Advances in Fluorescent Adjuncts in Pediatric Surgery: A Comprehensive Review of Applications of Indocyanine Green Across Surgical Specialties
Abstract
Highlights
- •
- While Indocyanine Green (ICG) is often associated with fluorescence of hepatobiliary pathologies and anatomy, its route and timing of administration can be titrated to target a wide variety of pathologies.
- •
- ICG fluorescence can aid in assessment of vascular anatomy, biliary anatomy, congenital lesions, tumor margins, tissue perfusion, and lymphatic flow.
- •
- ICG is a low-risk, low-cost adjunct that can aid in the surgical approach to numerous pathologies.
- •
- Preoperative planning is required to adequately target pathology of interest with proper route of administration, ICG dosage, and timing of administration.
Abstract
1. Introduction
2. Materials and Methods
3. Administration Considerations
| Target | Timing | Most Common Dosage | Outliers | Considerations | References |
|---|---|---|---|---|---|
| Angiography | Intraoperatively | 0.3–0.5 mg/kg | Doses as low as 0.02 mg/kg for renal microvasculature in transplant surgery. Others administer standardized doses of 2.5–5 mg rather than weight-based dosing. | Fluorescence is visible within 1 min. ICG has a 3 min half-life intravascularly. | [7,11,17,26,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] |
| Cholangiography | 3–7 h Preoperatively | 0.4–0.5 mg/kg | Doses as low as 0.1 mg/kg for infants and small children. Others administer a standardized dose of 2.5 mg for older children rather than weight-based dosing. Some administer ICG only 15 min preoperatively, whereas others administer up to 18 h preoperatively. | A shorter duration between administration and operation may result in a poor signal-to-noise ratio due to diffuse hepatic uptake. | [11,17,38,44,47,48,49,50,51] |
| Biliary Atresia | 24 h Preoperatively | 0.1 or 0.5 mg/kg | 0.05 mg/kg at 24 h preoperatively, 1 h preoperatively, or both. | ICG can be used as an adjunct for the diagnosis of biliary atresia and intraoperative guidance. | [17,38,52,53,54,55] |
| Choledochal Cyst | 4–8 h Preoperatively | 0.1 or 0.5 mg/kg | - | Preoperative dosing reduces background noise from the hepatic parenchyma. | [17,56,57] |
| Primary Hepatic Malignancy | 48–96 h Preoperatively | 0.5 mg/kg | ICG dosage ranged from 0.2 to 1.5 mg/kg, with timing ranging from 24 to 138 h preoperatively. | A shorter duration between administration and operation may result in a poor signal-to-noise ratio due to diffuse hepatic uptake. | [11,17,18,19,20,38,50,54,58,59,60,61,62,63,64,65,66] |
| Pulmonary Metastases | 24 h Preoperatively | 0.5 mg/kg | ICG dosage ranged from 0.2 to 1.5 mg/kg, with timing ranging from 0 to 96 h preoperatively. Some administered doses as high as 4.5 mg/kg for metastases from non-hepatic primaries. | - | [11,19,20,31,38,54,58,59,61,67,68,69,70,71,72,73,74,75,76,77,78] |
| Peritoneal Metastases from Hepatic Primary | 72 h Preoperatively | 0.5 mg/kg | - | - | [38,79] |
| Congenital Lung Malformations | Intraoperatively | 0.3 mg/kg | Dosage ranged from 0.1 mg/kg to 0.6 mg/kg. | - | [80,81,82,83] |
| Esophageal perfusion | Intraoperatively | 0.1 mg/kg | The standard angiography dose of 0.5 mg/kg has been successfully used as well. | - | [26,36,50,73,84,85,86,87] |
| Colorectal | Intraoperatively | 0.2–0.5 mg/kg | Doses as low as 0.01 mg/kg have been successfully used. Others administer a standard dose of 2.5 mg and will redose if there is inadequate fluorescence. | - | [31,34,88,89,90,91,92,93] |
| Pancreatic Lesions | Intraoperatively | 0.5 mg/kg or 2 mg/kg | - | Variable success rates with nodule fluorescence. | [50,71] |
| Splenic Cysts | Intraoperatively | 0.2–0.6 mg/kg | - | - | [94,95,96] |
| Renal Malignancies | 24 h Preoperatively | 1.5 mg/kg | Dosage ranges from 0.05 to 0.67 mg/kg. | If administered intraoperatively, malignancy may be hypo-fluorescent relative to healthy parenchyma. | [97,98] |
| Benign Renal Pathologies (Cyst, ureteral duplication, etc.) | Intraoperatively | 0.2–0.3 mg/kg | - | - | [14,38,99,100] |
| Sarcoma | 24 h Preoperatively | 4–5 mg/kg | - | Decreased tumor fluorescence at standard dosing. | [11] |
| Retroperitoneal Malignancies | Intraoperatively | 0.3 mg/kg | - | - | [101] |
| ENT Malignancies | 24 h Preoperatively | 1.5 mg/kg | - | - | [102] |
| Target | Timing | Most Common Dosage | Outliers | Considerations | References |
|---|---|---|---|---|---|
| Soft Tissue Malignancies (Melanoma, Sarcoma, Squamous Cell Carcinoma) Lymph Node Targeting | 30 min Preoperatively or Intraoperatively | 4–5 mg injected in total across multiple quadrants | 0.25 mg injected in 4 quadrants 4–24 h preoperatively. | Lymphatic vessels and nodes are fluorescent. | [103,104,105,106] |
| Nephroblastoma Lymph Node Targeting | Intraoperatively | 5 mg injected into the renal parenchyma or perihilar region. | - | Sentinel nodes are fluorescent. | [97,98,107] |
| Para-testicular Rhabdomyosarcoma Lymph Node Targeting | Intraoperatively | 10 mg injected within the spermatic cord. | - | Sentinel nodes are fluorescent. | [17,108] |
| Pulmonary metastases/nodules | Immediately Preoperatively | 0.0125–0.0625 mg injected into the target lesions under CT guidance. | - | Smaller lesions may require smaller volume injections. | [11,109,110] |
| Benign Cervico-facial Cysts (Thyroglossal, nasal, etc.) | Immediately Preoperatively | 0.5–5 mg | - | Dosage depending on cyst size | [111] |
| Target | Timing | Most Common Dosage | Outliers | Considerations | References |
|---|---|---|---|---|---|
| Congenital ascites or chylothorax | Intraoperatively | 0.1–0.25 mg injected into the first interdigital regions of hands and feet. | - | Fluorescence pattern directs interventional approach. | [25,112,113] |
| Postoperative Chylothorax | 1 h Preoperatively | 0.25 mg injected into the interweb spaces between the first and second toes. | Doses vary from 0.025 mg/extremity to 5 mg in the bilateral inguinal regions. | Identify fluorescent extravasation from the thoracic duct or another injured lymphatic vessel. | [11,17,38,114,115,116,117,118,119] |
| Primary/Congenital Lymphedema | Intraoperatively | 1 mg injected into the first and fourth interweb spaces of each hand and foot. | Doses vary from 0.0125 mg/extremity to 2.5 mg/extremity. | - | [38,112,120,121,122,123] |
| Lymphatic Malformations | Intraoperatively | 0.75 mg injected into the interdigital space of the ipsilateral extremity or 0.005–0.125 mg into multiple loci around the lymphatic malformation. | - | The fluorescence pattern may be used to quantify the severity of malformation. | [38,123,124,125,126] |
| Route | Target | Timing | Most Common Dosage | Outliers | Considerations | References |
|---|---|---|---|---|---|---|
| Enteral | Duodenal Atresia | Intraoperatively | 5 mL of 2.5 mg/mL ICG | - | Visualize the duodenal web through the intestinal wall. | [50,127] |
| Inhaled | Pulmonary Sequestration | 30–60 min Preoperatively | 0.25 mg/kg or 0.5 mg/kg | - | The region of sequestration does not fluoresce. | [24,128,129] |
| Intra-testicular | Varicocele | Intraoperatively | 2.5–6.25 mg | - | Laparoscopic Palomo technique is the most common approach. | [14,34,38,48,99,130] |
| Intra-ureter | Non-functional renal moiety | Intraoperatively | 10 mL 2.5 mg/mL ICG solution | - | - | [14] |
4. Clinical Applications
4.1. Hepatobiliary
4.1.1. Benign Gallbladder Pathologies
4.1.2. Biliary Malformations (Biliary Atresia and Choledochal Cysts)
4.1.3. Hepatic Malignancies
4.1.4. Transplantation
4.2. Gastrointestinal Perfusion
4.2.1. Esophageal
4.2.2. Small Bowel
4.2.3. Colorectal
4.3. Non-Hepatobiliary Oncology
4.3.1. Lymph Node Identification
4.3.2. Pancreatic
4.3.3. Adrenal/Neuroendocrine
4.4. Cardiothoracic
4.4.1. Pulmonary Metastases
4.4.2. Congenital Lung Malformations
4.4.3. Congenital Cardiac Defects
4.5. Urology
4.5.1. Varicocele
4.5.2. Testicular Torsion
4.5.3. Nephrectomy
4.5.4. Hemi-Nephrectomy
4.5.5. Congenital Malformations
4.5.6. Ureteral Identification/Reconstruction
4.5.7. Cyst De-Roofing
4.6. Lymphatic Pathologies
4.6.1. Chylothorax
4.6.2. Chylous Ascites
4.6.3. Lymphedema
4.6.4. Lymphangioma/Lymphatic Malformations
4.7. Gynecology
4.7.1. Ovarian Perfusion
4.7.2. Mayer-Rokitansky-Küster-Hauser Syndrome
4.8. Spleen
4.9. Soft Tissue/Flap Perfusion
4.10. Otolaryngology
4.11. Ophthalmology
4.12. Neurosurgery
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ICG | Indocyanine Green |
| NIR | Near-Infra Red |
| IOC | Intraoperative Cholangiography |
| CPAM | Congenital Pulmonary Airway Malformation |
References
- Lu, C.H.; Hsiao, J.K. Indocyanine green: An old drug with novel applications. Tzu Chi Med. J. 2021, 33, 317–322. [Google Scholar] [CrossRef]
- Fox, I.J.; Wood, E.H. Indocyanine green: Physical and physiologic properties. Proc. Staff. Meet. Mayo Clin. 1960, 35, 732–744. [Google Scholar] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5282412, Indocyanine Green. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Indocyanine-Green (accessed on 6 August 2025).
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef]
- Baker, K.J. Binding of sulfobromophthalein (BSP) sodium and indocyanine green (ICG) by plasma alpha-1 lipoproteins. Proc. Soc. Exp. Biol. Med. 1966, 122, 957–963. [Google Scholar] [CrossRef]
- Zeineddin, S.; Linton, S.; Inge, M.; De Boer, C.; Hu, A.; Goldstein, S.D.; Lautz, T.B. Fluorescence-guided surgery: National trends in adoption and application in pediatric surgery. J. Pediatr. Surg. 2023, 58, 689–694. [Google Scholar] [CrossRef]
- Engel, E.; Schraml, R.; Maisch, T.; Kobuch, K.; König, B.; Szeimies, R.M.; Hillenkamp, J.; Bäumler, W.; Vasold, R. Light-induced decomposition of indocyanine green. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1777–1783. [Google Scholar] [CrossRef]
- Yannuzzi, L.A. Indocyanine green angiography: A perspective on use in the clinical setting. Am. J. Ophthalmol. 2011, 151, 745–751.e741. [Google Scholar] [CrossRef]
- Lim, Z.Y.; Mohan, S.; Balasubramaniam, S.; Ahmed, S.; Siew, C.C.H.; Shelat, V.G. Indocyanine green dye and its application in gastrointestinal surgery: The future is bright green. World J. Gastrointest. Surg. 2023, 15, 1841–1857. [Google Scholar] [CrossRef]
- Goldstein, S.D.; Heaton, T.E.; Bondoc, A.; Dasgupta, R.; Abdelhafeez, A.; Davidoff, A.M.; Lautz, T.B. Evolving applications of fluorescence guided surgery in pediatric surgical oncology: A practical guide for surgeons. J. Pediatr. Surg. 2021, 56, 215–223. [Google Scholar] [CrossRef]
- Graves, C.; Ely, S.; Idowu, O.; Newton, C.; Kim, S. Direct Gallbladder Indocyanine Green Injection Fluorescence Cholangiography During Laparoscopic Cholecystectomy. J. Laparoendosc. Adv. Surg. Tech. 2017, 27, 1069–1073. [Google Scholar] [CrossRef]
- Esposito, C.; Rathod, K.J.; Cerulo, M.; Del Conte, F.; Saxena, R.; Coppola, V.; Sinha, A.; Esposito, G.; Escolino, M. Indocyanine green fluorescent cholangiography: The new standard practice to perform laparoscopic cholecystectomy in pediatric patients. A comparative study with conventional laparoscopic technique. Surgery 2024, 175, 498–504. [Google Scholar] [CrossRef]
- Esposito, C.; Masieri, L.; Cerulo, M.; Castagnetti, M.; Del Conte, F.; Di Mento, C.; Esposito, G.; Tedesco, F.; Carulli, R.; Continisio, L.; et al. Indocyanine green (ICG) fluorescence technology in pediatric robotic surgery. J. Robot. Surg. 2024, 18, 209. [Google Scholar] [CrossRef]
- Di Mitri, M.; Di Carmine, A.; Zen, B.; Collautti, E.; Bisanti, C.; D’Antonio, S.; Libri, M.; Gargano, T.; Lima, M. Advancing Pediatric Surgery with Indocyanine Green (ICG) Fluorescence Imaging: A Comprehensive Review. Children 2025, 12, 515. [Google Scholar] [CrossRef]
- Lau, C.T.; Au, D.M.; Wong, K.K.Y. Application of indocyanine green in pediatric surgery. Pediatr. Surg. Int. 2019, 35, 1035–1041. [Google Scholar] [CrossRef]
- Sincavage, J.; Gulack, B.C.; Zamora, I.J. Indocyanine green (ICG) fluorescence-enhanced applications in pediatric surgery. Semin. Pediatr. Surg. 2024, 33, 151384. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zheng, M.; Li, J.; Tan, T.; Yang, J.; Pan, J.; Hu, C.; Zou, Y.; Yang, T. Clinical Application of Indocyanine Green Fluorescence Imaging in the Resection of Hepatoblastoma: A Single Institution’s Experiences. Front. Surg. 2022, 9, 932721. [Google Scholar] [CrossRef]
- Souzaki, R.; Kawakubo, N.; Matsuura, T.; Yoshimaru, K.; Koga, Y.; Takemoto, J.; Shibui, Y.; Kohashi, K.; Hayashida, M.; Oda, Y.; et al. Navigation surgery using indocyanine green fluorescent imaging for hepatoblastoma patients. Pediatr. Surg. Int. 2019, 35, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafeez, A.; Talbot, L.; Murphy, A.J.; Davidoff, A.M. Indocyanine Green-Guided Pediatric Tumor Resection: Approach, Utility, and Challenges. Front. Pediatr. 2021, 9, 689612. [Google Scholar] [CrossRef] [PubMed]
- Speich, R.; Saesseli, B.; Hoffmann, U.; Neftel, K.A.; Reichen, J. Anaphylactoid reactions after indocyanine-green administration. Ann. Intern. Med. 1988, 109, 345–346. [Google Scholar] [CrossRef]
- Balogun, Z.; Wiener, A.; Berger, J.; Lesnock, J.; Garrett, A.A. Use of indocyanine green dye for sentinel lymph node mapping in patients with endometrial cancer and a history of iodinated contrast allergy. Gynecol. Oncol. Rep. 2024, 55, 101467. [Google Scholar] [CrossRef]
- McKibben, N.K.; Collawn, S.S. Diphenhydramine prophylaxis before using indocyanine green during flap surgery in patients with iodine allergies: A two-case report. AME Surg. J. 2025, 5, 4. [Google Scholar] [CrossRef]
- He, T.; Sun, X.; Che, G.; Luo, D.; Yuan, M.; Yang, G.; Cheng, K.; Xu, C. Thoracoscopic anatomical lesion resection by fluorescence imaging on congenital lung malformation. Asian J. Surg. 2024, 48, 2298–2301. [Google Scholar] [CrossRef]
- Shibasaki, J.; Hara, H.; Mihara, M.; Adachi, S.; Uchida, Y.; Itani, Y. Evaluation of lymphatic dysplasia in patients with congenital pleural effusion and ascites using indocyanine green lymphography. J. Pediatr. 2014, 164, 1116–1120.e1111. [Google Scholar] [CrossRef]
- Breuking, E.A.; van Varsseveld, O.C.; Harms, M.; Tytgat, S.; Hulscher, J.B.F.; Ruiterkamp, J. Safety and Feasibility of Indocyanine Green Fluorescence Angiography in Pediatric Gastrointestinal Surgery: A Systematic Review. J. Pediatr. Surg. 2023, 58, 1534–1542. [Google Scholar] [CrossRef]
- Yoo, K.Y.; Baek, H.Y.; Jeong, S.; Hallacoglu, B.; Lee, J. Intravenously administered indocyanine green may cause falsely high near-infrared cerebral oximetry readings. J. Neurosurg. Anesthesiol. 2015, 27, 57–60. [Google Scholar] [CrossRef]
- Pachl, M.J. Anaesthetic implications of intra-operative Indocyanine Green use in pediatric surgery. Photodiagnosis Photodyn. Ther. 2023, 44, 103817. [Google Scholar] [CrossRef] [PubMed]
- Dhua, A.K.; Jain, V.; Yadav, D.K.; Goel, P.; Agarwala, S. Harnessing Indocyanine Green Fluorescence for Checking Intraoperative Patency of Splenorenal Shunt for Extrahepatic Portal Venous Obstruction. J. Indian Assoc. Pediatr. Surg. 2025, 30, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, Y.; Hirayama, Y.; Yokoyama, N.; Otani, T.; Nitta, K.; Hashidate, H.; Yoshida, M.; Iida, H.; Masui, D.; Manabe, S. Intraoperative near-infrared indocyanine green fluorescence angiography (NIR-ICG AG) can predict delayed small bowel stricture after ischemic intestinal injury: Report of a case. J. Pediatr. Surg. 2013, 48, 1123–1128. [Google Scholar] [CrossRef]
- Shafy, S.Z.; Hakim, M.; Lynch, S.; Chen, L.; Tobias, J.D. Fluorescence Imaging Using Indocyanine Green Dye in the Pediatric Population. J. Pediatr. Pharmacol. Ther. 2020, 25, 309–313. [Google Scholar] [CrossRef]
- Hinchcliff, K.M.; Yao, A.; Taub, P.J. Laser-assisted indocyanine green imaging to assess perfusion of scalp closure in an infant. J. Craniofac. Surg. 2013, 24, 2004–2006. [Google Scholar] [CrossRef]
- Martins, D.B.; Farias-Eisner, G.; Mandelbaum, R.S.; Hoang, H.; Bradley, J.P.; Lee, J.C. Intraoperative Indocyanine Green Laser Angiography in Pediatric Autologous Ear Reconstruction. Plast. Reconstr. Surg. Glob. Open 2016, 4, e709. [Google Scholar] [CrossRef]
- Menon, R.; Jayakumar, T.K.; Nayak, S.; Saxena, R.; Jadhav, A.S.; Rathod, K.J.; Pathak, M.; Sinha, A. Advancing Pediatric Surgery with Indocyanine Green: Nonhepatobiliary Applications and Outcomes. J. Indian Assoc. Pediatr. Surg. 2025, 30, 52–58. [Google Scholar] [CrossRef]
- Le-Nguyen, A.; Bourque, C.J.; Trudeau, M.O.; Ducruet, T.; Faure, C.; Piché, N. Indocyanine green fluorescence angiography in pediatric intestinal resections: A first prospective mixed methods clinical trial. J. Pediatr. Surg. 2023, 58, 82–88. [Google Scholar] [CrossRef]
- Onishi, S.; Muto, M.; Yamada, K.; Murakami, M.; Kedoin, C.; Nagano, A.; Matsui, M.; Sugita, K.; Yano, K.; Harumatsu, T.; et al. Feasibility of delayed anastomosis for long gap esophageal atresia in the neonatal period using internal traction and indocyanine green-guided near-infrared fluorescence. Asian J. Endosc. Surg. 2022, 15, 877–881. [Google Scholar] [CrossRef]
- Han, T.; Sun, B.; Wang, W.; Cui, J.; Shen, W. The Role of ICG Angiography in Decision Making About Skin-Sparing in Pediatric Acute Trauma. Front. Pediatr. 2022, 10, 851270. [Google Scholar] [CrossRef]
- Le-Nguyen, A.; O’Neill Trudeau, M.; Dodin, P.; Keezer, M.R.; Faure, C.; Piché, N. The Use of Indocyanine Green Fluorescence Angiography in Pediatric Surgery: A Systematic Review and Narrative Analysis. Front. Pediatr. 2021, 9, 736242. [Google Scholar] [CrossRef]
- Pourmoghadam, K.K.; Bunnell, A.P.; O’Brien, M.C.; DeCampli, W.M. Avoiding coronary injury in congenital heart surgery by laser-assisted indocyanine green dye imaging. World J. Pediatr. Congenit. Heart Surg. 2014, 5, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Otake, K.; Uchida, K.; Inoue, M.; Koike, Y.; Narushima, M.; Kusunoki, M. Use of computed tomography-lymphangiography with direct injection of water-soluble contrast medium to identify the origin of chylous ascites. J. Vasc. Surg. Venous Lymphat. Disord. 2015, 3, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Gerken, A.L.H.; Nowak, K.; Meyer, A.; Kriegmair, M.C.; Weiss, C.; Krämer, B.K.; Glossner, P.; Heller, K.; Karampinis, I.; Kunath, F.; et al. Ureterovesical Anastomosis Complications in Kidney Transplantation: Definition, Risk Factor Analysis, and Prediction by Quantitative Fluorescence Angiography with Indocyanine Green. J. Clin. Med. 2022, 11, 6585. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, N.; Yoneda, A.; Ozeki, G.; Saito, T.; Fujiogi, M.; Kano, M.; Yamamoto, Y.; Ishimaru, T.; Kanamori, Y.; Fujino, A. Outcomes of nonrejection in weakly fluorescent intestine detected by indocyanine green fluorescence angiography: A case series of infants. Surg. Case Rep. 2024, 10, 97. [Google Scholar] [CrossRef]
- Iinuma, R.; Kato, H.; Yamada, T.; Sato, Y.; Tanaka, Y.; Kato, H.; Kuroki, M.; Shibata, H.; Kohyama, K.; Ohashi, T.; et al. Usefulness of intraoperative monitoring for mesenteric lymph node metastasis in transplanted free jejunum. Auris Nasus Larynx 2023, 50, 827–830. [Google Scholar] [CrossRef]
- Fernández-Bautista, B.; Mata, D.P.; Parente, A.; Pérez-Caballero, R.; De Agustín, J.C. First Experience with Fluorescence in Pediatric Laparoscopy. Eur. J. Pediatr. Surg. Rep. 2019, 7, e43–e46. [Google Scholar] [CrossRef] [PubMed]
- Kogon, B.; Fernandez, J.; Kanter, K.; Kirshbom, P.; Vincent, B.; Maher, K.; Guzetta, N. The role of intraoperative indocyanine green fluorescence angiography in pediatric cardiac surgery. Ann. Thorac. Surg. 2009, 88, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Said, S.M.; Marey, G.; Hiremath, G. Intraoperative fluorescence with indocyanine green in congenital cardiac surgery: Potential applications of a novel technology. JTCVS Tech. 2021, 8, 144–155. [Google Scholar] [CrossRef]
- Calabro, K.A.; Harmon, C.M.; Vali, K. Fluorescent Cholangiography in Laparoscopic Cholecystectomy and the Use in Pediatric Patients. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 586–589. [Google Scholar] [CrossRef]
- Esposito, C.; Settimi, A.; Del Conte, F.; Cerulo, M.; Coppola, V.; Farina, A.; Crocetto, F.; Ricciardi, E.; Esposito, G.; Escolino, M. Image-Guided Pediatric Surgery Using Indocyanine Green (ICG) Fluorescence in Laparoscopic and Robotic Surgery. Front. Pediatr. 2020, 8, 314. [Google Scholar] [CrossRef]
- Calabro, K.A.; Harmon, C.M.; Vali, K. Indocyanine Green Use During Pediatric Laparoscopic Cholecystectomy. Videoscopy 2019, 29. [Google Scholar] [CrossRef]
- Preziosi, A.; Paraboschi, I.; Giuliani, S. Evaluating the Development Status of Fluorescence-Guided Surgery (FGS) in Pediatric Surgery Using the Idea, Development, Exploration, Assessment, and Long-Term Study (IDEAL) Framework. Children 2023, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Boogerd, L.S.F.; Handgraaf, H.J.M.; Huurman, V.A.L.; Lam, H.D.; Mieog, J.S.D.; van der Made, W.J.; van de Velde, C.J.H.; Vahrmeijer, A.L. The Best Approach for Laparoscopic Fluorescence Cholangiography: Overview of the Literature and Optimization of Dose and Dosing Time. Surg. Innov. 2017, 24, 386–396. [Google Scholar] [CrossRef]
- Hirayama, Y.; Iinuma, Y.; Yokoyama, N.; Otani, T.; Masui, D.; Komatsuzaki, N.; Higashidate, N.; Tsuruhisa, S.; Iida, H.; Nakaya, K.; et al. Near-infrared fluorescence cholangiography with indocyanine green for biliary atresia. Real-time imaging during the Kasai procedure: A pilot study. Pediatr. Surg. Int. 2015, 31, 1177–1182. [Google Scholar] [CrossRef]
- Yanagi, Y.; Yoshimaru, K.; Matsuura, T.; Shibui, Y.; Kohashi, K.; Takahashi, Y.; Obata, S.; Sozaki, R.; Izaki, T.; Taguchi, T. The outcome of real-time evaluation of biliary flow using near-infrared fluorescence cholangiography with Indocyanine green in biliary atresia surgery. J. Pediatr. Surg. 2019, 54, 2574–2578. [Google Scholar] [CrossRef]
- Paraboschi, I.; De Coppi, P.; Stoyanov, D.; Anderson, J.; Giuliani, S. Fluorescence imaging in pediatric surgery: State-of-the-art and future perspectives. J. Pediatr. Surg. 2021, 56, 655–662. [Google Scholar] [CrossRef]
- Lim, Y.Z.; Mutore, K.; Bradd, M.V.; Pandya, S.; Corbitt, N. A Pilot Study for Biliary Atresia Diagnosis: Fluorescent Imaging of Indocyanine Green in Stool. J. Pediatr. Surg. 2024, 59, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Miguel, C.; López-Santamaría, M.; Hernández Oliveros, F. The Role of Indocyanine Green Navigation in Choledochal Cyst Surgery. Indian J. Surg. 2023, 85, 1272–1274. [Google Scholar] [CrossRef]
- García-Hernández, C.; Cabrera-González, H.; Carvajal-Figueroa, L.; Archivaldo-García, C.; Valero-Mamani, R.J.; Martinez-Flores, R.A. Usefulness of indocyanine green fluorescence in laparoscopic resection of choledochal cyst in children. J. Pediatr. Surg. Case Rep. 2022, 76, 102129. [Google Scholar] [CrossRef]
- Yamamichi, T.; Oue, T.; Yonekura, T.; Owari, M.; Nakahata, K.; Umeda, S.; Nara, K.; Ueno, T.; Uehara, S.; Usui, N. Clinical application of indocyanine green (ICG) fluorescent imaging of hepatoblastoma. J. Pediatr. Surg. 2015, 50, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, R.S.; Patel, K.R.; Yang, T.; Nguyen, H.N.; Masand, P.; Vasudevan, S.A. Pathologic correlation with near infrared-indocyanine green guided surgery for pediatric liver cancer. J. Pediatr. Surg. 2022, 57, 700–710. [Google Scholar] [CrossRef]
- Cho, Y.J.; Namgoong, J.M.; Kwon, H.H.; Kwon, Y.J.; Kim, D.Y.; Kim, S.C. The Advantages of Indocyanine Green Fluorescence Imaging in Detecting and Treating Pediatric Hepatoblastoma: A Preliminary Experience. Front. Pediatr. 2021, 9, 635394. [Google Scholar] [CrossRef]
- Lake, C.M.; Bondoc, A.J.; Dasgupta, R.; Jenkins, T.M.; Towbin, A.J.; Smith, E.A.; Alonso, M.H.; Geller, J.I.; Tiao, G.M. Indocyanine green is a sensitive adjunct in the identification and surgical management of local and metastatic hepatoblastoma. Cancer Med. 2021, 10, 4322–4343. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, E. Fluorescence Image-Guided Navigation Surgery Using Indocyanine Green for Hepatoblastoma. Children 2021, 8, 1015. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Uchida, K.; Irie, T.; Hirukawa, K.; Kadohisa, M.; Shimata, K.; Isono, K.; Shimojima, N.; Sugawara, Y.; Hibi, T. Recent advances in surgical strategies and liver transplantation for hepatoblastoma. Cancer Med. 2023, 12, 3909–3918. [Google Scholar] [CrossRef]
- Onishi, S.; Kawano, T.; Nishida, N.; Kedoin, C.; Nagano, A.; Murakami, M.; Sugita, K.; Harumatsu, T.; Muto, M.; Ieiri, S. Case report: Minimal tissue damage and low coagulation liver resection for hepatoblastoma using indocyanine green fluorescence and water-jet dissector. Front. Pediatr. 2023, 11, 1221596. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, R. Advances in the conventional clinical treatment for hepatoblastoma and therapeutic innovation. World J. Pediatr. Surg. 2021, 4, e000220. [Google Scholar] [CrossRef]
- Qiu, R.; Wu, Y.; Su, J.; Chen, L.; Liao, M.; Zhao, Z.; Lu, Z.; Xiong, X.; Jin, S.; Deng, X. Deploying Indocyanine Green Fluorescence-Guided Navigation System in Precise Laparoscopic Resection of Pediatric Hepatoblastoma. Cancers 2022, 14, 6057. [Google Scholar] [CrossRef]
- Chen-Yoshikawa, T.F.; Hatano, E.; Yoshizawa, A.; Date, H. Clinical application of projection mapping technology for surgical resection of lung metastasis. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 1010–1011. [Google Scholar] [CrossRef]
- Kitagawa, N.; Shinkai, M.; Mochizuki, K.; Usui, H.; Miyagi, H.; Nakamura, K.; Tanaka, M.; Tanaka, Y.; Kusano, M.; Ohtsubo, S. Navigation using indocyanine green fluorescence imaging for hepatoblastoma pulmonary metastases surgery. Pediatr. Surg. Int. 2015, 31, 407–411. [Google Scholar] [CrossRef]
- Yamada, Y.; Ohno, M.; Fujino, A.; Kanamori, Y.; Irie, R.; Yoshioka, T.; Miyazaki, O.; Uchida, H.; Fukuda, A.; Sakamoto, S.; et al. Fluorescence-Guided Surgery for Hepatoblastoma with Indocyanine Green. Cancers 2019, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Qin, H.; Yang, W.; Cheng, H.; Xu, J.; Han, J.; Mou, J.; Wang, H.; Ni, X. Tumor-Background Ratio is an effective method to identify tumors and false-positive nodules in indocyanine-green navigation surgery for pediatric liver cancer. Front. Pediatr. 2022, 10, 875688. [Google Scholar] [CrossRef]
- Delgado-Miguel, C.; Muñoz-Serrano, A.; Moratilla, L.; Sarmiento, M.D.C.; Miguel-Ferrero, M.; Leal, N.; Barrena, S.; Martínez, L. Indocyanine Green (ICG)-Guided Identification of Hypermetabolic Pancreatic Nodules in Focal Congenital Hyperinsulinism: A Case Report in a 3-Month-Old Infant. Eur. J. Pediatr. Surg. Rep. 2022, 10, e9–e12. [Google Scholar] [CrossRef] [PubMed]
- Yamamichi, T.; Nishikawa, M.; Takayama, K.; Takase, K.; Kim, K.; Umeda, S.; Tayama, A.; Tsukada, R.; Nomura, M.; Okuyama, H.; et al. Computed tomography-guided marking using a dye-staining method for preoperative localization of tiny pulmonary lesions in children. Pediatr. Surg. Int. 2021, 37, 1265–1272. [Google Scholar] [CrossRef]
- Sosnowska-Sienkiewicz, P.; Kowalewski, G.; Garnier, H.; Wojtylko, A.; Murawski, M.; Szczygieł, M.; Al-Ameri, M.; Czauderna, P.; Godzinski, J.; Kalicinski, P.; et al. Practical Guidelines for the Use of Indocyanine Green in Different Branches of Pediatric Surgery: A Polish Nationwide Multi-Center Retrospective Cohort Study. Health Sci. Rep. 2025, 8, e70586. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.C.; Choudhury, S.; Pachl, M. Early results of minimally invasive fluorescent guided pediatric oncology surgery with delivery of indocyanine green during induction of anesthesia. Photodiagnosis Photodyn. Ther. 2023, 42, 103639. [Google Scholar] [CrossRef]
- Delgado-Miguel, C.; Estefanía, K.; San Basilio, M.; Hernández Oliveros, F. Indocyanine green navigation in minimally invasive resection of multiple metachronous pulmonary metastases of hepatoblastoma. Thorac. Cancer 2023, 14, 528–532. [Google Scholar] [CrossRef]
- Kodikara, H.; Wanaguru, D.; Karpelowsky, J.; Singh, H. Systemic indocyanine green localization with thoracoscopic thermal sealing and suture: A useful method for subpleural pulmonary osteosarcoma metastectomy. J. Surg. Oncol. 2022, 126, 383–385. [Google Scholar] [CrossRef]
- Komatsu, S.; Terui, K.; Nakata, M.; Shibata, R.; Oita, S.; Kawaguchi, Y.; Yoshizawa, H.; Hirokawa, T.; Nakatani, E.; Hishiki, T. Combined Use of Three-Dimensional Construction and Indocyanine Green-Fluorescent Imaging for Resection of Multiple Lung Metastases in Hepatoblastoma. Children 2022, 9, 376. [Google Scholar] [CrossRef]
- Abdelhafeez, A.H.; Mothi, S.S.; Pio, L.; Mori, M.; Santiago, T.C.; McCarville, M.B.; Kaste, S.C.; Pappo, A.S.; Talbot, L.J.; Murphy, A.J.; et al. Feasibility of indocyanine green-guided localization of pulmonary nodules in children with solid tumors. Pediatr. Blood Cancer 2023, 70, e30437. [Google Scholar] [CrossRef]
- Takahashi, N.; Yamada, Y.; Hoshino, K.; Kawaida, M.; Mori, T.; Abe, K.; Fujimura, T.; Matsubara, K.; Hibi, T.; Shinoda, M.; et al. Living Donor Liver Re-Transplantation for Recurrent Hepatoblastoma in the Liver Graft following Complete Eradication of Peritoneal Metastases under Indocyanine Green Fluorescence Imaging. Cancers 2019, 11, 730. [Google Scholar] [CrossRef]
- Takenouchi, A.; Terui, K.; Komatsu, S.; Oita, S.; Kawaguchi, Y.; Nishimura, K.; Hishiki, T. Sublobar resection for pediatric intralobar pulmonary sequestration using indocyanine green fluorescence. Pediatr. Int. 2025, 67, e15874. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Agarwal, T.; Yadav, T.; Rathod, K.; Pathak, M. Bronchopulmonary malformation with multiple feeding vessels: Rare presentation and novel use of indocyanine green. J. Pediatr. Endosc. Surg. 2022, 4, 123–126. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, K.; Tabata, Y.; Kedoin, C.; Nagano, A.; Harumatsu, T.; Tsuruno, Y.; Murakami, M.; Sugita, K.; Onishi, S.; et al. Thoracoscopic Lobectomy for Right Upper Bronchial Atresia Combined with an Azygos Lobe of the Right Lower Lobe in an Infant Patient: A Case Report of a Rare Condition. Asian J. Endosc. Surg. 2025, 18, e70019. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Sun, X.; Che, G.; Luo, D.; Yuan, M.; Yang, G.; Cheng, K.; Xu, C. Fluorescence imaging-assisted thoracoscopic anatomical lesion resection in treating congenital lung malformation. Sci. Rep. 2025, 15, 755. [Google Scholar] [CrossRef]
- Meisner, J.W.; Kamran, A.; Staffa, S.J.; Mohammed, S.; Yasuda, J.L.; Ngo, P.; Manfredi, M.; Zurakowski, D.; Jennings, R.W.; Hamilton, T.E.; et al. Qualitative features of esophageal fluorescence angiography and anastomotic outcomes in children. J. Pediatr. Surg. 2023, 58, 1359–1367. [Google Scholar] [CrossRef]
- Kamran, A.; Zendejas, B.; Meisner, J.; Choi, S.S.; Munoz-San Julian, C.; Ngo, P.; Manfredi, M.; Yasuda, J.L.; Smithers, C.J.; Hamilton, T.E.; et al. Effect of Posterior Tracheopexy on Risk of Recurrence in Children after Recurrent Tracheo-Esophageal Fistula Repair. J. Am. Coll. Surg. 2021, 232, 690–698. [Google Scholar] [CrossRef]
- Paraboschi, I.; Privitera, L.; Loukogeorgakis, S.; Giuliani, S. Fluorescence-Guided Surgery (FGS) during a Laparoscopic Redo Nissen Fundoplication: The First Case in Children. Children 2022, 9, 947. [Google Scholar] [CrossRef] [PubMed]
- Tsuruno, Y.; Harumatsu, T.; Tabata, Y.; Kedoin, C.; Murakami, M.; Sugita, K.; Yano, K.; Onishi, S.; Kawano, T.; Ieiri, S. Specific Findings of Blood Perfusion on Anastomosed Esophagus of Neonatal Esophageal Atresia and Tracheoesophageal Fistula Using Indocyanine Green Fluorescence During Thoracoscopic Surgery. Asian J. Endosc. Surg. 2025, 18, e13422. [Google Scholar] [CrossRef] [PubMed]
- Bokova, E.; Elhalaby, I.; Saylors, S.; Lim, I.I.P.; Rentea, R.M. Utilization of Indocyanine Green (ICG) Fluorescence in Patients with Pediatric Colorectal Diseases: The Current Applications and Reported Outcomes. Children 2024, 11, 665. [Google Scholar] [CrossRef]
- Yada, K.; Migita, M.; Nakamura, R.; Abe, S.; Matsufuji, H. Indocyanine green fluorescence during pediatric stoma closure. J. Pediatr. Surg. Case Rep. 2020, 61, 101595. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Yokota, K.; Uchida, H.; Hinoki, A.; Shirota, C.; Tainaka, T.; Sumida, W.; Makita, S.; Amano, H.; Takimoto, A.; et al. Laparoscopic restorative proctocolectomy with ileal-J-pouch anal canal anastomosis without diverting ileostomy for total colonic and extensive aganglionosis is safe and feasible with combined Lugol’s iodine staining technique and indocyanine green fluorescence angiography. Front. Pediatr. 2022, 10, 1090336. [Google Scholar] [CrossRef]
- Srinivas, S.; Ahmad, H.; Knaus, M.E.; Pruitt, L.C.C.; Jimenez, A.N.; Read, M.; Liaqat, N.; Langer, J.C.; Levitt, M.A.; Diefenbach, K.A.; et al. Laparoscopic-Assisted Colonic Derotation in Patients with Hirschsprung Disease. J. Pediatr. Surg. 2024, 59, 161600. [Google Scholar] [CrossRef]
- Muto, M.; Onishi, S.; Murakami, M.; Yano, K.; Harumatsu, T.; Ieiri, S. Transanal Mesenteric Resection in Hirschsprung’s Disease Using ICG under Concept of NOTES Technique. Eur. J. Pediatr. Surg. Rep. 2022, 10, e115–e117. [Google Scholar] [CrossRef]
- Rentea, R.M.; Halleran, D.R.; Ahmad, H.; Sanchez, A.V.; Gasior, A.C.; McCracken, K.; Hewitt, G.D.; Alexander, V.; Smith, C.; Weaver, L.; et al. Preliminary Use of Indocyanine Green Fluorescence Angiography and Value in Predicting the Vascular Supply of Tissues Needed to Perform Cloacal, Anorectal Malformation, and Hirschsprung Reconstructions. Eur. J. Pediatr. Surg. 2020, 30, 505–511. [Google Scholar] [CrossRef]
- Bada-Bosch, I.; Mata, D.P.; de la Torre, M.; Ordóñez, J.; Blanco, M.D.; de Agustin, J. Laparoscopic Partial Splenectomy Assisted by Fluorescence in a 13-Year-Old Girl. Eur. J. Pediatr. Surg. Rep. 2020, 8, e81–e85. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; De Luca, U.; Cerulo, M.; Del Conte, F.; Bagnara, V.; Coppola, S.; Corcione, F.; Lepore, B.; Settimi, A.; Escolino, M. Twenty-Five-Year Experience with Minimally Invasive Splenectomy in Children: From Minilaparotomy to Use of Sealing Devices and Indocyanine Green Fluorescence Technology: Tips and Tricks and Technical Considerations. J. Laparoendosc. Adv. Surg. Tech. A 2022, 32, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Masuya, R.; Nakame, K.; Tahira, K.; Kai, K.; Hamada, T.; Yano, K.; Imamura, N.; Hiyoshi, M.; Nanashima, A.; Ieiri, S. Laparoscopic dome resection for pediatric nonparasitic huge splenic cyst safely performed using indocyanine green fluorescence and percutaneous needle grasper. Asian J. Endosc. Surg. 2022, 15, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafeez, A.H.; Murphy, A.J.; Brennan, R.; Santiago, T.C.; Lu, Z.; Krasin, M.J.; Bissler, J.J.; Gleason, J.M.; Davidoff, A.M. Indocyanine green–guided nephron-sparing surgery for pediatric renal tumors. J. Pediatr. Surg. 2022, 57, 174–178. [Google Scholar] [CrossRef]
- Feng, J.; Yang, W.; Qin, H.; Xu, J.; Liu, S.; Han, J.; Li, N.; He, L.; Wang, H. Clinical application of indocyanine green fluorescence imaging navigation for pediatric renal cancer. Front. Pediatr. 2023, 11, 1108997. [Google Scholar] [CrossRef]
- Esposito, C.; Coppola, V.; Del Conte, F.; Cerulo, M.; Esposito, G.; Farina, A.; Crocetto, F.; Castagnetti, M.; Settimi, A.; Escolino, M. Near-Infrared fluorescence imaging using indocyanine green (ICG): Emerging applications in pediatric urology. J. Pediatr. Urol. 2020, 16, 700–707. [Google Scholar] [CrossRef]
- Esposito, C.; Soria-Gondek, A.; Castagnetti, M.; Cerulo, M.; Del Conte, F.; Esposito, G.; Pecoraro, C.; Cicala, D.; Farina, A.; Escolino, M. Laparoscopic or Robotic Deroofing Guided by Indocyanine Green Fluorescence and Perirenal Fat Tissue Wadding Technique of Pediatric Simple Renal Cysts. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 471–476. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, H.; Wu, Q.; Chen, Y.; Ye, Z.; Liu, R.; Chai, C. Indocyanine green fluorescence imaging-assisted laparoscopy resection of retroperitoneal tumors in children: Case report and literature review. Front. Pediatr. 2024, 12, 1374919. [Google Scholar] [CrossRef]
- Richard, C.; White, S.; Williams, R.; Zaghloul, T.; Helmig, S.; Sheyn, A.; Abramson, Z.; Abdelhafeez, H. Indocyanine green near infrared-guided surgery in children, adolescents, and young adults with otolaryngologic malignancies. Auris Nasus Larynx 2023, 50, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, B.; van Scheltinga, C.; Smeele, L.E.; Tolboom, N.; Wijnen, M.; van der Steeg, A.F.W. Sentinel Lymph Node Procedure in Pediatric Patients with Melanoma, Squamous Cell Carcinoma, or Sarcoma Using Near-Infrared Fluorescence Imaging with Indocyanine Green: A Feasibility Trial. Ann. Surg. Oncol. 2023, 30, 2391–2398. [Google Scholar] [CrossRef]
- Campwala, I.; Vignali, P.D.A.; Seynnaeve, B.K.; Davit, A.J., 3rd; Weiss, K.; Malek, M.M. Utility of Indocyanine Green for Sentinel Lymph Node Biopsy in Pediatric Sarcoma and Melanoma. J. Pediatr. Surg. 2024, 59, 1326–1333. [Google Scholar] [CrossRef]
- Pio, L.; Richard, C.; Zaghloul, T.; Murphy, A.J.; Davidoff, A.M.; Abdelhafeez, A.H. Sentinel lymph node mapping with Indocyanine green fluorescence (ICG) for pediatric and adolescent tumors: A prospective observational study. Sci. Rep. 2024, 14, 30135. [Google Scholar] [CrossRef]
- Johnston, M.E., 2nd; Farooqui, Z.A.; Nagarajan, R.; Pressey, J.G.; Turpin, B.; Dasgupta, R. Fluorescent-guided surgery and the use of indocyanine green sentinel lymph node mapping in the pediatric and young adult oncology population. Cancer 2023, 129, 3962–3970. [Google Scholar] [CrossRef]
- Pachl, M.J. Fluorescent Guided Lymph Node Harvest in Laparoscopic Wilms Nephroureterectomy. Urology 2021, 158, 189–192. [Google Scholar] [CrossRef]
- Mansfield, S.A.; Murphy, A.J.; Talbot, L.; Prajapati, H.; Maller, V.; Pappo, A.; Singhal, S.; Krasin, M.J.; Davidoff, A.M.; Abdelhafeez, A. Alternative approaches to retroperitoneal lymph node dissection for paratesticular rhabdomyosarcoma. J. Pediatr. Surg. 2020, 55, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, H.; Kato, T.; Hu, H.P.; Patel, P.; Wada, H.; Fujino, K.; Weersink, R.; Nguyen, E.; Cypel, M.; Pierre, A.; et al. A novel minimally invasive near-infrared thoracoscopic localization technique of small pulmonary nodules: A phase I feasibility trial. J. Thorac. Cardiovasc. Surg. 2017, 154, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Kodikara, H.; Laing, A. Direct Parenchymal Injection of Indocyanine Green for Identification and Resection of an Osteosarcoma Pulmonary Metastasis. Pediatr. Blood Cancer 2025, 72, e31447. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Lepore, B.; Cerulo, M.; Del Conte, F.; Coppola, V.; Esposito, G.; Carulli, R.; Carraturo, F.; Escolino, M. Applications of indocyanine green (ICG) fluorescence technology in open surgery: Preliminary experience in pediatric surgery. Front. Surg. 2023, 10, 1238487. [Google Scholar] [CrossRef]
- Mihara, M.; Hara, H.; Shibasaki, J.; Seki, Y.; Hayashi, A.; Iida, T.; Adachi, S.; Uchida, Y.; Kaneko, H.; Haragi, M.; et al. Indocyanine green lymphography and lymphaticovenous anastomosis for generalized lymphatic dysplasia with pleural effusion and ascites in neonates. Ann. Vasc. Surg. 2015, 29, 1111–1122. [Google Scholar] [CrossRef]
- Yokoyama, S.; Nakaoka, T. Successful use of intraoperative ICG fluorescence lymphography and fibrin sealant with PGA felt for refractory chylous ascites in an infant: A novel procedure. Pediatr. Int. 2020, 62, 862–863. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.I.; Chen, Y.S.; Huang, S.C. Intraoperative indocyanine green fluorescence lymphography to detect chylous leakage sites after congenital heart surgery. J. Thorac. Cardiovasc. Surg. 2014, 148, 739–740. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Shirota, C.; Uchida, H.; Amano, H.; Hinoki, A.; Sumida, W.; Makita, S.; Okamoto, M.; Takimoto, A.; Yasui, A.; et al. Management of Congenital and Postoperative Chylothorax: Use of Thoracoscopic Lymphatic Leak Ligations with Intraoperative ICG Lymphangiography. J. Pediatr. Surg. 2023, 58, 1754–1761. [Google Scholar] [CrossRef]
- Tan, I.C.; Balaguru, D.; Rasmussen, J.C.; Guilliod, R.; Bricker, J.T.; Douglas, W.I.; Sevick-Muraca, E.M. Investigational lymphatic imaging at the bedside in a pediatric postoperative chylothorax patient. Pediatr. Cardiol. 2014, 35, 1295–1300. [Google Scholar] [CrossRef]
- Ishizu, K.; Araki, K.; Kagisaki, K.; Ozawa, H. The Usefulness of Intraoperative Indocyanine Green Fluorescence Imaging in Surgical Treatment of Refractory Chylothorax in Pediatric Patients: A Case Report. Surg. Case Rep. 2025, 11, cr.24–0112. [Google Scholar] [CrossRef]
- Shirotsuki, R.; Uchida, H.; Tanaka, Y.; Shirota, C.; Yokota, K.; Murase, N.; Hinoki, A.; Oshima, K.; Chiba, K.; Sumida, W.; et al. Novel thoracoscopic navigation surgery for neonatal chylothorax using indocyanine-green fluorescent lymphography. J. Pediatr. Surg. 2018, 53, 1246–1249. [Google Scholar] [CrossRef]
- Vecchiato, M.; Martino, A.; Sponza, M.; Uzzau, A.; Ziccarelli, A.; Marchesi, F.; Petri, R. Thoracic duct identification with indocyanine green fluorescence during minimally invasive esophagectomy with patient in prone position. Dis. Esophagus 2020, 33, doaa030. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Liu, T.T. Lymphedema microsurgery improved outcomes of pediatric primary extremity lymphedema. Microsurgery 2020, 40, 766–775. [Google Scholar] [CrossRef]
- Greives, M.R.; Aldrich, M.B.; Sevick-Muraca, E.M.; Rasmussen, J.C. Near-Infrared Fluorescence Lymphatic Imaging of a Toddler with Congenital Lymphedema. Pediatrics 2017, 139, e20154456. [Google Scholar] [CrossRef]
- Ogata, F.; Narushima, M.; Mihara, M.; Azuma, R.; Morimoto, Y.; Koshima, I. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann. Plast. Surg. 2007, 59, 180–184. [Google Scholar] [CrossRef]
- Drobot, A.; Ganam, S.; Karra, N.; Bickel, A.; Abu Shakra, I.; Kakiashvili, E. Resection of an axillary macrocystic lymphatic malformation in a 14-year-old girl using intraoperative indocyanine green lymphography. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 504–507. [Google Scholar] [CrossRef]
- Kato, M.; Watanabe, S.; Iida, T.; Watanabe, A. Flow Pattern Classification in Lymphatic Malformations by Indocyanine Green Lymphography. Plast. Reconstr. Surg. 2019, 143, 558e–564e. [Google Scholar] [CrossRef]
- Shirota, C.; Hinoki, A.; Takahashi, M.; Tanaka, Y.; Tainaka, T.; Sumida, W.; Murase, N.; Oshima, K.; Shirotsuki, R.; Chiba, K.; et al. New Navigation Surgery for Resection of Lymphatic Malformations Using Indocyanine Green Fluorescence Imaging. Am. J. Case Rep. 2017, 18, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Watanabe, S.; Iida, T.; Watanabe, A.; Megumi, F. Peri-orbital lymphangioma treated by lymphatic-venous anastomosis with indocyanine green lymphography analysis. J. Pediatr. Surg. Case Rep. 2017, 23, 9–14. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Zhang, Y.; Liao, J.; Hua, K.; Gu, Y.; Wang, D.; Tian, J.; Huang, J. Indocyanine green localization for laparoscopic duodenal web excision. Photodiagnosis Photodyn. Ther. 2022, 38, 102842. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhang, G.; Li, W.; Zhuansun, D.; Xiong, X.; Li, Y.; He, Y.; Wang, W.; Zhu, T.; Feng, J. Atomized inhalation of indocyanine green in thoracoscopic surgery for intralobar pulmonary sequestration: A multicenter study. Respir. Res. 2024, 25, 403. [Google Scholar] [CrossRef]
- Zhu, T.; Li, Y.; Zhang, J.; Xiong, X.; Yin, Y.; Zhuansun, D.; He, Y.; Feng, J. Precise Thoracoscopic Pneumonectomy Using Fluorescence Imaging After Aerosolized Indocyanine Green Inhalation: A Novel Strategy for Treating Congenital Pulmonary Airway Malformation. J. Pediatr. Surg. 2025, 60, 162063. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Del Conte, F.; Cerulo, M.; Gargiulo, F.; Izzo, S.; Esposito, G.; Spagnuolo, M.I.; Escolino, M. Clinical application and technical standardization of indocyanine green (ICG) fluorescence imaging in pediatric minimally invasive surgery. Pediatr. Surg. Int. 2019, 35, 1043–1050. [Google Scholar] [CrossRef]
- Esposito, C.; Corcione, F.; Settimi, A.; Farina, A.; Centonze, A.; Esposito, G.; Spagnuolo, M.I.; Escolino, M. Twenty-Five Year Experience with Laparoscopic Cholecystectomy in the Pediatric Population-From 10 mm Clips to Indocyanine Green Fluorescence Technology: Long-Term Results and Technical Considerations. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 1185–1191. [Google Scholar] [CrossRef]
- Esposito, C.; Settimi, A.; Cerulo, M.; Escolino, M. Efficacy of indocyanine green (ICG) fluorescent cholangiography to improve intra-operative visualization during laparoscopic cholecystectomy in pediatric patients: A comparative study between ICG-guided fluorescence and standard technique. Surg. Endosc. 2022, 36, 4369–4375. [Google Scholar] [CrossRef]
- Ishizawa, T.; Bandai, Y.; Kokudo, N. Fluorescent cholangiography using indocyanine green for laparoscopic cholecystectomy: An initial experience. Arch. Surg. 2009, 144, 381–382. [Google Scholar] [CrossRef]
- Reeves, J.J.; Broderick, R.C.; Lee, A.M.; Blitzer, R.R.; Waterman, R.S.; Cheverie, J.N.; Jacobsen, G.R.; Sandler, B.J.; Bouvet, M.; Doucet, J.; et al. The price is right: Routine fluorescent cholangiography during laparoscopic cholecystectomy. Surgery 2022, 171, 1168–1176. [Google Scholar] [CrossRef]
- Osayi, S.N.; Wendling, M.R.; Drosdeck, J.M.; Chaudhry, U.I.; Perry, K.A.; Noria, S.F.; Mikami, D.J.; Needleman, B.J.; Muscarella, P., 2nd; Abdel-Rasoul, M.; et al. Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg. Endosc. 2015, 29, 368–375. [Google Scholar] [CrossRef]
- Cazares, J.; Koga, H.; Yamataka, A. Advances in the surgical technique of Kasai portoenterostomy. Semin. Pediatr. Surg. 2024, 33, 151481. [Google Scholar] [CrossRef] [PubMed]
- Shirota, C.; Hinoki, A.; Togawa, T.; Ito, S.; Sumida, W.; Makita, S.; Amano, H.; Takimoto, A.; Takada, S.; Okamoto, M.; et al. Intraoperative indocyanine green fluorescence cholangiography can rule out biliary atresia: A preliminary report. Front. Pediatr. 2022, 10, 1005879. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Okada, A.; Fukui, Y.; Kawahara, H.; Imura, K.; Kamata, S. Indocyanine green test is a reliable indicator of postoperative liver function in biliary atresia. J. Pediatr. Gastroenterol. Nutr. 1993, 16, 61–65. [Google Scholar] [CrossRef]
- Onishi, S.; Yamada, K.; Murakami, M.; Kedoin, C.; Muto, M.; Ieiri, S. Co-injection of Bile and Indocyanine Green for Detecting Pancreaticobiliary Maljunction of Choledochal Cyst. Eur. J. Pediatr. Surg. Rep. 2022, 10, e127–e130. [Google Scholar] [CrossRef] [PubMed]
- Pio, L.; Wijnen, M.; Giuliani, S.; Sarnacki, S.; Davidoff, A.M.; Abdelhafeez, A.H. Identification of Pediatric Tumors Intraoperatively Using Indocyanine Green (ICG). Ann. Surg. Oncol. 2023, 30, 7789–7798. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.H.Y.; Chok, K.S.H.; Wong, K.K.Y. Indocyanine green fluorescence-assisted laparoscopic hepatectomy for hepatocellular carcinoma in a pre-adolescent girl: A case report. Hong Kong Med. J. 2020, 26, 342–344. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, X.; Pan, S.; Li, T.; Zhou, J. Effectiveness of indocyanine green fluorescence imaging in resection of hepatoblastoma. Pediatr. Surg. Int. 2023, 39, 181. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Shimizu, T.; Mori, M.; Okamatsu, C.; Nakamura, N.; Watanabe, T. Rim-type indocyanine green fluorescence pattern in a child with undifferentiated embryonal sarcoma of the liver treated with navigation surgery. Pediatr. Blood Cancer 2022, 69, e29799. [Google Scholar] [CrossRef] [PubMed]
- Lake, C.M.; Tiao, G.M.; Bondoc, A.J. Surgical management of locally-advanced and metastatic hepatoblastoma. Semin. Pediatr. Surg. 2019, 28, 150856. [Google Scholar] [CrossRef]
- Mitani, Y.; Kubota, A.; Ueno, M.; Takifuji, K.; Watanabe, T.; Hayami, S.; Kounami, S.; Tsujimoto, H.; Yamaue, H. Real-time identification of hepatoblastoma using a near infrared imaging with indocyanine green. J. Pediatr. Surg. Case Rep. 2014, 2, 180–183. [Google Scholar] [CrossRef]
- Kokudo, N.; Ishizawa, T. Clinical application of fluorescence imaging of liver cancer using indocyanine green. Liver Cancer 2012, 1, 15–21. [Google Scholar] [CrossRef]
- Hanaki, T.; Tokuyasu, N.; Sakamoto, T.; Fujiwara, Y. Hepatectomy guided by indocyanine green fluorescent imaging for visualizing bile leakage (with video). Clin. Case Rep. 2022, 10, e05942. [Google Scholar] [CrossRef]
- Hanaki, T.; Goto, K.; Morimoto, M.; Murakami, Y.; Matsunaga, T.; Yamamoto, M.; Tokuyasu, N.; Sakamoto, T.; Hasegawa, T.; Fujiwara, Y. Surgical Administration of Indocyanine Green in Hepatectomy for Improved Bile Leakage Detection. Anticancer. Res. 2022, 42, 4787–4793. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, C.P.; Lautz, T.B.; Superina, R. Indocyanine green fluorescence imaging as an adjunct for the localization of a bile leak after split liver transplantation. Pediatr. Transplant. 2023, 27, e14431. [Google Scholar] [CrossRef] [PubMed]
- Kisaoglu, A.; Demiryilmaz, I.; Dandin, O.; Ozkan, O.; Aydinli, B. Management of reperfusion deficiency with indocyanine green fluorescence imaging during deceased donor liver transplantation in a pediatric recipient. HPB 2020, 22, 633. [Google Scholar] [CrossRef]
- Chittmittrapap, S.; Spitz, L.; Kiely, E.M.; Brereton, R.J. Anastomotic leakage following surgery for esophageal atresia. J. Pediatr. Surg. 1992, 27, 29–32. [Google Scholar] [CrossRef]
- Delgado-Miguel, C.; Camps, J.; Hernandez Oliveros, F. The Role of Indocyanine Green in Pediatric Gastrointestinal Surgery: Systematic Review. Eur. J. Pediatr. Surg. 2024, 34, 2–8. [Google Scholar] [CrossRef]
- Sugita, K.; Onishi, S.; Kedoin, C.; Matsui, M.; Murakami, M.; Yano, K.; Harumatsu, T.; Yamada, K.; Yamada, W.; Matsukubo, M.; et al. A safe and effective laparoscopic Ladd’s procedure technique involving the confirmation of mesenteric vascular perfusion by fluorescence imaging using indocyanine green: A case report of an infant. Asian J. Endosc. Surg. 2022, 15, 410–414. [Google Scholar] [CrossRef]
- Paraboschi, I.; Privitera, L.; Loukogeorgakis, S.; Giuliani, S. Indocyanine Green-Based Fluorescence-Guided Surgery in a Male Infant with Anorectal Malformation. Eur. J. Pediatr. Surg. Rep. 2022, 10, e122–e125. [Google Scholar] [CrossRef]
- Dall’Igna, P.; De Corti, F.; Alaggio, R.; Cecchetto, G. Sentinel node biopsy in pediatric patients: The experience in a single institution. Eur. J. Pediatr. Surg. 2014, 24, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Pachl, M. Intraparenchymal Indocyanine Green Use Improves Nodal Yield During Minimally Invasive Tumor Nephrectomy in Children. J. Laparoendosc. Adv. Surg. Tech. A 2024, 34, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Pio, L.; Zaghloul, T.; Abdelhafeez, A.H. Indocyanine green fluorescence-guided lymphadenectomy with single site retroperitoneoscopy in children. J. Pediatr. Urol. 2023, 19, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.N.; Trout, A.T.; Shenoy, A.; Abu-El-Haija, M.; Nathan, J.D. Solid pancreatic masses in children: A review of current evidence and clinical challenges. Front. Pediatr. 2022, 10, 966943. [Google Scholar] [CrossRef]
- Kodikara, H.; Dodgshun, A. Intraoperative Indocyanine Green to Localize a Microscopic Focus of Paraganglioma. Pediatr. Blood Cancer 2025, 72, e31513. [Google Scholar] [CrossRef]
- Fung, C.H.; Lau, C.T.; Wong, K.K.Y. Indocyanine green fluorescence-guided pulmonary wedge resection in a child: A case report. Hong Kong Med. J. 2020, 26, 345–347. [Google Scholar] [CrossRef]
- Rothenberg, S.S.; Shipman, K.; Kay, S.; Kadenhe-Chiweshe, A.; Thirumoorthi, A.; Garcia, A.; Czauderna, P.; Kravarusic, D.; Freud, E. Thoracoscopic segmentectomy for congenital and acquired pulmonary disease: A case for lung-sparing surgery. J. Laparoendosc. Adv. Surg. Tech. A 2014, 24, 50–54. [Google Scholar] [CrossRef]
- Esposito, C.; Turrà, F.; Del Conte, F.; Izzo, S.; Gargiulo, F.; Farina, A.; Severino, G.; Cerulo, M.; Escolino, M. Indocyanine Green Fluorescence Lymphography: A New Technique to Perform Lymphatic Sparing Laparoscopic Palomo Varicocelectomy in Children. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 564–567. [Google Scholar] [CrossRef]
- Tomita, K.; Kageyama, S.; Hanada, E.; Yoshida, T.; Okinaka, Y.; Kubota, S.; Nagasawa, M.; Johnin, K.; Narita, M.; Kawauchi, A. Indocyanine Green Angiography-assisted Laparoendoscopic Single-site Varicocelectomy. Urology 2017, 106, 221–225. [Google Scholar] [CrossRef]
- Azizoglu, M.; Yuksel, S.; Kayrancioglu, B.; Karakas, E.; Karaaslan, B.; Sarac, F. Indocyanine Green (ICG) Assisted Laparoscopic Palomo Varicocelectomy. J. Pediatr. Surg. 2025, 60, 162134. [Google Scholar] [CrossRef]
- Esposito, C.; Borgogni, R.; Chiodi, A.; Cerulo, M.; Autorino, G.; Esposito, G.; Coppola, V.; Del Conte, F.; Di Mento, C.; Escolino, M. Indocyanine green (ICG)-GUIDED lymphatic sparing laparoscopic varicocelectomy in children and adolescents. Is intratesticular injection of the dye safe? A mid-term follow-up study. J. Pediatr. Urol. 2024, 20, 282.e1–282.e6. [Google Scholar] [CrossRef]
- Monje Fuente, S.; Fernandez Bautista, B.; Blanco Verdu, M.D.; Bada Bosch, I.; Ortiz Rodriguez, R.; Burgos Lucena, L.; De Agustin, J.C.; Angulo Madero, J.M. Usefulness of indocyanine green in the laparoscopic Palomo technique: A comparative study. Cir. Pediatr. 2024, 37, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, Y.; Kawashima, M.; Kimura, T.; Yamanaka, H.; Hatta, K.; Branch, J.; Matsuda, Y. Successful salvage of torsion testis by means of intraoperative indocyanine green fluorescence imaging. Surg. Case Rep. 2022, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Terui, K.; Takenouchi, A.; Kawaguchi, Y.; Nishimura, K.; Oita, S.; Yoshizawa, H.; Takiguchi, S.; Hishiki, T. Indocyanine green fluorescence imaging as a predictor of long-term testicular atrophy in testicular torsion: A pilot study. Surg. Today 2025, 55, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, Y.; Yoshimura, S.; Matsufuji, H.; Umeyama, T.; Machigashira, S.; Yada, K. Criteria for Preserving Grossly Ischemic Torsed Testicles Using Indocyanine Green Fluorescence Imaging: A Single-Center Case Series. Urology 2023, 178, 133–137. [Google Scholar] [CrossRef]
- Doery, A.J.; Ang, E.; Ditchfield, M.R. Duplex kidney: Not just a drooping lily. J. Med. Imaging Radiat. Oncol. 2015, 59, 149–153. [Google Scholar] [CrossRef]
- Herz, D.; DaJusta, D.; Ching, C.; McLeod, D. Segmental arterial mapping during pediatric robot-assisted laparoscopic heminephrectomy: A descriptive series. J. Pediatr. Urol. 2016, 12, 266.e1–266.e6. [Google Scholar] [CrossRef]
- Esposito, C.; Borgogni, R.; Autorino, G.; Cerulo, M.; Carulli, R.; Esposito, G.; Del Conte, F.; Escolino, M. Applications of Indocyanine Green-Guided Near-Infrared Fluorescence Imaging in Pediatric Minimally Invasive Surgery Urology: A Narrative Review. J. Laparoendosc. Adv. Surg. Tech. A 2022, 32, 1280–1287. [Google Scholar] [CrossRef]
- Paraboschi, I.; Gnech, M.; Minoli, D.G.; De Marco, E.A.; Parente, G.; Mantica, G.; Manzoni, G.; Berrettini, A. Indocyanine Green (ICG)-Guided Onlay Preputial Island Flap Urethroplasty for the Single-Stage Repair of Hypospadias in Children: A Case Report. Int. J. Environ. Res. Public. Health 2023, 20, 6246. [Google Scholar] [CrossRef]
- Raines, A.; Fernandez, N.; Ahn, J.; Cain, M.; Joyner, B.; Kieran, K.; Merguerian, P.; Shnorhavorian, M. Preputial pedicle flap ICG blood flow assessment during proximal hypospadias repair: Development of a standardized protocol. J. Pediatr. Urol. 2024, 20, 691.e1–691.e7. [Google Scholar] [CrossRef] [PubMed]
- Paraboschi, I.; Gnech, M.; Minoli, D.G.; De Marco, E.A.; Parente, G.; Mantica, G.; Bagnara, V.; Manzoni, G.; Leclair, M.D.; Berrettini, A. Indocyanine Green (ICG)-Guided One-Stage Delayed Bladder Closure and Radical Soft-Tissue Mobilization (Kelly Procedure) For Bladder Exstrophy Repair: The First Experience. Res. Rep. Urol. 2023, 15, 375–380. [Google Scholar] [CrossRef]
- Kaefer, M.; Saad, K.; Gargollo, P.; Whittam, B.; Rink, R.; Fuchs, M.; Bowen, D.; Reddy, P.; Cheng, E.; Jayanthi, R.; et al. Intraoperative laser angiography in bladder exstrophy closure: A simple technique to monitor penile perfusion. J. Pediatr. Urol. 2022, 18, 746 e1–746 e7. [Google Scholar] [CrossRef] [PubMed]
- Kodikara, H. Intraureteral Instillation of Indocyanine Green for Intraoperative Identification of the Ureter During Laparoscopy. J. Pediatr. Surg. 2025, 60, 162100. [Google Scholar] [CrossRef] [PubMed]
- Locke, R.A.; Kwenda, E.P.; Archer, J.; Bergamo, J.; Domino, M.P.; DeMarco, R.T.; Bayne, C.E. Pediatric Robot-Assisted Laparoscopic and Ureteroscopic Ureterolithotomy and Ureteroplasty. J. Endourol. Case Rep. 2020, 6, 264–267. [Google Scholar] [CrossRef]
- De Pauw, V.; Daelemans, S.; Depoorter, L.; Brussaard, C.; Smets, D. Refractory chylothorax after severe vomiting and coughing in a 4-year-old child. J. Surg. Case Rep. 2023, 2023, rjad466. [Google Scholar] [CrossRef]
- Chang, E.I.; Skoracki, R.J.; Chang, D.W. Lymphovenous Anastomosis Bypass Surgery. Semin. Plast. Surg. 2018, 32, 22–27. [Google Scholar] [CrossRef]
- Esposito, C.; Di Mento, C.; Chiodi, A.; Cerulo, M.; Coppola, V.; Del Conte, F.; Carraturo, F.; Esposito, G.; Escolino, M. Indocyanine Green (ICG) Fluorescence-Assisted Open Surgery Using the Rubina(®) Lens System in the Pediatric Population: A Single-Center Prospective Case Series. Children 2023, 11, 54. [Google Scholar] [CrossRef]
- Kaneshi, Y.; Shibasaki, J.; Aida, N.; Shimokaze, T.; Toyoshima, K. Indocyanine green lymphography for congenital lymphatic dysplasia with tuberous sclerosis complex: A case report. Pediatr. Int. 2020, 62, 234–236. [Google Scholar] [CrossRef]
- Ciro, E.; Vincenzo, C.; Mariapina, C.; Fulvia, D.C.; Vincenzo, B.; Giorgia, E.; Roberto, C.; Lepore, B.; Castagnetti, M.; Califano, G.; et al. Review of a 25-Year Experience in the Management of Ovarian Masses in Neonates, Children and Adolescents: From Laparoscopy to Robotics and Indocyanine Green Fluorescence Technology. Children 2022, 9, 1219. [Google Scholar] [CrossRef]
- Mungadi, I.A.; Ahmad, Y.; Yunusa, G.H.; Agwu, N.P.; Ismail, S. Mayer-rokitansky-kuster-hauser syndrome: Surgical management of two cases. J. Surg. Tech. Case Rep. 2010, 2, 39–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herlin, M.K.; Petersen, M.B.; Brännström, M. Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome: A comprehensive update. Orphanet J. Rare Dis. 2020, 15, 214. [Google Scholar] [CrossRef]
- Saxena, R.; Agarwal, T.; Pathak, M.; Sinha, A. Novel use of indocyanine green fluorescence in total laparoscopic sigmoid colon vaginoplasty. J. Pediatr. Endosc. Surg. 2022, 4, 181–184. [Google Scholar] [CrossRef]
- Sicard, R.; Oleru, O.; Doan, J.; Seyidova, N.; Taub, P.J. Efficacy and dosing of indocyanine green in pediatric plastic and reconstructive surgery. Microsurgery 2024, 44, e31188. [Google Scholar] [CrossRef]
- Tomioka, Y.K.; Narushima, M.; Yamashita, S.; Ito, A.; Okazaki, M. Foot Web Space Transfer for Congenital Syndactyly. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3292. [Google Scholar] [CrossRef] [PubMed]
- Fried, F.W.; Beier, J.P.; Bohr, C.; Iro, H.; Horch, R.E.; Arkudas, A. Free Latissimus Dorsi Myocutaneous Flap in a 6-Month-Old Child for Reconstruction of a Temporal Fossa Defect After Teratoma Resection. Ann. Plast. Surg. 2019, 82, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Jiang, W.; Jiang, S.; Huang, S.; Shen, W. Monitoring lymphatic reconstitution in free latissimus dorsi flap for lower extremity defects repair in pediatric patients: A case series. Transl. Pediatr. 2024, 13, 2034–2042. [Google Scholar] [CrossRef]
- Keren, S.; Dotan, G.; Leibovitch, L.; Selva, D.; Leibovitch, I. Indocyanine green assisted removal of orbital lacrimal duct cysts in children. J. Ophthalmol. 2015, 2015, 130215. [Google Scholar] [CrossRef]
- Jacobs, D.S.; Cox, T.A.; Wagoner, M.D.; Ariyasu, R.G.; Karp, C.L. Capsule staining as an adjunct to cataract surgery: A report from the American Academy of Ophthalmology. Ophthalmology 2006, 113, 707–713. [Google Scholar] [CrossRef]
- Pandey, S.K.; Werner, L.; Escobar-Gomez, M.; Roig-Melo, E.A.; Apple, D.J. Dye-enhanced cataract surgery: Part 1: Anterior capsule staining for capsulorhexis in advanced/white cataract11None of the authors has a financial or proprietary interest in any material or method mentioned. J. Cataract. Refract. Surg. 2000, 26, 1052–1059. [Google Scholar] [CrossRef]
- Kuroda, S.; Houkin, K. Moyamoya disease: Current concepts and future perspectives. Lancet Neurol. 2008, 7, 1056–1066. [Google Scholar] [CrossRef]
- Mishra, S.; Katiyar, V.; Narwal, P.; Sharma, R. Superficial Temporal Artery to Middle Cerebral Artery Bypass for Moyamoya Disease: Surgical Nuances. World Neurosurg. 2022, 161, 54. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Kimiwada, T.; Tominaga, K.; Endo, H. Intraoperative Superficial Temporal Artery-middle Cerebral Artery Bypass Failure during Combined Bypass Surgery in Children with Moyamoya Disease. Neurol. Med. Chir. 2025, 65, 133–140. [Google Scholar] [CrossRef]
- Uchino, H.; Kazumata, K.; Ito, M.; Nakayama, N.; Kuroda, S.; Houkin, K. Intraoperative assessment of cortical perfusion by indocyanine green videoangiography in surgical revascularization for moyamoya disease. Acta. Neurochir. 2014, 156, 1753–1760. [Google Scholar] [CrossRef]
- Horie, N.; Fukuda, Y.; Izumo, T.; Hayashi, K.; Suyama, K.; Nagata, I. Indocyanine green videoangiography for assessment of postoperative hyperperfusion in moyamoya disease. Acta. Neurochir. 2014, 156, 919–926. [Google Scholar] [CrossRef]
- Nagamitsu, S.; Kaneko, N.; Nagatsuna, T.; Yasuda, H.; Urakawa, M.; Fujii, M.; Yamashita, T. Idiopathic dissecting cerebral aneurysm of the distal anterior cerebral artery in an infant successfully treated with aneurysmectomy: Illustrative case. J. Neurosurg. Case Lessons 2021, 1, case20142. [Google Scholar] [CrossRef] [PubMed]
- Wali, A.R.; Kang, K.M.; Rennert, R.; Santiago-Dieppa, D.; Khalessi, A.A.; Levy, M. First-in-Human Clinical Experience Using High-Definition Exoscope with Intraoperative Indocyanine Green for Clip Reconstruction of Unruptured Large Pediatric Aneurysm. World Neurosurg. 2021, 151, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, S.; Qian, H.; Shi, X. Internal Maxillary Bypass for Complex Pediatric Aneurysms. World Neurosurg. 2017, 103, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Park, Y.S.; Nakagawa, I.; Nishimura, F.; Motoyama, Y.; Nakase, H. Effectiveness of intraoperative indocyanine green videoangiography in direct surgical treatment of pediatric intracranial pial arteriovenous fistula. J. Neurosurg. Pediatr. 2015, 15, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Buiza, M.J.; Rivero-Garvia, M.; Márquez-Rivas, J. Microvascular Fluorescence in Spinal Cord Repair. World Neurosurg. 2019, 125, 245–246. [Google Scholar] [CrossRef] [PubMed]


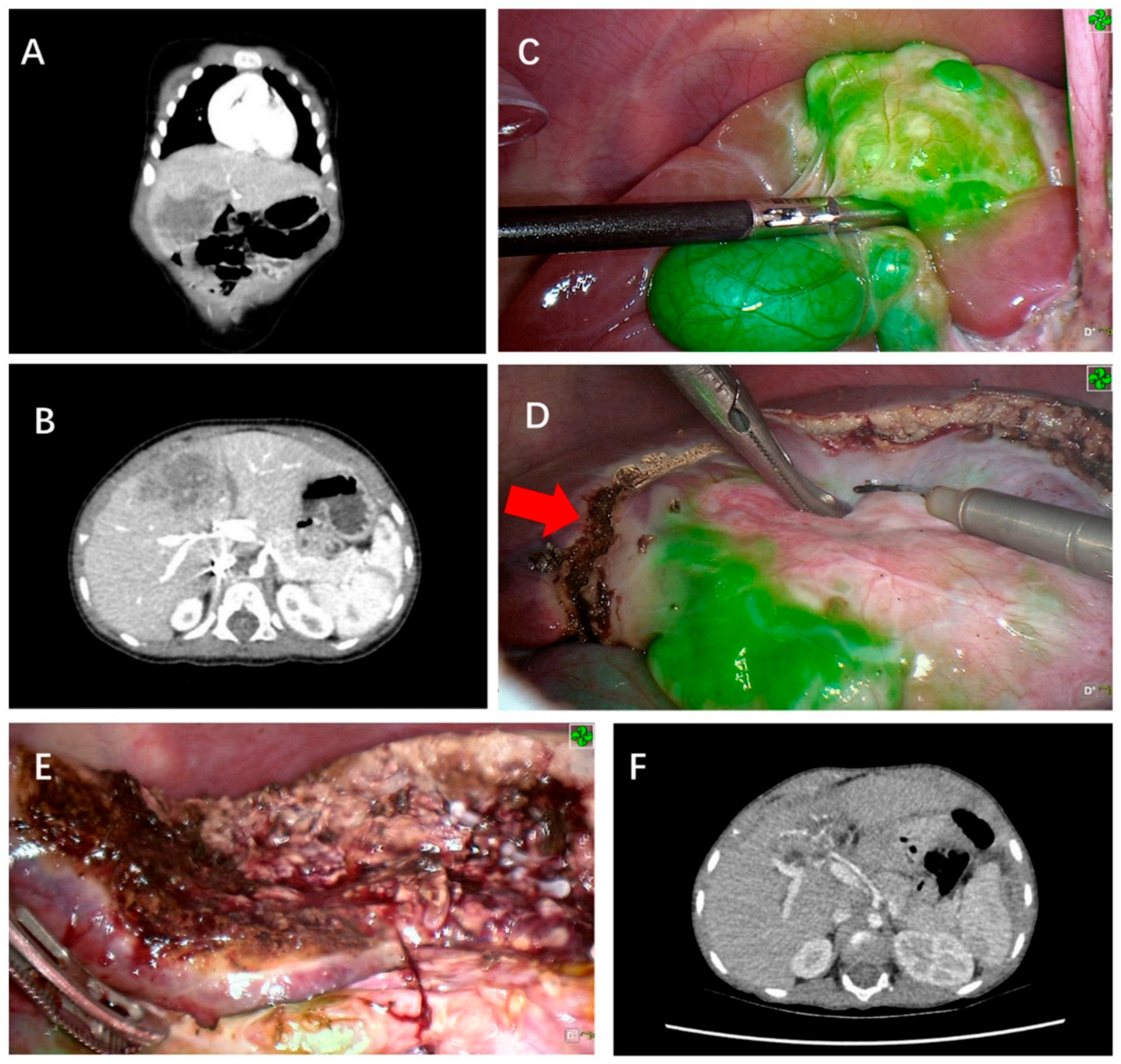
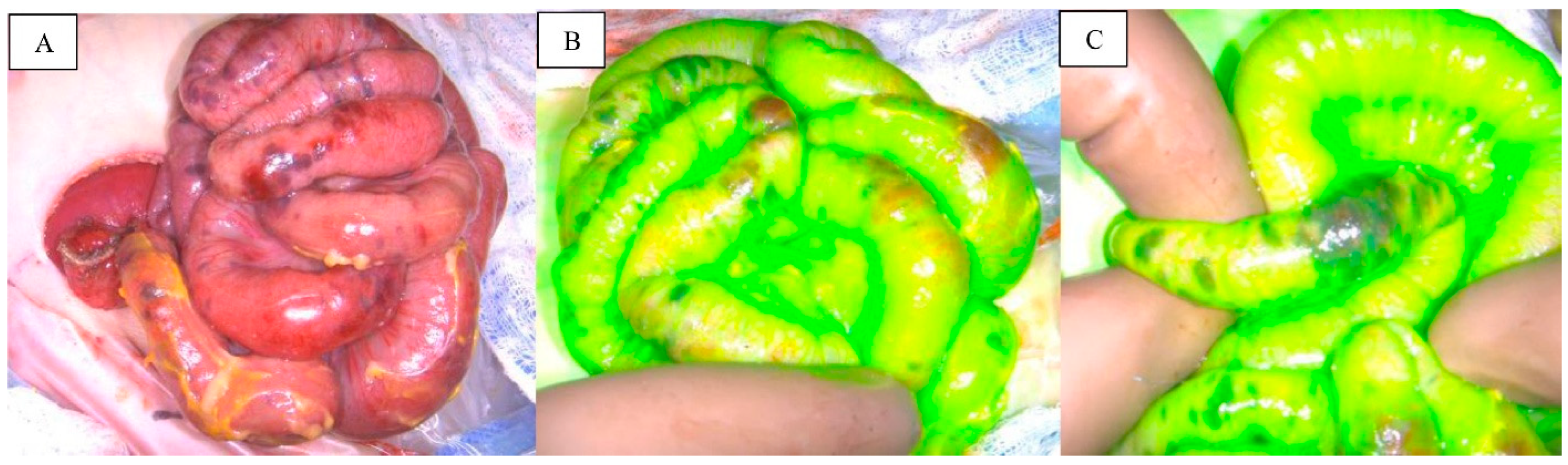


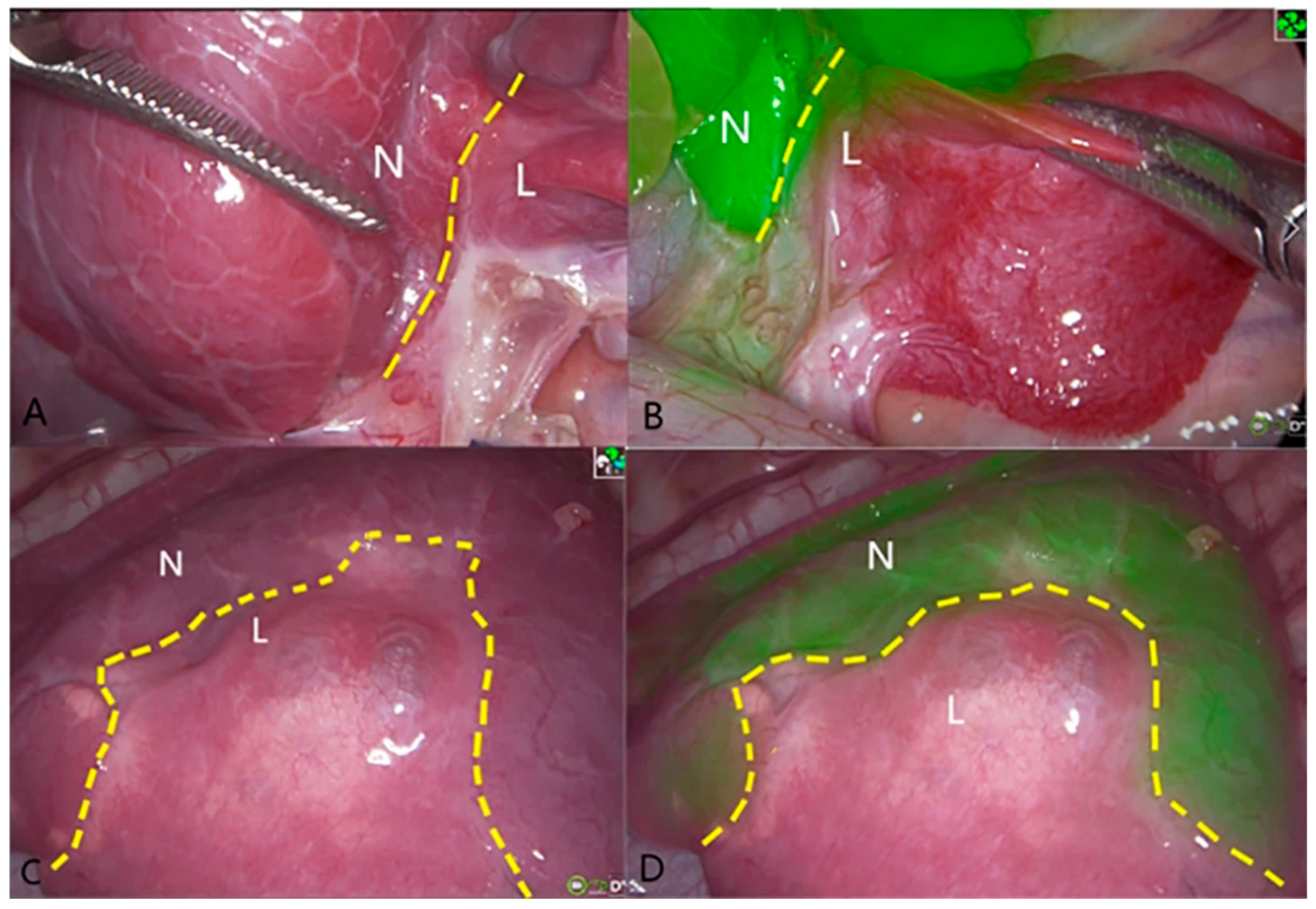

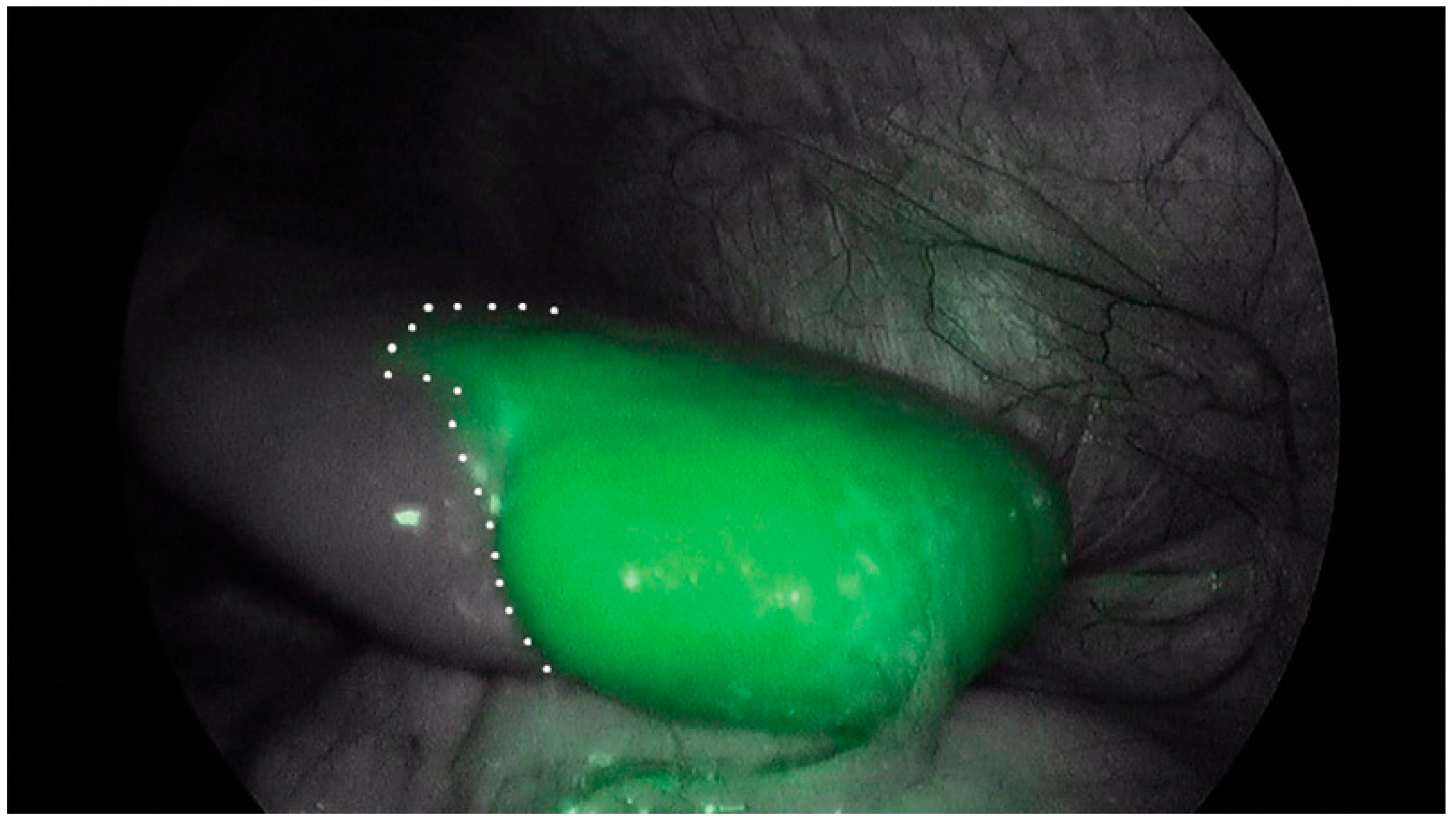
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Patient population ≤18 years old | Patient population >18 years old |
| Use of ICG for preoperative planning or diagnostics | Use of alternative fluorescent dye (e.g., fluorescein) |
| Use of ICG for intraoperative guidance or diagnostics | Full manuscript not available in English |
| Use of ICG for postoperative diagnostics or prognostication |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, N.J.; Corona, A.M.; Sanan, A.; Perez, E.A.; Huerta, C.T. Advances in Fluorescent Adjuncts in Pediatric Surgery: A Comprehensive Review of Applications of Indocyanine Green Across Surgical Specialties. Children 2025, 12, 1048. https://doi.org/10.3390/children12081048
Iglesias NJ, Corona AM, Sanan A, Perez EA, Huerta CT. Advances in Fluorescent Adjuncts in Pediatric Surgery: A Comprehensive Review of Applications of Indocyanine Green Across Surgical Specialties. Children. 2025; 12(8):1048. https://doi.org/10.3390/children12081048
Chicago/Turabian StyleIglesias, Nicholas Jose, Andres Mauricio Corona, Akshat Sanan, Eduardo Alfonso Perez, and Carlos Theodore Huerta. 2025. "Advances in Fluorescent Adjuncts in Pediatric Surgery: A Comprehensive Review of Applications of Indocyanine Green Across Surgical Specialties" Children 12, no. 8: 1048. https://doi.org/10.3390/children12081048
APA StyleIglesias, N. J., Corona, A. M., Sanan, A., Perez, E. A., & Huerta, C. T. (2025). Advances in Fluorescent Adjuncts in Pediatric Surgery: A Comprehensive Review of Applications of Indocyanine Green Across Surgical Specialties. Children, 12(8), 1048. https://doi.org/10.3390/children12081048






