-compulsive disorder and the 3ʹ region of neuronal glutamate transporter gene SLC1A1. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013, 162, 367–379. [Google Scholar] [CrossRef]
- International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC); OCD Collaborative Genetics Association Studies (OCGAS). Revealing the complex genetic architecture of obsessive–compulsive disorder using meta-analysis. Mol. Psychiatry 2017, 23, 1181–1188. [Google Scholar] [CrossRef]
- Hyman, S.E. PANDAS: Too Narrow a View of the Neuroimmune Landscape. Am. J. Psychiatry 2021, 178, 5–7. [Google Scholar] [CrossRef]
- Bechter, K. The Challenge of Assessing Mild Neuroinflammation in Severe Mental Disorders. Front. Psychiatry 2020, 11, 773. [Google Scholar] [CrossRef]
- Orlovska, S.; Vestergaard, C.H.; Bech, B.H.; Nordentoft, M.; Vestergaard, M.; Benros, M.E. Association of Streptococcal Throat Infection with Mental Disorders: Testing Key Aspects of the PANDAS Hypothesis in a Nationwide Study. JAMA Psychiatry 2017, 74, 740–746. [Google Scholar] [CrossRef]
- Westwell-Roper, C.; Williams, K.A.; Samuels, J.; Bienvenu, O.J.; Cullen, B.; Goes, F.S.; Grados, M.A.; Geller, D.; Greenberg, B.D.; Knowles, J.A.; et al. Immune-Related Comorbidities in Childhood-Onset Obsessive Compulsive Disorder: Lifetime Prevalence in the Obsessive Compulsive Disorder Collaborative Genetics Association Study. J. Child Adolesc. Psychopharmacol. 2019, 29, 615–624. [Google Scholar] [CrossRef]
- Endres, D.; Pollak, T.A.; Bechter, K.; Denzel, D.; Pitsch, K.; Nickel, K.; Runge, K.; Pankratz, B.; Klatzmann, D.; Tamouza, R.; et al. Immunological causes of obsessive-compulsive disorder: Is it time for the concept of an “autoimmune OCD” subtype? Transl. Psychiatry 2022, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Involvement of virus infections and antiviral agents in the schizophrenia. Psychol. Med. 2025, 55, e73. [Google Scholar] [CrossRef]
- Chang, K.; Frankovich, J.; Cooperstock, M.; Cunningham, M.W.; Latimer, M.E.; Murphy, T.K.; Pasternack, M.; Thienemann, M.; Williams, K.; Walter, J.; et al. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): Recommendations from the 2013 PANS Consensus Conference. J. Child Adolesc. Psychopharmacol. 2015, 25, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Gerentes, M.; Pelissolo, A.; Rajagopal, K.; Tamouza, R.; Hamdani, N. Obsessive-Compulsive Disorder: Autoimmunity and Neuroinflammation. Curr. Psychiatry Rep. 2019, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vigil, A.; Fernández de la Cruz, L.; Brander, G.; Isomura, K.; Gromark, C.; Mataix-Cols, D. The link between autoimmune diseases and obsessive-compulsive and tic disorders: A systematic review. Neurosci. Biobehav. Rev. 2016, 71, 542–562. [Google Scholar] [CrossRef]
- Endres, D.; Maier, V.; Leypoldt, F.; Wandinger, K.P.; Lennox, B.; Pollak, T.A.; Nickel, K.; Maier, S.; Feige, B.; Domschke, K.; et al. Autoantibody-associated psychiatric syndromes: A systematic literature review resulting in 145 cases. Psychol. Med. 2020, 7, 1135–1146. [Google Scholar] [CrossRef]
- Foroughipour, M.; Behdani, F.; Hebrani, P.; Marvast, M.N.; Esmatinia, F.; Akhavanrezayat, A. Frequency of obsessive-compulsive disorder in patients with multiple sclerosis: A cross-sectional study. J. Res. Med. Sci. 2012, 17, 248–253. [Google Scholar]
- Wang, L.Y.; Chen, S.F.; Chiang, J.H.; Hsu, C.Y.; Shen, Y.C. Systemic autoimmune diseases are associated with an increased risk of obsessive-compulsive disorder: A nationwide population-based cohort study. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 507–516. [Google Scholar] [CrossRef]
- Mataix-Cols, D.; Frans, E.; Pérez-Vigil, A.; Kuja-Halkola, R.; Gromark, C.; Isomura, K.; Fernández de la Cruz, L.; Serlachius, E.; Leckman, J.F.; Crowley, J.J.; et al. A total-population multigenerational family clustering study of autoimmune diseases in obsessive-compulsive disorder and Tourette’s/chronic tic disorders. Mol. Psychiatry 2018, 23, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Cappi, C.; Brentani, H.; Lima, L.; Sanders, S.J.; Zai, G.; Diniz, B.J.; Reis, V.N.; Hounie, A.G.; Conceição do Rosário, M.; Mariani, D.; et al. Whole-exome sequencing in obsessive-compulsive disorder identifies rare mutations in immunological and neurodevelopmental pathways. Transl. Psychiatry 2016, 6, e764. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.; Morer, A.; González-Navarro, E.A.; Gassó, P.; Boloc, D.; Serra-Pagès, C.; Lafuente, A.; Lazaro, L.; Mas, S. Human-leukocyte antigen class II genes in early-onset obsessive-compulsive disorder. World J. Biol. Psychiatry 2019, 20, 352–358. [Google Scholar] [CrossRef]
- Fabricius, R.A.; Sørensen, C.B.; Skov, L.; Debes, N.M. Cytokine profile of pediatric patients with obsessive-compulsive and/or movement disorder symptoms: A review. Front. Pediatr. 2022, 10, 893815. [Google Scholar] [CrossRef] [PubMed]
- Sarmin, N.; Roknuzzaman, A.S.M.; Sarker, R.; Rashid, M.O.; Hasan, A.; Qusar, M.M.A.S.; Kabir, E.R.; Islam, M.R.; Mahmud, Z.A. Exploring the role of interleukin-1β and interleukin-6 in the pathophysiology of obsessive-compulsive disorder. PLoS ONE 2024, 19, e0306125. [Google Scholar] [CrossRef]
- Cosco, T.D.; Pillinger, T.; Emam, H.; Solmi, M.; Budhdeo, S.; Matthew Prina, A.; Maes, M.; Stein, D.J.; Stubbs, B.; Carvalho, A.F. Immune Aberrations in Obsessive-Compulsive Disorder: A Systematic Review and Meta-Analysis. Mol. Neurobiol. 2019, 56, 4751–4759. [Google Scholar] [CrossRef]
- Raposo-Lima, C.; Morgado, P. The role of stress in obsessive-compulsive disorder: A narrative review. Harv. Rev. Psychiatry 2020, 28, 356–370. [Google Scholar] [CrossRef]
- Pallanti, S.; Hollander, E. Obsessive-compulsive disorder spectrum as a scientific “metaphor”. CNS Spectr. 2008, 13, 6–15. [Google Scholar] [CrossRef]
- von der Marwitz, T. Etiology, psychoanalytical diagnosis and treatment of obsessive-compulsive disorder in childhood and adolescence. Praxis Kinderpsychol. Kinderpsychiatr. 2008, 57, 468–485. [Google Scholar]
- Chamberlain, S.R.; Leppink, E.W.; Redden, S.A.; Grant, J.E. Are obsessive-compulsive symptoms impulsive, compulsive or both? Compr. Psychiatry 2016, 68, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; He, L.; Wen, R.; Verguts, T.; Seger, C.A.; Chen, Q. Obsessive-compulsive disorder is characterized by decreased Pavlovian influence on instrumental behavior. PLoS Comput. Biol. 2022, 18, e1009945. [Google Scholar] [CrossRef]
- Morein-Zamir, S.; Fineberg, N.A.; Robbins, T.W.; Sahakian, B.J. Inhibition of thoughts and actions in obsessive-compulsive disorder: Extending the endophenotype? Psychol. Med. 2010, 40, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Whitton, A.E.; Henry, J.D.; Grisham, J.R. Moral rigidity in obsessive-compulsive disorder: Do abnormalities in inhibitory control, cognitive flexibility and disgust play a role? J. Behav. Ther. Exp. Psychiatry 2014, 45, 152–159. [Google Scholar] [CrossRef]
- Moritz, S.; Kloss, M.; Jelinek, L. Negative priming (cognitive inhibition) in obsessive-compulsive disorder (OCD). J. Behav. Ther. Exp. Psychiatry 2010, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nikodijevic, A.; Moulding, R.; Anglim, J.; Aardema, F.; Nedeljkovic, M. Fear of self, doubt and obsessive compulsive symptoms. J. Behav. Ther. Exp. Psychiatry 2015, 49, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Ettelt, S.; Ruhrmann, S.; Barnow, S.; Buthz, F.; Hochrein, A.; Meyer, K.; Kraft, S.; Reck, C.; Pukrop, R.; Klosterkötter, J.; et al. Impulsiveness in obsessive-compulsive disorder: Results from a family study. Acta Psychiatr. Scand. 2007, 115, 41–47. [Google Scholar] [CrossRef]
- Mathews, C. Obsessive-compulsive disorders. Continuum 2021, 27, 1764–1784. [Google Scholar] [CrossRef]
- Kalenzaga, S.; Clarys, D.; Jaafari, N. The memory deficit hypothesis of compulsive checking in OCD: What are we really talking about? A narrative review. Memory 2020, 28, 1089–1103. [Google Scholar] [CrossRef]
- Heinzel, S.; Kaufmann, C.; Grützmann, R.; Hummel, R.; Klawohn, J.; Riesel, A.; Bey, K.; Lennertz, L.; Wagner, M.; Kathmann, N. Neural correlates of working memory deficits and associations to response inhibition in obsessive compulsive disorder. Neuroimage Clin. 2017, 17, 426–434. [Google Scholar] [CrossRef]
- Jelinek, L.; Rietschel, L.; Kellner, M.; Muhtz, C.; Moritz, S. The effect of practice on the recall of salient information in obsessive-compulsive disorder. Psychiatry Res. 2012, 198, 89–93. [Google Scholar] [CrossRef]
- Holaway, R.M.; Heimberg, R.G.; Coles, M.E. A comparison of intolerance of uncertainty in analogue obsessive-compulsive disorder and generalized anxiety disorder. J. Anxiety Disord. 2006, 20, 158–174. [Google Scholar] [CrossRef]
- Steketee, G.; Frost, R.O.; Cohen, I. Beliefs in obsessive-compulsive disorder. J. Anxiety Disord. 1998, 12, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Trak, E.; İnözü, M. Obsessive beliefs prospectively predict adherence to safety behaviours related to COVID-19 through obsessive-compulsive symptoms and COVID-19 distress: A serial multiple mediator analysis. Int. J. Psychol. 2022, 57, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.G., Jr.; Riemann, B.C.; Wetterneck, C.T.; Cisler, J.M. Obsessive beliefs predict cognitive behavior therapy outcome for obsessive compulsive disorder. Cogn. Behav. Ther. 2012, 41, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Brakoulias, V.; Starcevic, V. The characterization of beliefs in obsessive-compulsive disorder. Psychiatric Q. 2011, 82, 151–161. [Google Scholar] [CrossRef]
- Moritz, S.; Von Mühlenen, A.; Randjbar, S.; Fricke, S.; Jelinek, L. Evidence for an attentional bias for washing- and checking-relevant stimuli in obsessive-compulsive disorder. J. Int. Neuropsychol. Soc. 2009, 15, 365–371. [Google Scholar] [CrossRef]
- Sip, K.E.; Muratore, A.F.; Stern, E.R. Effects of context on risk taking and decision times in obsessive-compulsive disorder. J. Psychiatr. Res. 2016, 75, 82–90. [Google Scholar] [CrossRef]
- Dar, K.A.; Iqbal, N. Worry and rumination in generalized anxiety disorder and obsessive compulsive disorder. J. Psychol. 2015, 149, 866–880. [Google Scholar] [CrossRef]
- Myers, S.G.; Wells, A. Obsessive-compulsive symptoms: The contribution of metacognitions and responsibility. J. Anxiety Disord. 2005, 19, 806–817. [Google Scholar] [CrossRef]
- Hermans, D.; Engelen, U.; Grouwels, L.; Joos, E.; Lemmens, J.; Pieters, G. Cognitive confidence in obsessive-compulsive disorder: Distrusting perception, attention and memory. Behav. Res. Ther. 2008, 46, 98–113. [Google Scholar] [CrossRef]
- Salkovskis, P.M. Obsessional-compulsive problems: A cognitive-behavioural analysis. Behav. Res. Ther. 1985, 23, 571–583. [Google Scholar] [CrossRef]
- Gillan, C.M.; Morein-Zamir, S.; Durieux, A.M.; Fineberg, N.A.; Sahakian, B.J.; Robbins, T.W. Obsessive–compulsive disorder patients have a reduced sense of control on the illusion of control task. Front. Psychol. 2014, 5, 204. [Google Scholar] [CrossRef]
- Vaghi, M.M.; Cardinal, R.N.; Apergis-Schoute, A.M.; Fineberg, N.A.; Sule, A.; Robbins, T.W. Action-outcome knowledge dissociates from behavior in obsessive-compulsive disorder following contingency degradation. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Banca, P.; Vestergaard, M.D.; Rankov, V.; Baek, K.; Mitchell, S.; Lapa, T.; Castelo-Branco, M.; Voon, V. Evidence accumulation in obsessive-compulsive disorder: The role of uncertainty and monetary reward on perceptual decision-making thresholds. Neuropsychopharmacology 2015, 40, 1192–1202. [Google Scholar] [CrossRef]
- Hauser, T.U.; Allen, M.; Rees, G.; Dolan, R.J. Metacognitive impairments extend perceptual decision making weaknesses in compulsivity. Sci. Rep. 2017, 7, 6614. [Google Scholar] [CrossRef]
- Myers, S.G.; Fisher, P.L.; Wells, A. An empirical test of the metacognitive model of obsessive-compulsive symptoms: Fusion beliefs, beliefs about rituals, and stop signals. J. Anxiety Disord. 2009, 23, 436–442. [Google Scholar] [CrossRef]
- Shafran, R.; Rachman, S. Thought-action fusion: A review. J. Behav. Ther. Exp. Psychiatry 2004, 35, 87–107. [Google Scholar] [CrossRef]
- Williams, A.D.; Lau, G.; Grisham, J.R. Thought-action fusion as a mediator of religiosity and obsessive-compulsive symptoms. J. Behav. Ther. Exp. Psychiatry 2013, 44, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Agorastos, A.; Metscher, T.; Huber, C.G.; Jelinek, L.; Vitzthum, F.; Muhtz, C.; Kellner, M.; Moritz, S. Religiosity, magical ideation, and paranormal beliefs in anxiety disorders and obsessive-compulsive disorder: A cross-sectional study. J. Nerv. Ment. Dis. 2012, 200, 876–884. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, S.J. Thought-action fusion as predictors of obsessive-compulsive symptom dimensions. Psychiatry Investig. 2020, 17, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Moritz, S.; Rietschel, L.; Jelinek, L.; Bäuml, K.H. Are patients with obsessive-compulsive disorder generally more doubtful? Doubt is warranted! Psychiatry Res. 2011, 189, 265–269. [Google Scholar] [CrossRef]
- Rachman, S.; Thordarson, D.S.; Shafran, R.; Woody, S.R. Perceived responsibility: Structure and significance. Behav. Res. Ther. 1995, 33, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, S.J.; Choi, M. Psychological inflexibility, cognitive fusion, and thought-action fusion as a transdiagnostic construct: Direct comparisons among major depressive disorder, obsessive-compulsive disorder, and healthy controls. Psychiatry Investig. 2025, 22, 93–101. [Google Scholar] [CrossRef]
- Julien, D.; O’Connor, K.; Aardema, F. The inference-based approach to obsessive-compulsive disorder: A comprehensive review of its etiological model, treatment efficacy, and model of change. J. Affect. Disord. 2016, 202, 187–196. [Google Scholar] [CrossRef]
- Wong, S.F.; Grisham, J.R. Inverse reasoning processes in obsessive-compulsive disorder. J. Anxiety Disord. 2017, 47, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.F.; Aardema, F.; Grisham, J.R. Inverse reasoning processes in obsessive-compulsive disorder: Replication in a clinical sample. J. Anxiety Disord. 2019, 63, 1–8. [Google Scholar] [CrossRef]
- Aminaee, M.; Khosravani, V.; Moulding, R.; Aardema, F.; Wong, S.F.; Samimi Ardestani, S.M. The role of feared possible selves in the relationship between inferential confusion and obsessive-compulsive symptoms: A replication and extension in a clinical sample. Br. J. Clin. Psychol. 2024, 63, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Suzer Gamli, I.; Kacar, A.; Eyupoglu, N.; Karakus, O.B.; Adak, I. Emotion regulation, coping, and alexithymia in adolescents with obsessive-compulsive disorder: A mediational analysis. Clin. Psychol. Psychother. 2025, 32, e70083. [Google Scholar] [CrossRef]
- Nielsen, S.K.K.; Stuart, A.C.; Winding, C.; Pedersen, M.Ø.; Daniel, S.I.F.; Vangkilde, S.; Rosenberg, N.; Hageman, I.; Petersen, A.; Jørgensen, M.B. Adult attachment style, emotion regulation and obsessive-compulsive disorder—A preliminary cross-sectional mediational investigation of an attachment-based model. Clin. Psychol. Psychother. 2025, 32, e70031. [Google Scholar] [CrossRef]
- Dar, R.; Lazarov, A.; Liberman, N. How can I know what I’m feeling? Obsessive-compulsive tendencies and induced doubt are related to reduced access to emotional states. J. Behav. Ther. Exp. Psychiatry 2016, 52, 128–137. [Google Scholar] [CrossRef]
- Dostal, A.L.; Pilkington, P.D. Early maladaptive schemas and obsessive-compulsive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2023, 336, 42–51. [Google Scholar] [CrossRef]
- Coles, M.E.; Schofield, C.A.; Pietrefesa, A.S. Behavioral inhibition and obsessive-compulsive disorder. J. Anxiety Disord. 2006, 20, 1118–1132. [Google Scholar] [CrossRef]
- Kiverstein, J.; Rietveld, E.; Slagter, H.A.; Denys, D. Obsessive compulsive disorder: A pathology of self-confidence? Trends Cogn. Sci. 2019, 23, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Zhang, Z.L.; Li, H. Double conflicts model and anxiety ratification therapy hypotheses of obsessive-compulsive disorder. Med. Hypotheses 2010, 75, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Early Life Stress and Gut Microbiome Dysbiosis: A Narrative Review. Stresses 2025, 5, 38. [Google Scholar] [CrossRef]
- Morina, N.; Sulaj, V.; Schnyder, U.; Klaghofer, R.; Müller, J.; Martin-Sölch, C.; Rufer, M. Obsessive-compulsive and posttraumatic stress symptoms among civilian survivors of war. BMC Psychiatry 2016, 16, 115. [Google Scholar] [CrossRef]
- Krebs, G.C.; Hannigan, L.J.; Gregory, A.M.; Rijsdijk, F.V.; Maughan, B.; Eley, T.C. Are punitive parenting and stressful life events environmental risk factors for obsessive-compulsive symptoms in youth? A longitudinal twin study. Eur. Psychiatry 2019, 56, 35–42. [Google Scholar] [CrossRef]
- Real, E.; Labad, J.; Alonso, P.; Segalàs, C.; Jiménez-Murcia, S.; Bueno, B.; Subirà, M.; Vallejo, J.; Menchón, J.M. Stressful life events at onset of obsessive-compulsive disorder are associated with a distinct clinical pattern. Depress. Anxiety 2011, 28, 367–376. [Google Scholar] [CrossRef]
- Goldberg, X.; Soriano-Mas, C.; Alonso, P.; Segalàs, C.; Real, E.; López-Solà, C.; Subirà, M.; Via, E.; Jiménez-Murcia, S.; Menchón, J.M.; et al. Predictive value of familiality, stressful life events and gender on the course of obsessive-compulsive disorder. J. Affect. Disord. 2015, 185, 129–134. [Google Scholar] [CrossRef]
- Barzilay, R.; Patrick, A.; Calkins, M.E.; Moore, T.M.; Gur, R.C.; Gur, R.E. Association between early-life trauma and obsessive compulsive symptoms in community youth. Depress. Anxiety 2019, 36, 586–595. [Google Scholar] [CrossRef]
- Briggs, E.S.; Price, I.R. The relationship between adverse childhood experience and obsessive-compulsive symptoms and beliefs: The role of anxiety, depression, and experiential avoidance. J. Anxiety Disord. 2009, 23, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Rachman, S. Betrayal: A psychological analysis. Behav. Res. Ther. 2010, 48, 304–311. [Google Scholar] [CrossRef]
- Pace, S.M.; Thwaites, R.; Freeston, M.H. Exploring the role of external criticism in Obsessive Compulsive Disorder: A narrative review. Clin. Psychol. Rev. 2011, 31, 361–370. [Google Scholar] [CrossRef]
- Abregú-Crespo, R.; Garriz-Luis, A.; Ayora, M.; Martín-Martínez, N.; Cavone, V.; Carrasco, M.Á.; Fraguas, D.; Martín-Babarro, J.; Arango, C.; Díaz-Caneja, C.M. School bullying in children and adolescents with neurodevelopmental and psychiatric conditions: A systematic review and meta-analysis. Lancet Child Adolesc. Health 2024, 8, 122–134. [Google Scholar] [CrossRef]
- Hidayati, E.; Pragholapati, A.; Ismail, S. Bullying in children: A concept analysis. J. Pak. Med. Assoc. 2024, 74, S84–S87. [Google Scholar] [CrossRef]
- Kowalski, R.M. Bullying in the classroom: Teachers as perpetrators. J. Adolesc. Health 2024, 75, 377–378. [Google Scholar] [CrossRef]
- Fernández, S.; Saguy, T.; Halperin, E. The paradox of humiliation: The acceptance of an unjust devaluation of the self. Pers. Soc. Psychol. Bull. 2015, 41, 976–988. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Fernández, S. Humiliation and its relationship with bullying victimization: A narrative review. Psychol. Soc. Educ. 2024, 16, 42–51. [Google Scholar] [CrossRef]
- Elshout, M.; Nelissen, R.M.A.; van Beest, I. Conceptualising humiliation. Cogn. Emot. 2017, 31, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Visvalingam, S.; Crone, C.; Street, S.; Oar, E.L.; Gilchrist, P.; Norberg, M.M. The causes and consequences of shame in obsessive-compulsive disorder. Behav. Res. Ther. 2022, 151, 104064. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, L.; Chung, M.C. Childhood trauma in obsessive compulsive disorder: The roles of alexithymia and attachment. Psychol. Psychother. 2011, 84, 367–388. [Google Scholar] [CrossRef]
- Leong, A.; Colah, Z.A.; Guzick, A.G.; Chen, E.Y.; Shah, S.S.; Fall, D.A.; Chen, R.; Zhang, Y.; Zhang, C.; Cepeda, S.L.; et al. COVID-19-related intrusive thoughts and associated ritualistic behaviors. Bull. Menn. Clin. 2023, 87, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A. Impact of the COVID-19 pandemic on obsessive-compulsive disorder: A mini-review. Nov. Trends Ment. Health 2024, 1, 31–38. [Google Scholar]
- Ivarsson, T.; Saavedra, F.; Granqvist, P.; Broberg, A.G. Traumatic and adverse attachment childhood experiences are not characteristic of OCD but of depression in adolescents. Child Psychiatry Hum. Dev. 2016, 47, 270–280. [Google Scholar] [CrossRef]
- Pol-Fuster, J.; Fernández de la Cruz, L.; Isomura, K.; Sidorchuk, A.; Kuja-Halkola, R.; Lichtenstein, P.; D’Onofrio, B.M.; Brikell, I.; Larsson, H.; de Schipper, E.; et al. Association between bullying victimization and obsessive-compulsive disorder: A population-based, genetically informative study. Mol. Psychiatry 2025, 30, 2457–2462. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Q.; Xu, T.; Gu, Q.; Liu, Q.; Wang, Y.; Lin, G.N.; Wang, Z. Interaction between PGRN gene and the early trauma on clinical characteristics in patients with obsessive-compulsive disorder. J. Affect. Disord. 2020, 263, 134–140. [Google Scholar] [CrossRef]
- Kılıç, A.; Görmez, A.; Yeni Elbay, R.; Özer, B.U. Internalized stigma in obsessive compulsive disorder: Correlates and associations with quality of life. Arch. Psychiatr. Nurs. 2022, 39, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Spencer, L.; Carel, H. ‘Isn’t everyone a little OCD?’: The epistemic harms of wrongful depathologization. Philos. Med. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Ansari, E.; Mishra, S.; Tripathi, A.; Kar, S.K.; Dalal, P.K. Cross-sectional study of internalised stigma and medication adherence in patients with obsessive compulsive disorder. Gen. Psychiatry 2020, 33, e100180. [Google Scholar] [CrossRef]
- Morgiève, M.; N’Diaye, K.; Fernandez-Vidal, S.; Clair, A.H.; Mallet, L. Obsessive-compulsive disorder as seen by those who are confronted with it: A survey of patients, relatives and clinicians. L’encephale 2017, 43, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.T.; Jahn, M.E. Obsessive-compulsive disorder in African American children and adolescents: Risks, resiliency, and barriers to treatment. Am. J. Orthopsychiatry 2017, 87, 291–303. [Google Scholar] [CrossRef]
- Rejtô, N.; Papp, G.; Molnar, J. The etiology of obsessive-compulsive disorder from the aspects of attachment theory, with special regard to perceived parental treatment, attachment patterns and emotion regulation difficulties. Psychiatr. Hung. 2017, 32, 145–157. [Google Scholar]
- Haciomeroglu, B.; Karanci, A.N. Perceived parental rearing behaviours, responsibility attitudes and life events as predictors of obsessive compulsive symptomatology: Test of a cognitive model. Behav. Cogn. Psychother. 2014, 42, 641–652. [Google Scholar] [CrossRef]
- Van Noppen, B.; Steketee, G. Testing a conceptual model of patient and family predictors of obsessive compulsive disorder (OCD) symptoms. Behav. Res. Ther. 2009, 47, 18–25. [Google Scholar] [CrossRef]
- Lebowitz, E.R.; Panza, K.E.; Bloch, M.H. Family accommodation in obsessive-compulsive and anxiety disorders: A five-year update. Expert Rev. Neurother. 2016, 16, 45–53. [Google Scholar] [CrossRef]
- Storch, E.A.; Geffken, G.R.; Merlo, L.J.; Jacob, M.L.; Murphy, T.K.; Goodman, W.K.; Larson, M.J.; Fernandez, M.; Grabill, K. Family accommodation in pediatric obsessive-compulsive disorder. J. Clin. Child Adolesc. Psychol. 2007, 36, 207–216. [Google Scholar] [CrossRef]
- Champion, S.M.; Grisham, J.R. How can we stop caregiver reassurance? Investigating predictors of family accommodation in obsessive-compulsive scenarios. J. Obsessive Compuls. Relat. Disord. 2022, 34, 100741. [Google Scholar] [CrossRef]
- Shimshoni, Y.; Shrinivasa, B.; Cherian, A.V.; Lebowitz, E.R. Family accommodation in psychopathology: A synthesized review. Indian J. Psychiatry 2019, 61, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Lebowitz, E.R.; Panza, K.E.; Su, J.; Bloch, M.H. Family accommodation in obsessive-compulsive disorder. Expert. Rev. Neurother. 2012, 12, 229–238. [Google Scholar] [CrossRef]
- Caporino, N.E.; Morgan, J.; Beckstead, J.; Phares, V.; Murphy, T.K.; Storch, E.A. A structural equation analysis of family accommodation in pediatric obsessive-compulsive disorder. J. Abnorm. Child Psychol. 2012, 40, 133–143. [Google Scholar] [CrossRef]
- Guzick, A.G.; Geller, D.A.; Small, B.J.; Murphy, T.K.; Wilhelm, S.; Storch, E.A. Irritability in Children and Adolescents with OCD. Behav. Ther. 2021, 52, 883–896. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Microbial Dysbiosis in the Skin Microbiome and Its Psychological Consequences. Microorganisms 2024, 12, 1908. [Google Scholar] [CrossRef]
- Rahman, S.M.; Abduelmula, A.; Jafferany, M. Psychopathological symptoms in dermatology: A basic approach toward psychocutaneous disorders. Int. J. Dermatol. 2023, 62, 346–356. [Google Scholar] [CrossRef]
- Brierley, M.E.; Albertella, L.; Christensen, E.; Rotaru, K.; Jacka, F.N.; Segrave, R.A.; Richardson, K.E.; Lee, R.S.; Kayayan, E.; Hughes, S.; et al. Lifestyle risk factors for obsessive-compulsive symptoms and related phenomena: What should lifestyle interventions target? Aust. N. Z. J. Psychiatry 2023, 57, 379–390. [Google Scholar] [CrossRef]
- Holmberg, A.; Martinsson, L.; Lidin, M.; Rück, C.; Mataix-Cols, D.; Fernández de la Cruz, L. General somatic health and lifestyle habits in individuals with obsessive-compulsive disorder: An international survey. BMC Psychiatry 2024, 24, 98. [Google Scholar] [CrossRef]
- Holmberg, A.; Pol-Fuster, J.; Kuja-Halkola, R.; Larsson, H.; Lichtenstein, P.; Chang, Z.; D’Onofrio, B.M.; Brikell, I.; Sidorchuk, A.; Isomura, K.; et al. Multigenerational family coaggregation study of obsessive-compulsive disorder and cardiometabolic disorders. BMJ Ment. Health 2025, 28, e301323. [Google Scholar] [CrossRef]
- Isomura, K.; Brander, G.; Chang, Z.; Kuja-Halkola, R.; Rück, C.; Hellner, C.; Lichtenstein, P.; Larsson, H.; Mataix-Cols, D.; Fernández de la Cruz, L. Metabolic and cardiovascular complications in obsessive-compulsive disorder: A total population, sibling comparison study with long-term follow-up. Biol. Psychiatry 2018, 84, 324–331. [Google Scholar] [CrossRef]
- Pérez-Vigil, A.; Fernández de la Cruz, L.; Brander, G.; Isomura, K.; Jangmo, A.; Feldman, I.; Hesselmark, E.; Serlachius, E.; Lázaro, L.; Rück, C.; et al. Association of obsessive-compulsive disorder with objective indicators of educational attainment: A nationwide register-based sibling control study. JAMA Psychiatry 2018, 75, 47–55. [Google Scholar] [CrossRef]
- Das, P.; Babaei, P.; Nielsen, J. Metagenomic analysis of microbe-mediated vitamin metabolism in the human gut microbiome. BMC Genom. 2019, 20, 208. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Della Vecchia, A.; Marazziti, D. Back to the Future: The Role of Infections in Psychopathology. Focus on OCD. Clin. Neuropsychiatry 2022, 19, 248–263. [Google Scholar]
- Flegr, J.; Horáček, J. Toxoplasma-infected subjects report an Obsessive-Compulsive Disorder diagnosis more often and score higher in Obsessive-Compulsive Inventory. Eur. Psychiatry 2017, 40, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Johnco, C.; Kugler, B.B.; Murphy, T.K.; Storch, E.A. Obsessive-compulsive symptoms in adults with Lyme disease. Gen. Hosp. Psychiatry 2018, 51, 85–89. [Google Scholar] [CrossRef]
- Köhler-Forsberg, O.; Petersen, L.; Gasse, C.; Mortensen, P.B.; Dalsgaard, S.; Yolken, R.H.; Mors, O.; Benros, M.E. A Nationwide Study in Denmark of the Association Between Treated Infections and the Subsequent Risk of Treated Mental Disorders in Children and Adolescents. JAMA Psychiatry 2019, 76, 271–279. [Google Scholar] [CrossRef]
- Jung, T.D.; Jung, P.S.; Raveendran, L.; Farbod, Y.; Dvorkin-Gheva, A.; Sakic, B.; Surette, M.G.; Szechtman, H. Changes in gut microbiota during development of compulsive checking and locomotor sensitization induced by chronic treatment with the dopamine agonist quinpirole. Behav. Pharmacol. 2018, 29, 211–224. [Google Scholar] [CrossRef]
- Scheepers, I.M.; Cryan, J.F.; Bastiaanssen, T.F.S.; Rea, K.; Clarke, G.; Jaspan, H.B.; Harvey, B.H.; Hemmings, S.M.J.; Santana, L.; van der Sluis, R.; et al. Natural compulsive-like behaviour in the deer mouse (Peromyscus maniculatus bairdii) is associated with altered gut microbiota composition. Eur. J. Neurosci. 2020, 51, 1419–1427. [Google Scholar] [CrossRef]
- Quagliariello, A.; Del Chierico, F.; Russo, A.; Reddel, S.; Conte, G.; Lopetuso, L.R.; Ianiro, G.; Dallapiccola, B.; Cardona, F.; Gasbarrini, A.; et al. Gut Microbiota Profiling and Gut-Brain Crosstalk in Children Affected by Pediatric Acute-Onset Neuropsychiatric Syndrome and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections. Front. Microbiol. 2018, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Turna, J.; Grosman Kaplan, K.; Anglin, R.; Patterson, B.; Soreni, N.; Bercik, P.; Surette, M.G.; Van Ameringen, M. The gut microbiome and inflammation in obsessive-compulsive disorder patients compared to age- and sex-matched controls: A pilot study. Acta Psychiatr. Scand. 2020, 142, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Domènech, L.; Willis, J.; Alemany-Navarro, M.; Morell, M.; Real, E.; Escaramís, G.; Bertolín, S.; Sánchez Chinchilla, D.; Balcells, S.; Segalàs, C.; et al. Changes in the stool and oropharyngeal microbiome in obsessive-compulsive disorder. Sci. Rep. 2022, 12, 1448. [Google Scholar] [CrossRef]
- D’Addario, C.; Pucci, M.; Bellia, F.; Girella, A.; Sabatucci, A.; Fanti, F.; Vismara, M.; Benatti, B.; Ferrara, L.; Fasciana, F.; et al. Regulation of oxytocin receptor gene expression in obsessive-compulsive disorder: A possible role for the microbiota-host epigenetic axis. Clin. Epigenetics 2022, 14, 47. [Google Scholar] [CrossRef]
- Dai, J.; Li, M.; He, J.; Duan, L.; Zhu, X.; Liu, L.; Meng, M.; Shao, X.; Zhu, G. Gut microbiota changes are associated with abnormal metabolism activity in children and adolescents with obsessive-compulsive disorder. J. Psychiatr. Res. 2025, 181, 728–737. [Google Scholar] [CrossRef]
- Swedo, S.E.; Seidlitz, J.; Kovacevic, M.; Latimer, M.E.; Hommer, R.; Lougee, L.; Grant, P. Clinical presentation of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections in research and community settings. J. Child Adolesc. Psychopharmacol. 2015, 25, 26–30. [Google Scholar] [CrossRef]
- Tagi, V.M.; Tosi, M.; Greco, I.P.; Stucchi, E.; Verduci, E.; Zuccotti, G. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections and gut microbiota composition: What do we know? Front. Nutr. 2025, 11, 1477893. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.Y.E.; Letchumanan, V.; Tan, L.T.H.; Law, J.W.F. Gut Microbiome in Obsessive Compulsive Disorder: Potential of Probiotics as an Adjuvant Therapy. Prog. Microbes Mol. Biol. 2022, 5, a0000272. [Google Scholar] [CrossRef]
- Figee, M.; de Koning, P.; Klaassen, S.; Vulink, N.; Mantione, M.; van den Munckhof, P.; Schuurman, R.; van Wingen, G.; van Amelsvoort, T.; Booij, J.; et al. Deep brain stimulation induces striatal dopamine release in obsessive-compulsive disorder. Biol. Psychiatry 2014, 75, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Vulink, N.C.; Denys, D.; Fluitman, S.B.; Meinardi, J.C.; Westenberg, H.G. Quetiapine augments the effect of citalopram in non-refractory obsessive-compulsive disorder: A randomized, double-blind, placebo-controlled study of 76 patients. J. Clin. Psychiatry 2009, 70, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; Labaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef]
- Johnson, D.; Thurairajasingam, S.; Letchumanan, V.; Chan, K.G.; Lee, L.H. Exploring the Role and Potential of Probiotics in the Field of Mental Health: Major Depressive Disorder. Nutrients 2021, 13, 1728. [Google Scholar] [CrossRef]
- He, M.; Zhang, H.; Luo, Z.; Duan, X.; Zhao, F.; Su, P.; Zen, Z.; Zhou, L.; Chen, C.; Qiu, J. Causal link between gut microbiota and obsessive-compulsive disorder: A two-sample Mendelian randomization analysis. J. Affect. Disord. 2025, 379, 852–860. [Google Scholar] [CrossRef]
- Bendriss, G.; MacDonald, R.; McVeigh, C. Microbial Reprogramming in Obsessive-Compulsive Disorders: A Review of Gut-Brain Communication and Emerging Evidence. Int. J. Mol. Sci. 2023, 24, 11978. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Neurodevelopmental Disorders Associated with Gut Microbiome Dysbiosis in Children. Children 2024, 11, 796. [Google Scholar] [CrossRef]
- Codagnone, M.G.; Stanton, C.; O’Mahony, S.M.; Dinan, T.G.; Cryan, J.F. Microbiota and Neurodevelopmental Trajectories: Role of Maternal and Early-Life Nutrition. Ann. Nutr. Metab. 2019, 74, 16–27. [Google Scholar] [CrossRef]
- Troyer, E.A.; Kohn, J.N.; Ecklu-Mensah, G.; Aleti, G.; Rosenberg, D.R.; Hong, S. Searching for host immune-microbiome mechanisms in obsessive-compulsive disorder: A narrative literature review and future directions. Neurosci. Biobehav. Rev. 2021, 125, 517–534. [Google Scholar] [CrossRef]
- Huus, K.E.; Petersen, C.; Finlay, B.B. Diversity and dynamism of IgA-microbiota interactions. Nat. Rev. Immunol. 2021, 21, 514–525. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Isung, J.; Williams, K.; Isomura, K.; Gromark, C.; Hesselmark, E.; Lichtenstein, P.; Larsson, H.; Fernández de la Cruz, L.; Sidorchuk, A.; Mataix-Cols, D. Association of Primary Humoral Immunodeficiencies with Psychiatric Disorders and Suicidal Behavior and the Role of Autoimmune Diseases. JAMA Psychiatry 2020, 77, 1147–1154. [Google Scholar] [CrossRef]
- Catanzaro, J.R.; Strauss, J.D.; Bielecka, A.; Porto, A.F.; Lobo, F.M.; Urban, A.; Schofield, W.B.; Palm, N.W. IgA-deficient humans exhibit gut microbiota dysbiosis despite secretion of compensatory IgM. Sci. Rep. 2019, 9, 13574. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.R.; Sanz, Y.; Walder, K.; Maes, M. The Role of the Microbial Metabolites Including Tryptophan Catabolites and Short Chain Fatty Acids in the Pathophysiology of Immune-Inflammatory and Neuroimmune Disease. Mol. Neurobiol. 2017, 54, 4432–4451. [Google Scholar] [CrossRef] [PubMed]
- Waclawiková, B.; El Aidy, S. Role of Microbiota and Tryptophan Metabolites in the Remote Effect of Intestinal Inflammation on Brain and Depression. Pharmaceuticals 2018, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Szebik, H.; Miskolczi, C.; Bruzsik, B.; Balla, G.; Szabó, S.; Biró, L.; Mikics, É. Dynamic changes of serotonin transporter expression in the prefrontal cortex evoked by aggressive social interactions. Neurobiol. Stress 2025, 36, 100722. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Melancholic microbes: A link between gut microbiota and depression? Neurogastroenterol. Motil. 2013, 25, 713–719. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The impact of gut microbiota on brain and behaviour: Implications for psychiatry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 552–558. [Google Scholar] [CrossRef]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef]
- Rosenberg, D.R.; MacMaster, F.P.; Keshavan, M.S.; Fitzgerald, K.D.; Stewart, C.M.; Moore, G.J. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.W.; Shekhar, A.; Whiteman, A.F.; McDougle, C.J. Serotoninergic mechanisms in the treatment of obsessive-compulsive disorder. Drug Discov. Today 2008, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Cruz, N.J.; Kang, D.W.; Gandal, M.J.; Wang, B.; Kim, Y.M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e1617. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, T.; Prete, R.; Battista, N.; Corsetti, A.; De Jaco, A. Exposure to Antibiotics and Neurodevelopmental Disorders: Could Probiotics Modulate the Gut-Brain Axis? Antibiotics 2022, 11, 1767. [Google Scholar] [CrossRef] [PubMed]
- Njotto, L.L.; Simin, J.; Fornes, R.; Odsbu, I.; Mussche, I.; Callens, S.; Engstrand, L.; Bruyndonckx, R.; Brusselaers, N. Maternal and Early-Life Exposure to Antibiotics and the Risk of Autism and Attention-Deficit Hyperactivity Disorder in Childhood: A Swedish Population-Based Cohort Study. Drug Saf. 2023, 46, 467–478. [Google Scholar] [CrossRef]
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 2016, 6, e774. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Early-life microbiome development and its potential long-term impact on health outcomes. Microbiome Res. Rep. 2025, 4, 20. [Google Scholar] [CrossRef]
- Martin, A.F.; Jassi, A.; Cullen, A.E.; Broadbent, M.; Downs, J.; Krebs, G. Co-occurring obsessive-compulsive disorder and autism spectrum disorder in young people: Prevalence, clinical characteristics and outcomes. Eur. Child Adolesc. Psychiatry 2020, 29, 1603–1611. [Google Scholar] [CrossRef]
- Aymerich, C.; Pacho, M.; Catalan, A.; Yousaf, N.; Pérez-Rodríguez, V.; Hollocks, M.J.; Parellada, M.; Krebs, G.; Clark, B.; Salazar de Pablo, G. Prevalence and Correlates of the Concurrence of Autism Spectrum Disorder and Obsessive Compulsive Disorder in Children and Adolescents: A Systematic Review and Meta-Analysis. Brain Sci. 2024, 14, 379. [Google Scholar] [CrossRef]
- O’Loghlen, J.; McKenzie, M.; Lang, C.; Paynter, J. Repetitive Behaviors in Autism and Obsessive-Compulsive Disorder: A Systematic Review. J. Autism Dev. Disord. 2024, 23, 2307–2321. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Nardi, B.; Bonelli, C.; Amatori, G.; Pereyra, M.A.; Massimetti, E.; Cremone, I.M.; Pini, S.; Carpita, B. Autistic Traits as Predictors of Increased Obsessive-Compulsive Disorder Severity: The Role of Inflexibility and Communication Impairment. Brain Sci. 2024, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Abramovitch, A.; Dar, R.; Mittelman, A.; Wilhelm, S. Comorbidity Between Attention Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder Across the Lifespan: A Systematic and Critical Review. Harv. Rev. Psychiatry 2015, 23, 245–262. [Google Scholar] [CrossRef]
- Cabarkapa, S.; King, J.A.; Dowling, N.; Ng, C.H. Co-Morbid Obsessive-Compulsive Disorder and Attention Deficit Hyperactivity Disorder: Neurobiological Commonalities and Treatment Implications. Front. Psychiatry 2019, 10, 557. [Google Scholar] [CrossRef]
- Martinez, S.; Stoyanov, K.; Carcache, L. Unraveling the spectrum: Overlap, distinctions, and nuances of ADHD and ASD in children. Front. Psychiatry 2024, 15, 1387179. [Google Scholar] [CrossRef]
- Brem, S.; Grünblatt, E.; Drechsler, R.; Riederer, P.; Walitza, S. The neurobiological link between OCD and ADHD. Atten. Defic. Hyperact. Disord. 2014, 6, 175–202. [Google Scholar] [CrossRef]
- Guzick, A.G.; McNamara, J.P.H.; Reid, A.M.; Balkhi, A.M.; Storch, E.A.; Murphy, T.K.; Goodman, W.K.; Bussing, R.; Geffken, G.R. The link between ADHD-like inattention and obsessions and compulsions during treatment of youth with OCD. J. Obsessive Compuls. Relat. Disord. 2017, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, H.Y.; Chien, Y.; Tung, Y.H.; Ni, Y.H.; Gau, S.S. Altered gut microbiota correlates with behavioral problems but not gastrointestinal symptoms in individuals with autism. Brain Behav. Immun. 2022, 106, 161–178. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, K.; Liu, X.; Dai, Y.; Liu, Y.; Zhang, L.; Du, X.; Zhu, T.; Yu, J.; Fang, S.; et al. Gut Microbial Profile Is Associated with the Severity of Social Impairment and IQ Performance in Children with Autism Spectrum Disorder. Front. Psychiatry 2021, 12, 789864. [Google Scholar] [CrossRef]
- Niu, M.; Li, Q.; Zhang, J.; Wen, F.; Dang, W.; Duan, G.; Li, H.; Ruan, W.; Yang, P.; Guan, C.; et al. Characterization of Intestinal Microbiota and Probiotics Treatment in Children with Autism Spectrum Disorders in China. Front. Neurol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Szopinska-Tokov, J.; Dam, S.; Naaijen, J.; Konstanti, P.; Rommelse, N.; Belzer, C.; Buitelaar, J.; Franke, B.; Bloemendaal, M.; Aarts, E.; et al. Investigating the Gut Microbiota Composition of Individuals with Attention-Deficit/Hyperactivity Disorder and Association with Symptoms. Microorganisms 2020, 8, 406. [Google Scholar] [CrossRef]
- Wan, L.; Ge, W.R.; Zhang, S.; Sun, Y.L.; Wang, B.; Yang, G. Case-Control Study of the Effects of Gut Microbiota Composition on Neurotransmitter Metabolic Pathways in Children with Attention Deficit Hyperactivity Disorder. Front. Neurosci. 2020, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Yang, C.Y.; Chou, W.J.; Lee, M.J.; Chou, M.C.; Kuo, H.C.; Yeh, Y.M.; Lee, S.Y.; Huang, L.H.; Li, S.C. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2020, 29, 287–297. [Google Scholar] [CrossRef]
- Zhai, Q.; Cen, S.; Jiang, J.; Zhao, J.; Zhang, H.; Chen, W. Disturbance of trace element and gut microbiota profiles as indicators of autism spectrum disorder: A pilot study of Chinese children. Environ. Res. 2019, 171, 501–509. [Google Scholar] [CrossRef]
- Zou, R.; Xu, F.; Wang, Y.; Duan, M.; Guo, M.; Zhang, Q.; Zhao, H.; Zheng, H. Changes in the Gut Microbiota of Children with Autism Spectrum Disorder. Autism Res. 2020, 13, 1614–1625. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Levkova, M.; Chervenkov, T.; Pancheva, R. Genus-Level Analysis of Gut Microbiota in Children with Autism Spectrum Disorder: A Mini Review. Children 2023, 10, 1103. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Zhang, X.; Yu, Z.H.; Zhang, Z.; Deng, M.; Zhao, J.-H.; Ruan, B. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef]
- Chakraborty, P.; Laird, A.S. Understanding activity of butyrate at a cellular level. Neural Regen. Res. 2025, 20, 2323–2324. [Google Scholar] [CrossRef]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef] [PubMed]
- Knuesel, T.; Mohajeri, M.H. The Role of the Gut Microbiota in the Development and Progression of Major Depressive and Bipolar Disorder. Nutrients 2022, 14, 256. [Google Scholar] [CrossRef]
- Kovtun, A.S.; Averina, O.V.; Angelova, I.Y.; Yunes, R.A.; Zorkina, Y.A.; Morozova, A.Y.; Pavlichenko, A.V.; Syunyakov, T.S.; Karpenko, O.A.; Kostyuk, G.P.; et al. Alterations of the Composition and Neurometabolic Profile of Human Gut Microbiota in Major Depressive Disorder. Biomedicines 2022, 10, 2162. [Google Scholar] [CrossRef]
- Li, N.; Yang, J.; Zhang, J.; Liang, C.; Wang, Y.; Chen, B.; Zhao, C.; Wang, J.; Zhang, G.; Zhao, D.; et al. Correlation of Gut Microbiome Between ASD Children and Mothers and Potential Biomarkers for Risk Assessment. Genom. Proteom. Bioinform. 2019, 17, 26–38. [Google Scholar] [CrossRef]
- Swierkosz-Lenart, K.; Dos Santos, J.F.A.; Elowe, J.; Clair, A.H.; Bally, J.F.; Riquier, F.; Bloch, J.; Draganski, B.; Clerc, M.T.; Pozuelo Moyano, B.; et al. Therapies for obsessive-compulsive disorder: Current state of the art and perspectives for approaching treatment-resistant patients. Front. Psychiatry 2023, 14, 1065812. [Google Scholar] [CrossRef]
- Grassi, G.; Cecchelli, C.; Vignozzi, L.; Pacini, S. Investigational and Experimental Drugs to Treat Obsessive-Compulsive Disorder. J. Exp. Pharmacol. 2021, 12, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Nezgovorova, V.; Reid, J.; Fineberg, N.A.; Hollander, E. Optimizing first line treatments for adults with OCD. Compr. Psychiatry 2022, 115, 152305. [Google Scholar] [CrossRef]
- Schüller, T.; Kohl, S.; Dembek, T.; Tittgemeyer, M.; Huys, D.; Visser-Vandewalle, V.; Li, N.; Wehmeyer, L.; Barbe, M.; Kuhn, J.; et al. Internal Capsule/Nucleus Accumbens Deep Brain Stimulation Increases Impulsive Decision Making in Obsessive-Compulsive Disorder. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging 2023, 8, 281–289. [Google Scholar] [CrossRef] [PubMed]
- van Roessel, P.J.; Grassi, G.; Aboujaoude, E.N.; Menchón, J.M.; Van Ameringen, M.; Rodríguez, C.I. Treatment-resistant OCD: Pharmacotherapies in adults. Compr. Psychiatry 2023, 120, 152352. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Psicobióticos: Una nueva perspectiva para el tratamiento del estrés, de la ansiedad y de la depresión. Ansiedad Y Estrés 2024, 30, 79–93. [Google Scholar] [CrossRef]
- Talbott, S.M.; Talbott, J.A.; Stephens, B.J.; Oddou, M.P. Effect of coordinated probiotic/prebiotic/phytobiotic supplementation on microbiome balance and psychological mood state in healthy stressed adults. Funct. Foods Health Dis. 2019, 9, 265–274. [Google Scholar] [CrossRef]
- Tabouy, L.; Getselter, D.; Ziv, O.; Karpuj, M.; Tabouy, T.; Lukic, I.; Maayouf, R.; Werbner, N.; Ben-Amram, H.; Nuriel-Ohayon, M.; et al. Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain Behav. Immun. 2018, 73, 310–319. [Google Scholar] [CrossRef]
- Szklany, K.; Wopereis, H.; de Waard, C.; van Wageningen, T.; An, R.; van Limpt, K.; Knol, J.; Garssen, J.; Knippels, L.M.J.; Belzer, C.; et al. Supplementation of dietary non-digestible oligosaccharides from birth onwards improve social and reduce anxiety-like behaviour in male BALB/c mice. Nutr. Neurosci. 2020, 23, 896–910. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Al-Ayadhi, L.; Hassan, W.M.; Bhat, R.S.; Alonazi, M.A.; El-Ansary, A. Bee Pollen and Probiotics May Alter Brain Neuropeptide Levels in a Rodent Model of Autism Spectrum Disorders. Metabolites 2022, 12, 562. [Google Scholar] [CrossRef]
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef]
- Kobliner, V.; Mumper, E.; Baker, S.M. Reduction in Obsessive Compulsive Disorder and Self-Injurious Behavior with Saccharomyces boulardii in a Child with Autism: A Case Report. Integr. Med. 2018, 17, 38–41. [Google Scholar]
- Ghuge, S.; Rahman, Z.; Bhale, N.A.; Dikundwar, A.G.; Dandekar, M.P. Multistrain probiotic rescinds quinpirole-induced obsessive-compulsive disorder phenotypes by reshaping of microbiota gut-brain axis in rats. Pharmacol. Biochem. Behav. 2023, 232, 173652. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.; Stanford, J.L. Give us this day our daily germs. Immunol. Today 1998, 19, 113–116. [Google Scholar] [CrossRef]
- Gonda, X.; Jekkel, E.; Varga, A.; Miklósi, M.; Forintos, D.P. Advantage of obsessive-compulsive symptoms from the aspect of individual selection and group selection: An evolutionary psychological approach to obsessive-compulsive disorder. Neuropsychopharmacol. Hung. 2008, 10, 225–232. [Google Scholar]
- Leckman, J.F.; Denys, D.; Simpson, H.B.; Mataix-Cols, D.; Hollander, E.; Saxena, S.; Miguel, E.C.; Rauch, S.L.; Goodman, W.K.; Phillips, K.A.; et al. Obsessive-compulsive disorder: A review of the diagnostic criteria and possible subtypes and dimensional specifiers for DSM-V. Depress. Anxiety 2010, 27, 507–527. [Google Scholar] [CrossRef] [PubMed]
- Summerfeldt, L.J.; Kloosterman, P.H.; Antony, M.M.; Swinson, R.P. Examining an obsessive-compulsive core dimensions model: Structural validity of harm avoidance and incompleteness. J. Obs. Compuls. Relat. Disord. 2014, 3, 83–94. [Google Scholar] [CrossRef]
- Mataix-Cols, D.; Rosario-Campos, M.C.; Leckman, J.F. A multidimensional model of obsessive-compulsive disorder. Am. J. Psychiatry 2005, 162, 228–238. [Google Scholar] [CrossRef]
- Abramowitz, J.S.; Jacoby, R.J. Obsessive-compulsive disorder in the DSM-5. Clin. Psychol. Sci. Pract. 2014, 21, 221–235. [Google Scholar] [CrossRef]
- Abramowitz, J.S.; Jacoby, R.J. Obsessive-compulsive and related disorders: A critical review of the new diagnostic class. Annu. Rev. Clin. Psychol. 2015, 11, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Lochner, C.; Stein, D.J. Does work on obsessive-compulsive spectrum disorders contribute to understanding the heterogeneity of obsessive-compulsive disorder? Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 353–361. [Google Scholar] [CrossRef]
- Phillips, K.A.; Stein, D.J.; Rauch, S.L.; Hollander, E.; Fallon, B.A.; Barsky, A.; Fineberg, N.; Mataix-Cols, D.; Ferrão, Y.A.; Saxena, S.; et al. Should an obsessive-compulsive spectrum grouping of disorders be included in DSM-V? Depress. Anxiety 2010, 27, 528–555. [Google Scholar] [CrossRef]
- Sica, C.; Bottesi, G.; Caudek, C.; Orsucci, A.; Ghisi, M. “Not Just Right Experiences” as a psychological endophenotype for obsessive-compulsive disorder: Evidence from an Italian family study. Psychiatry Res. 2016, 245, 27–35. [Google Scholar] [CrossRef]
- Riesel, A.; Endrass, T.; Auerbach, L.A.; Kathmann, N. Overactive performance monitoring as an endophenotype for obsessive-compulsive disorder: Evidence from a treatment study. Am. J. Psychiatry 2015, 172, 665–673. [Google Scholar] [CrossRef]
- Russo, M.; Naro, A.; Mastroeni, C.; Morgante, F.; Terranova, C.; Muscatello, M.R.; Zoccali, R.; Calabrò, R.S.; Quartarone, A. Obsessive-compulsive disorder: A “sensory-motor” problem? Int. J. Psychophysiol. 2014, 92, 74–78. [Google Scholar] [CrossRef]
- Subirà, M.; Sato, J.R.; Alonso, P.; do Rosário, M.C.; Segalàs, C.; Batistuzzo, M.C.; Real, E.; Lopes, A.C.; Cerrillo, E.; Diniz, J.B.; et al. Brain structural correlates of sensory phenomena in patients with obsessive-compulsive disorder. J. Psychiatry Neurosci. 2015, 40, 232–240. [Google Scholar] [CrossRef]
- Kamble, S.R.; Dandekar, M.P. Implication of microbiota gut-brain axis in the manifestation of obsessive-compulsive disorder: Preclinical and clinical evidence. Eur. J. Pharmacol. 2023, 957, 176014. [Google Scholar] [CrossRef]
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Kärkkäinen, O.; Layé, S.; Delzenne, N.; Hanhineva, K. Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes 2022, 14, 2102878. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J. Inflammation, Obsessive-Compulsive Disorder, and Related Disorders. Curr. Top. Behav. Neurosci. 2021, 49, 31–53. [Google Scholar] [PubMed]
- Turna, J.; Grosman Kaplan, K.; Patterson, B.; Bercik, P.; Anglin, R.; Soreni, N.; Van Ameringen, M. Higher prevalence of irritable bowel syndrome and greater gastrointestinal symptoms in obsessive-compulsive disorder. J. Psychiatr. Res. 2019, 118, 1–6. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Human gut microbiome, diet, and mental disorders. Int. Microbiol. 2025, 28, 1–15. [Google Scholar] [CrossRef]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B.; et al. Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef]
- Biazzo, M.; Deidda, G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. J. Clin. Med. 2022, 11, 4119. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Fecal Microbiota Transplantation as a Tool for Therapeutic Modulation of Neurological and Mental Disorders. SciBase Neurol. 2024, 2, 1018. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Nutritional Psychiatry: A Novel Approach to the Treatment of Mental Health Disorders. Actas Esp. Psiquiatr. 2025, 53, 443–445. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A. Una revisión crítica sobre la influencia de la dieta vegetariana en la salud mental. Rev. Esp. Nutr. Comunitaria 2024, 30. Available online: https://hdl.handle.net/20.500.14468/25971 (accessed on 30 May 2025).
- Macy, A.S.; Theo, J.N.; Kaufmann, S.C.; Ghazzaoui, R.B.; Pawlowski, P.A.; Fakhry, H.I.; Cassmassi, B.J.; IsHak, W.W. Quality of life in obsessive compulsive disorder. CNS Spectr. 2013, 18, 21–33. [Google Scholar] [CrossRef]
- Friedman-Ezra, A.; Keydar-Cohen, K.; van Oppen, P.; Eikelenboom, M.; Schruers, K.; Anholt, G.E. Loneliness in OCD and its determinants. Psychiatry Res. 2024, 337, 115963. [Google Scholar] [CrossRef] [PubMed]
- Timpano, K.R.; Çek, D.; Rubenstein, L.M.; Murphy, D.; Schmidt, N.B. Exploring the association between obsessive-compulsive symptoms and loneliness: Consideration of specificity and gender. J. Cogn. Psychother. 2014, 28, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Durna, G.; Yorulmaz, O.; Aktaç, A. Public stigma of obsessive compulsive disorder and schizophrenic disorder: Is there really any difference? Psychiatry Res. 2019, 271, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.M.; Cameron, R.; Jorgensen, A. Germaphobia! Does Our Relationship with and Knowledge of Biodiversity Affect Our Attitudes Toward Microbes? Front. Psychol. 2021, 12, 678752. [Google Scholar] [CrossRef]
- Jalalifar, E.; Arad, A.; Rastkar, M.; Beheshti, R. The COVID-19 pandemic and obsessive-compulsive disorder: A systematic review of comparisons between males and females. Acta Neuropsychiatr. 2023, 35, 270–291. [Google Scholar] [CrossRef]
- Algadeeb, J.; Alramdan, M.J.; AlGadeeb, R.B.; Almusawi, K.N. The Impact of COVID-19 on Patients with Obsessive-Compulsive Disorder in Gulf Countries: A Narrative Review. Cureus 2024, 16, e67381. [Google Scholar] [CrossRef]
- D’Urso, G.; Magliacano, A.; Manzo, M.; Pomes, M.V.; Iuliano, C.; Iasevoli, F.; Dell’Osso, B.; de Bartolomeis, A. Impact of Benzodiazepines and Illness Duration on Obsessive-Compulsive Disorder during COVID-19 in Italy: Exploring Symptoms’ Evolutionary Benefits. Brain Sci. 2024, 14, 338. [Google Scholar] [CrossRef]
- Nahidi, M.; Mirza Hoseinzadeh Moghaddam, Z.; Tabesh, H.; Afshari Saleh, L.; Rohani, F.; Shoib, S. Prevalence of obsessive-compulsive symptoms and their psychosocial correlates among medical students during COVID-19 pandemic. Int. Clin. Psychopharmacol. 2024, 39, 174–180. [Google Scholar] [CrossRef]
- Pozza, A.; Ragucci, F.; Angelo, N.L.; Pugi, D.; Cuomo, A.; Garcia-Hernandez, M.D.; Rosa-Alcazar, A.I.; Fagiolini, A.; Starcevic, V. Worldwide prevalence of obsessive-compulsive symptoms during the COVID-19 pandemic: A systematic review and meta-analysis. J. Psychiatr. Res. 2024, 172, 360–381. [Google Scholar] [CrossRef]
- Demaria, F.; Pontillo, M.; Vicari, S. Hand Washing: When Ritual Behavior Protects! COVID-19 Experience in Children and Adolescents with (and without) Obsessive-Compulsive Disorder. Actas Esp. Psiquiatr. 2024, 52, 595–597. [Google Scholar] [CrossRef]
- Henein, A.; Pascual-Sanchez, A.; Corciova, S.; Hodes, M. Obsessive-compulsive disorder in treatment-seeking children and adolescents during the COVID-19 pandemic. Eur. Child Adolesc. Psychiatry 2024, 33, 629–632. [Google Scholar] [CrossRef]
- Blendermann, M.; Ebalu, T.I.; Obisie-Orlu, I.C.; Fried, E.I.; Hallion, L.S. A narrative systematic review of changes in mental health symptoms from before to during the COVID-19 pandemic. Psychol. Med. 2024, 54, 43–66. [Google Scholar] [CrossRef]
- Khalkhali, M.; Zarvandi, P.; Mohammadpour, M.; Alavi, S.M.K.; Khalkhali, P.; Farrahi, H. The anxiety response of patients with severe psychiatric disorders to the recent public health crisis. BMC Psychiatry 2024, 24, 302. [Google Scholar] [CrossRef] [PubMed]
- Chaharshughi, B.T.; Izadi, R.; Naghavi, A. Tele-psychotherapy for individuals with obsessive-compulsive disorder during the COVID-19 outbreak: A qualitative study. J. Educ. Health Promot. 2024, 13, 112. [Google Scholar] [CrossRef]
- Momani, M.A.A.L.; Abdalrahim, M.S.; Shoqirat, N.; Zeilani, R.S.; Dardas, L.A. In the shadows of OCD: Jordanian patients’ experiences during the COVID-19 quarantine. J. Psychiatr. Ment. Health Nurs. 2024, 32, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Human Oral Microbiome and Its Influence on Mental Health and Brain Disorders. AIMS Microbiol. 2025, 11, 242–294. [Google Scholar] [CrossRef] [PubMed]
- Martinovic, D.; Cernak, M.; Lasic, S.; Puizina, E.; Lesin, A.; Rakusic, M.; Lupi-Ferandin, M.; Jurina, L.; Kulis, E.; Bozic, J. The Association Between Oral Health and the Tendencies to Obsessive-Compulsive Behavior in Biomedical Students—A Questionnaire Based Study. Medicina 2025, 61, 593. [Google Scholar] [CrossRef]
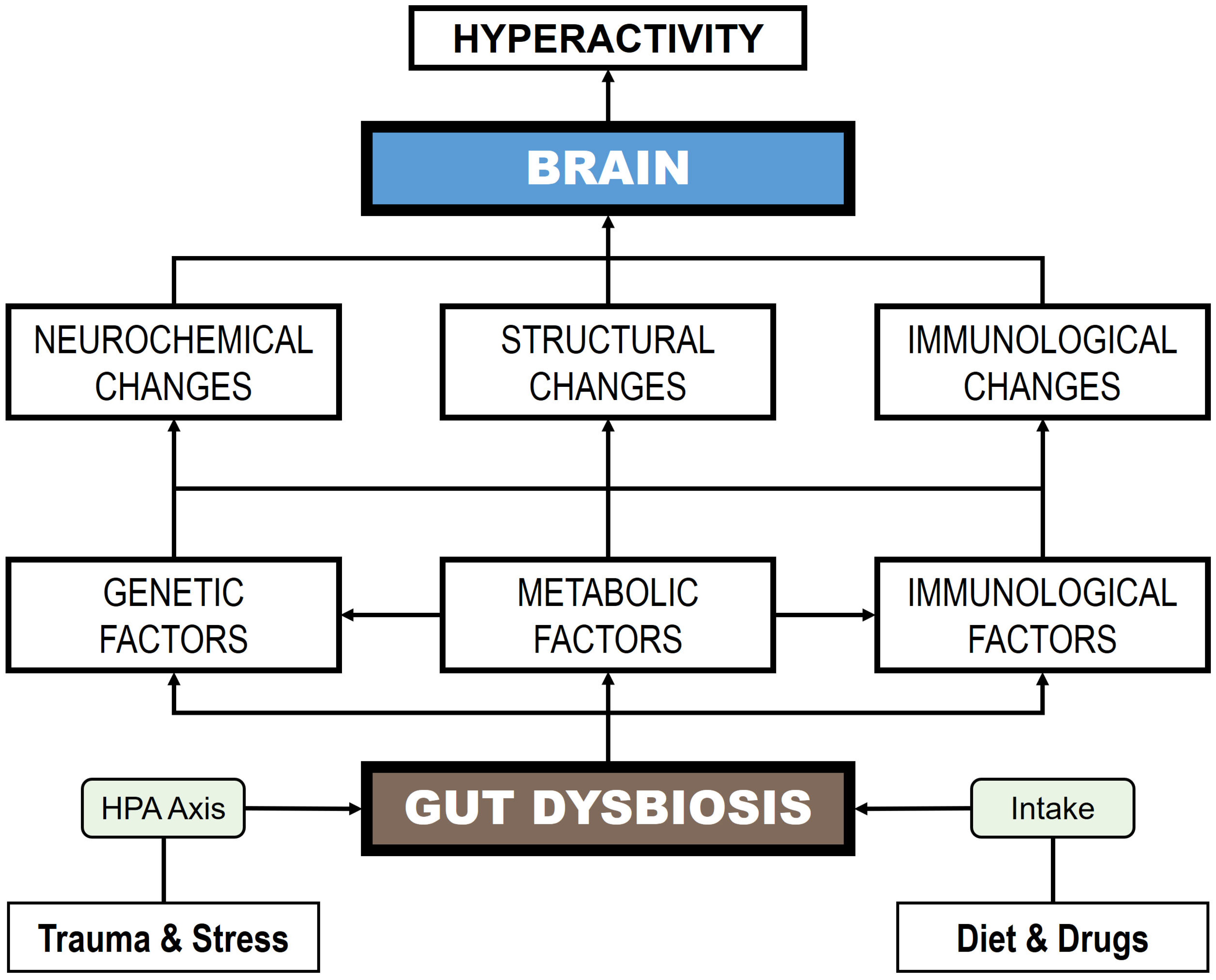

| References | Sample | Decrease | Increase |
|---|---|---|---|
| Preclinical | |||
| Jung et al. [217] | Long-Evans rats treated with quinpirol (N = 15). | NE | Lachnospiraceae Ruminococcaceae |
| Scheepers et al. [218] | Apodemus sylvaticus mice (N = 11). | Anaeroplasma Prevotella | Aestuariispira Desulfovermiculus Holdemanella Peptococcus |
| Clinical | |||
| Quagliariello et al. [219] | OCD patients with PANS/PANDAS (N = 30). | Coprococcus Dorea Roseburia Turicibacter | Bacteroides Odoribacter Oscillospira |
| Turna et al. [220] | OCD patients (N = 21). | Anaerostipes Odoribacter Oscillospira | NE |
| Domènech et al. [221] | OCD patients (N = 38). | Agathobacter Coprococcus Prevotellaceae | Alistipes |
| D’Addario et al. [222] | OCD patients (N = 64) | Fusobacteriota/Actiomycetota ratio | Actinobacter Bacteroidota |
| Dai et al. [223] | OCD children (N = 49) | Escherichia Shigella Romboutsia Subdoligranulum Terrisporobacter Mitsuokella Coprococcus Ruminococcus | Bacteroides Lachnospira Parabacteroides Parasutterella |
| References | Studies | Treatment | Outcomes |
|---|---|---|---|
| Preclinical | |||
| Kantak et al. [32] | BALB/cJ mice (N = 12). | Probiotic: L. rhamnosus strain GG for 2–4 weeks. | Attenuates OCD-like behaviors (increased perseverative locomotion in the open field, stereotypic movements, and marble burying). |
| Tabouy et al. [295] | Shank3 KO mice (N = 31). | Probiotic: L. reuteri strain MM4-1A for 3 weeks. | Reduces repetitive and antisocial behaviors and GABA receptor expression. |
| Sanikhani et al. [34] | Wistar rats (N = 30). | Probiotic: L. casei strain Shirota + Fluoxetine for 4 weeks. | Modulates genes related to serotonin. Exerts an increase in BDNF. |
| Ghuge et al. [300] | Wistar rats (N = 30) treated with quinpirole | Probiotic cocktail: Bacillus coagulans strain Unique IS-2, L. rhamnosus strain UBLR-58, L. plantarum strain UBLP-40, B. lactis strain UBBLa-70, B. infantis strain UBBI-01, and B. breve strainUBBr-01 for 8 weeks | Probiotic cocktail reverses quinpirole-induced OCD-like phenotypes. Restores the dysregulated inflammatory mediators and neurotransmitters |
| Szklany et al. [296] | BALB/cByJ mice (N = 10). | Prebiotic: GOS + FOS for 11 weeks. | Reduces anxiety and improves social behaviors. Exerts changes in the serotonergic system. |
| Alghamdi et al. [297] | Wistar rats (N = 36). | Synbiotic: probiotic ProtexinR (B. longum subsp. infantis, B. breve, L. acidophilus, L. delbrueckii subsp. bulgaricus, L. casei, L. rhamnosus, and Streptococcus thermophilus) + prebiotic (bee pollen) for 3 weeks. | Reverses the effects of propionic acid on cognitive dysfunction. Protects from the neurotoxic effects of propionic acid. |
| Clinical | |||
| Messaoudi et al. [298] | Healthy patients (N = 25) and OCD patients (N = 3). | Probiotic: L. helveticus strain R0052 and B. longum strain R0175 for 4 weeks. | Reduces anxiety and depression. Decreases the symptom severity index (Hopkins checklist). |
| Kobliner et al. [299] | A patient with OCD, ASD, and self-injurious behavior (N = 1). | Probiotic: Saccharomyces boulardii. The duration of treatment is not established. | Reduces symptoms related to OCD and self-injurious behaviors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrego-Ruiz, A.; Borrego, J.J. Biological, Psychosocial, and Microbial Determinants of Childhood-Onset Obsessive–Compulsive Disorder: A Narrative Review. Children 2025, 12, 1063. https://doi.org/10.3390/children12081063
Borrego-Ruiz A, Borrego JJ. Biological, Psychosocial, and Microbial Determinants of Childhood-Onset Obsessive–Compulsive Disorder: A Narrative Review. Children. 2025; 12(8):1063. https://doi.org/10.3390/children12081063
Chicago/Turabian StyleBorrego-Ruiz, Alejandro, and Juan J. Borrego. 2025. "Biological, Psychosocial, and Microbial Determinants of Childhood-Onset Obsessive–Compulsive Disorder: A Narrative Review" Children 12, no. 8: 1063. https://doi.org/10.3390/children12081063
APA StyleBorrego-Ruiz, A., & Borrego, J. J. (2025). Biological, Psychosocial, and Microbial Determinants of Childhood-Onset Obsessive–Compulsive Disorder: A Narrative Review. Children, 12(8), 1063. https://doi.org/10.3390/children12081063





