Attention Deficit and Memory Function in Children with Bronchial Asthma: A Systematic Review and Meta-Analysis of 104,975 Patients with Trial Sequential Analysis
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Data Extraction
2.3. Endpoints and Subgroup Analyses
2.4. Quality Assessment
2.5. Statistical Analysis
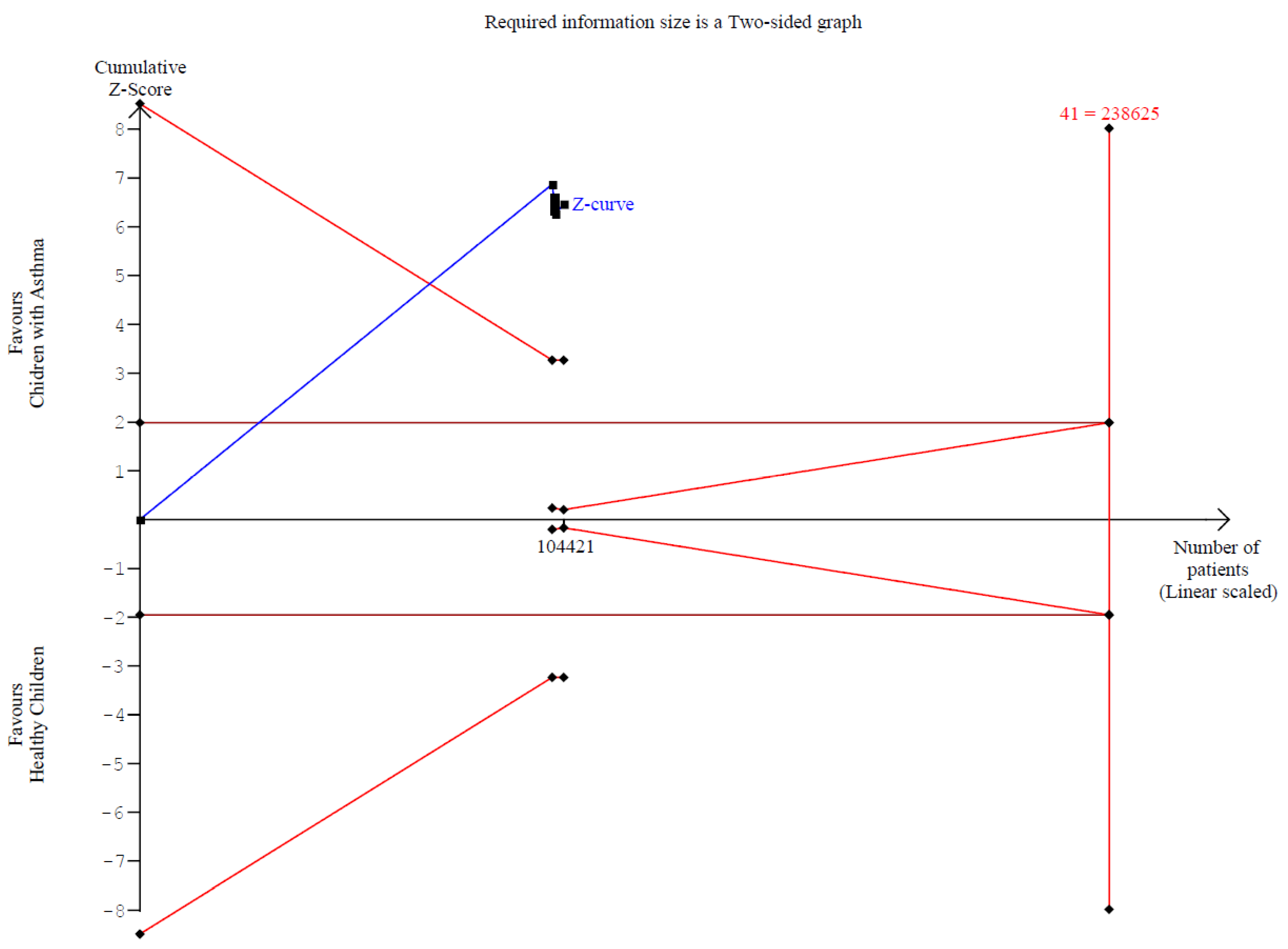
2.6. Trial Sequential Analysis (TSA)
3. Results
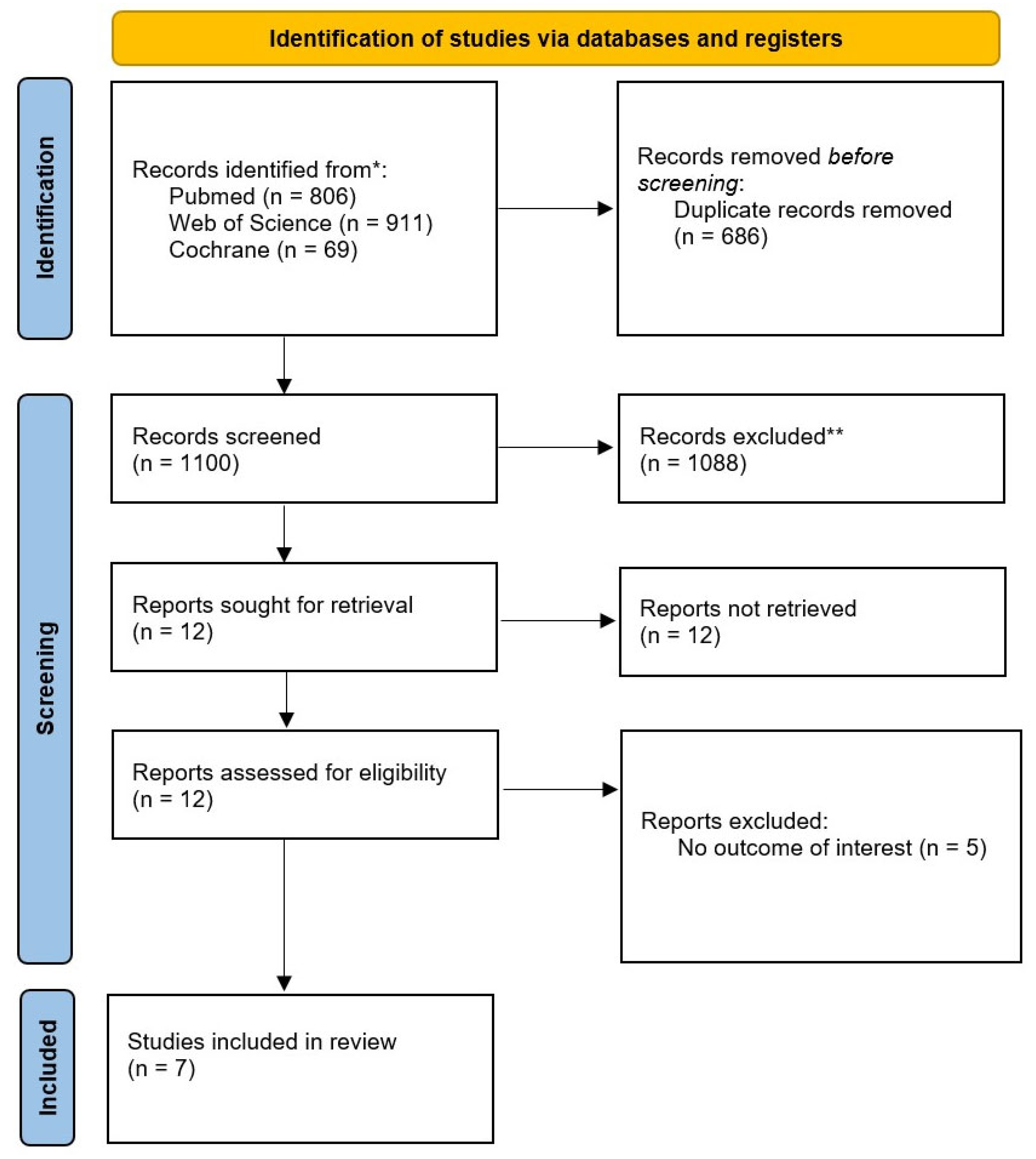
3.1. Study Selection and Baseline Characteristics
3.2. Pooled Analyses of All Included Studies
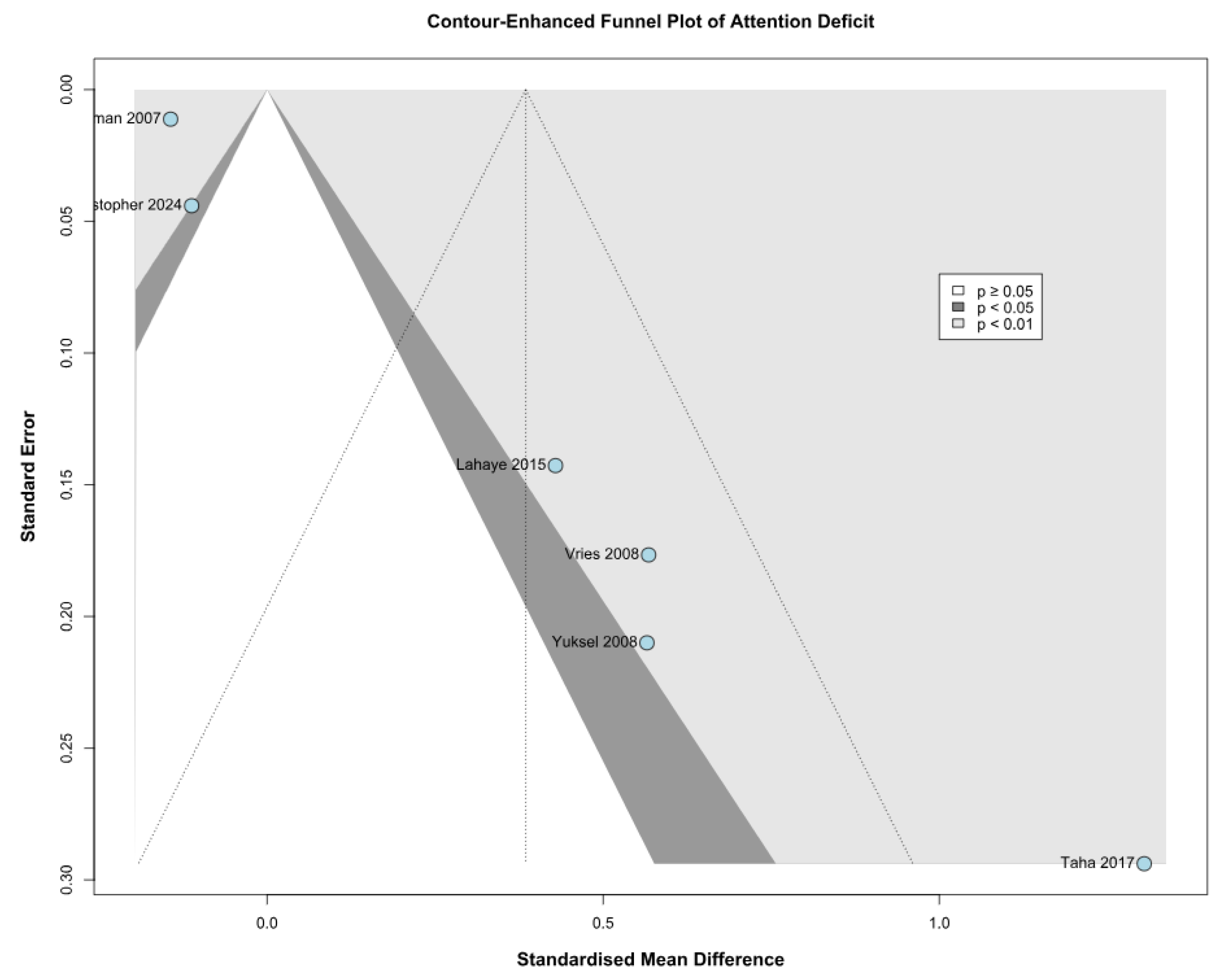
3.2.1. Attention Deficit
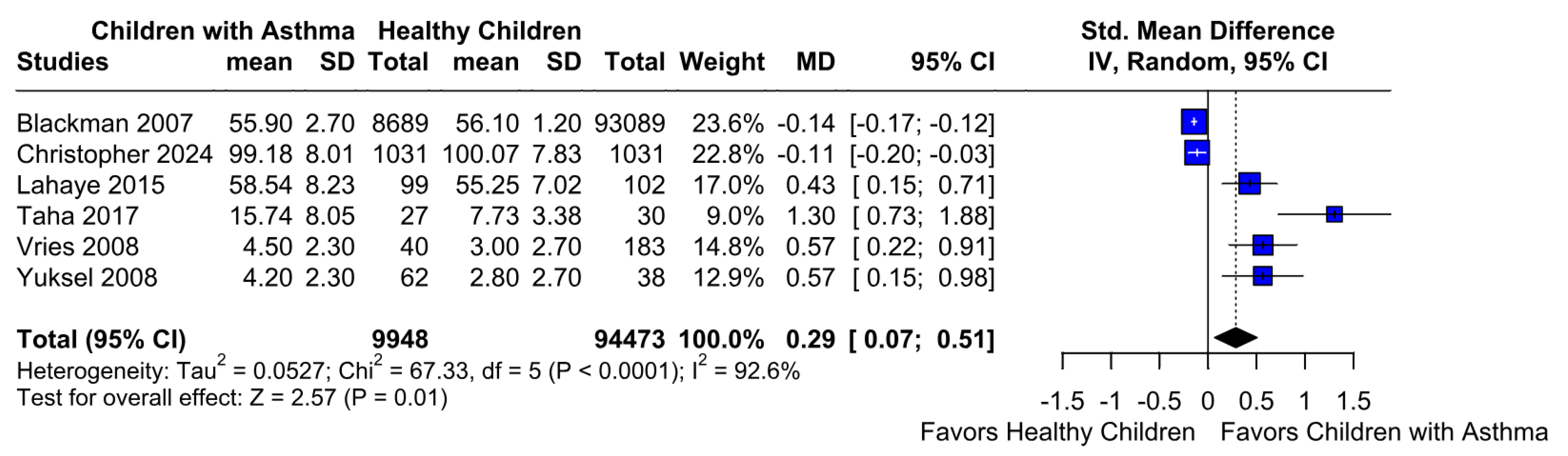
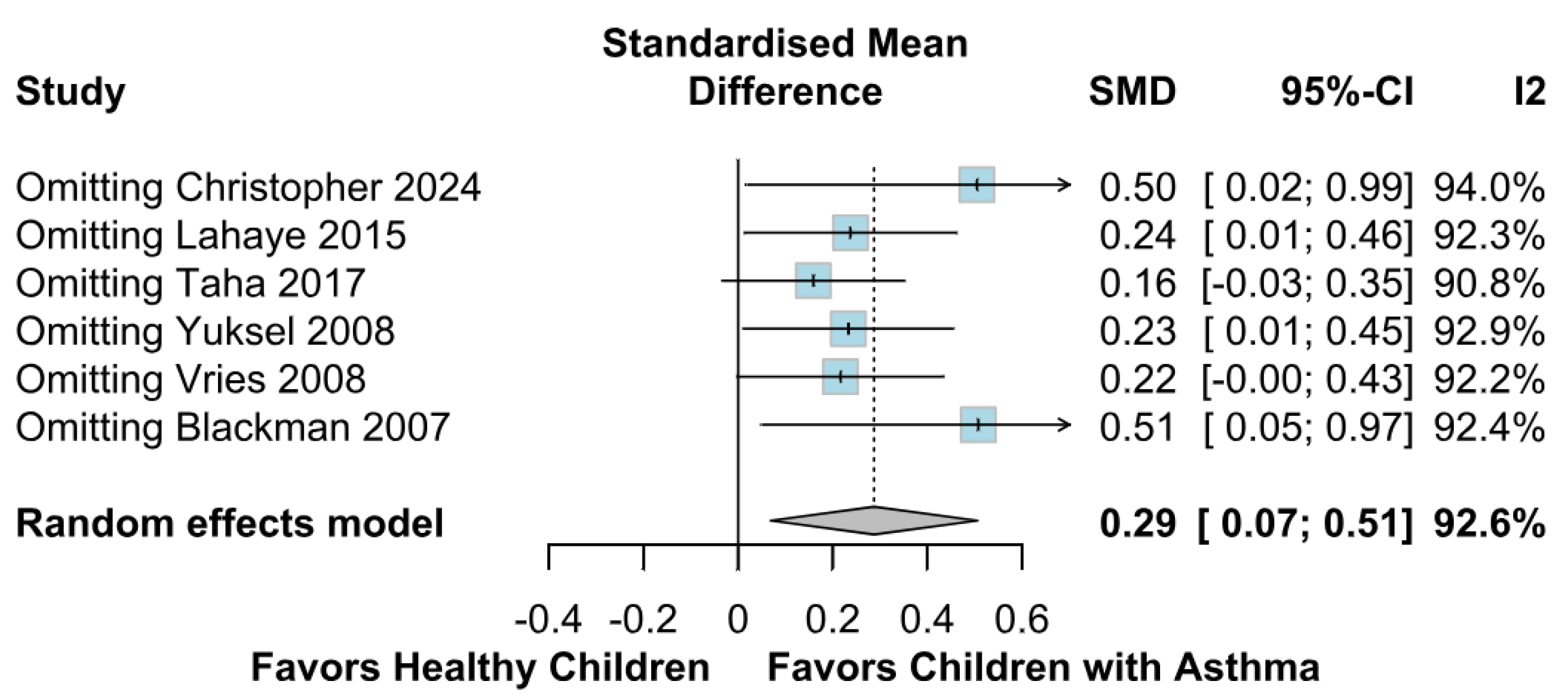
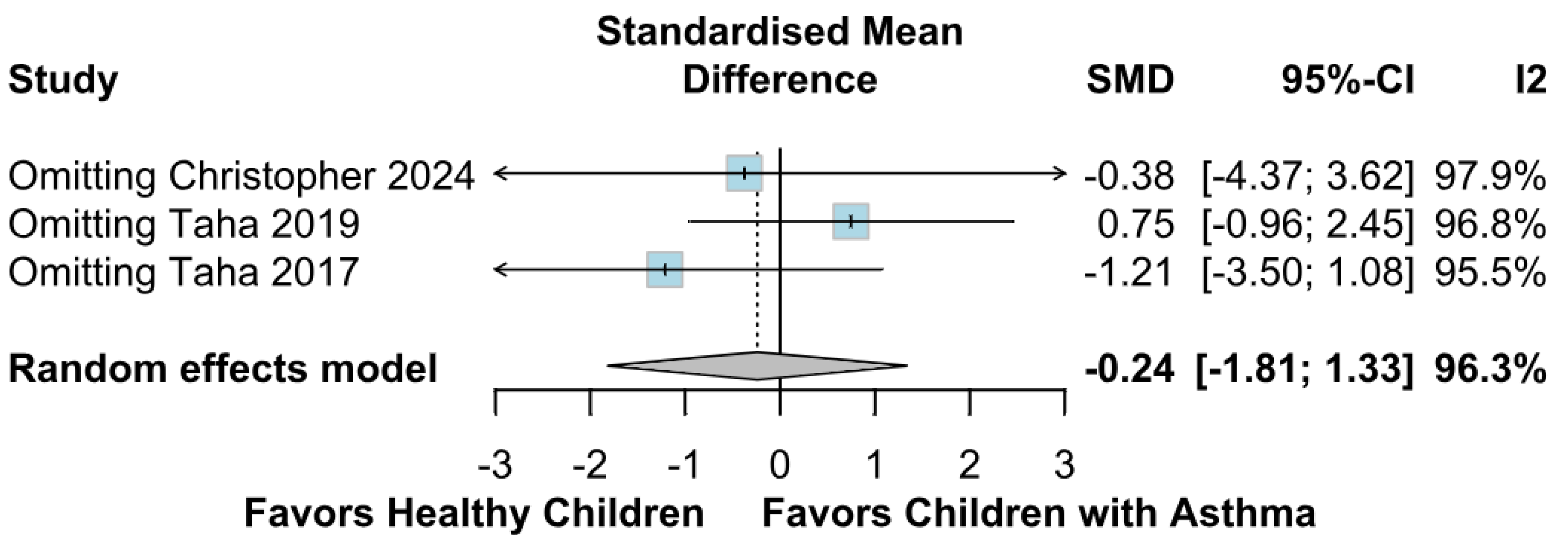
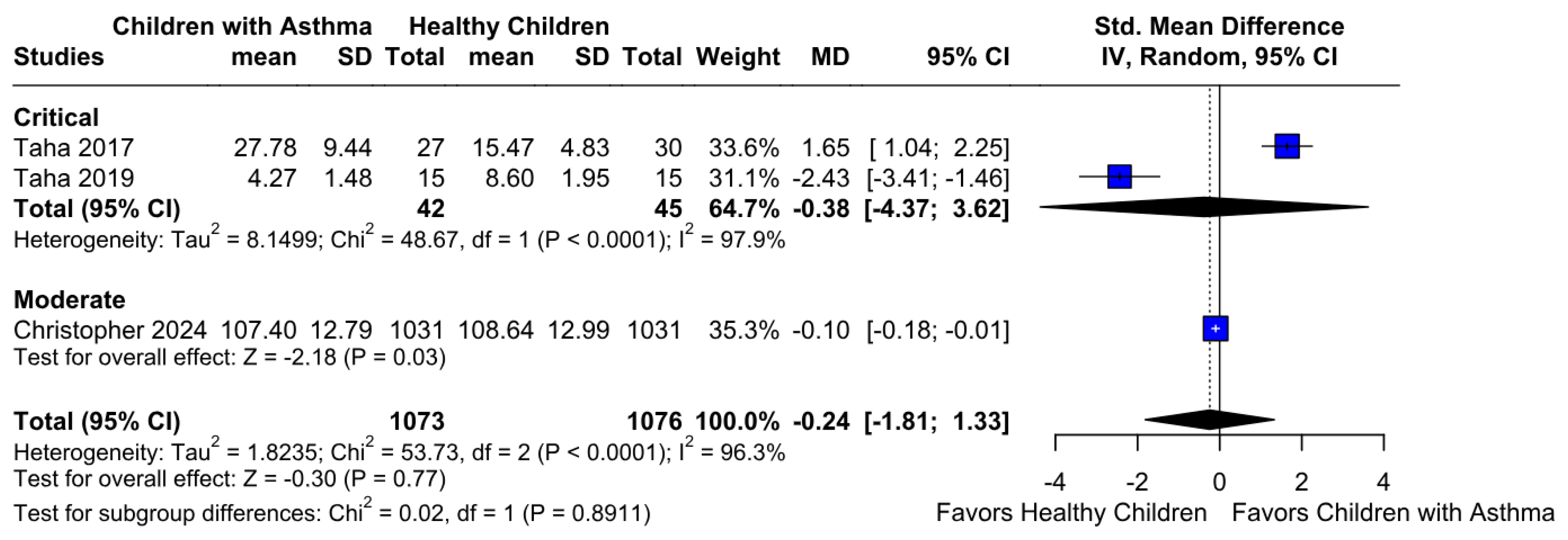
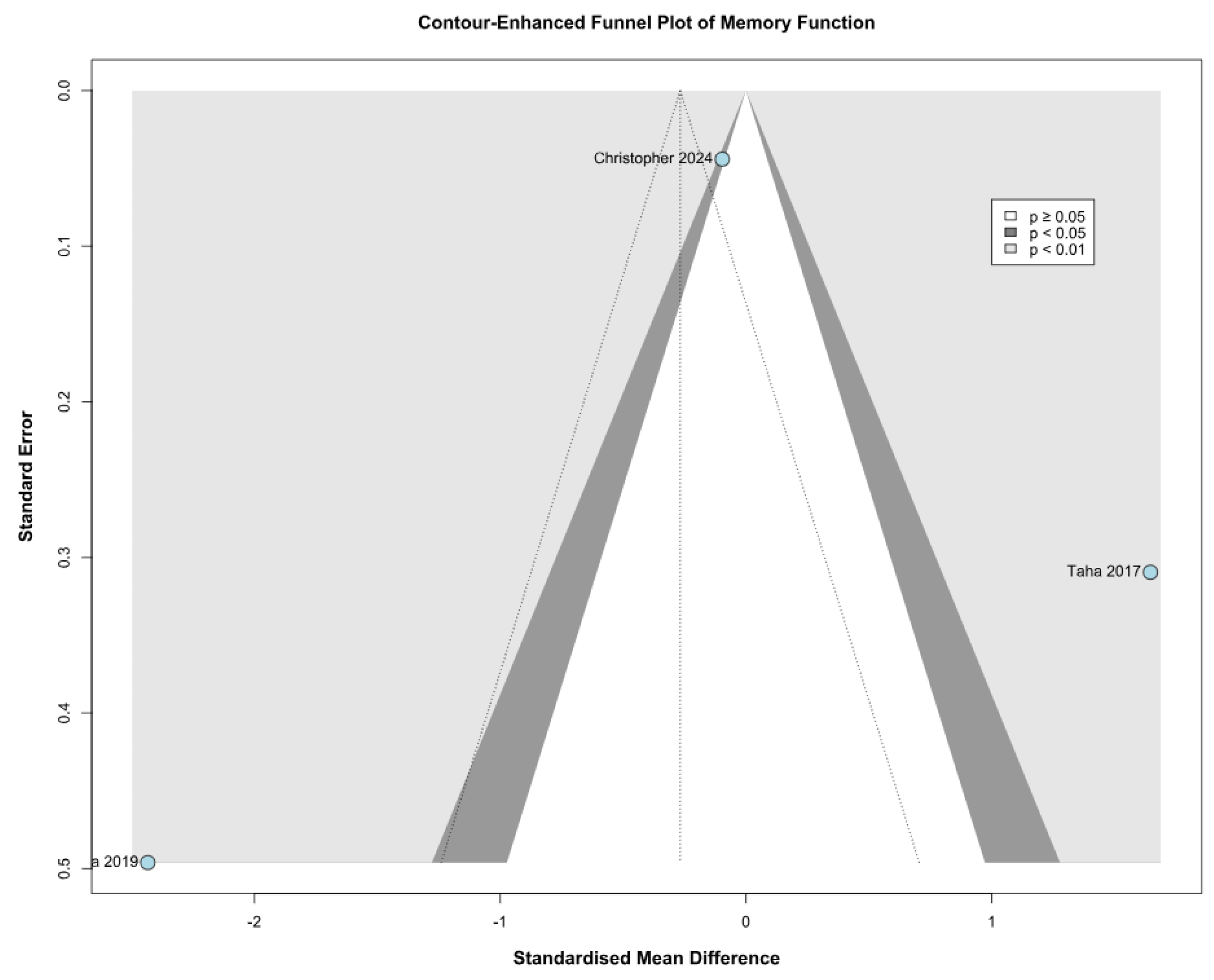
3.2.2. Memory Function
3.3. Subgroup Analyses
3.3.1. Attention Deficit Based on the Risk of Bias
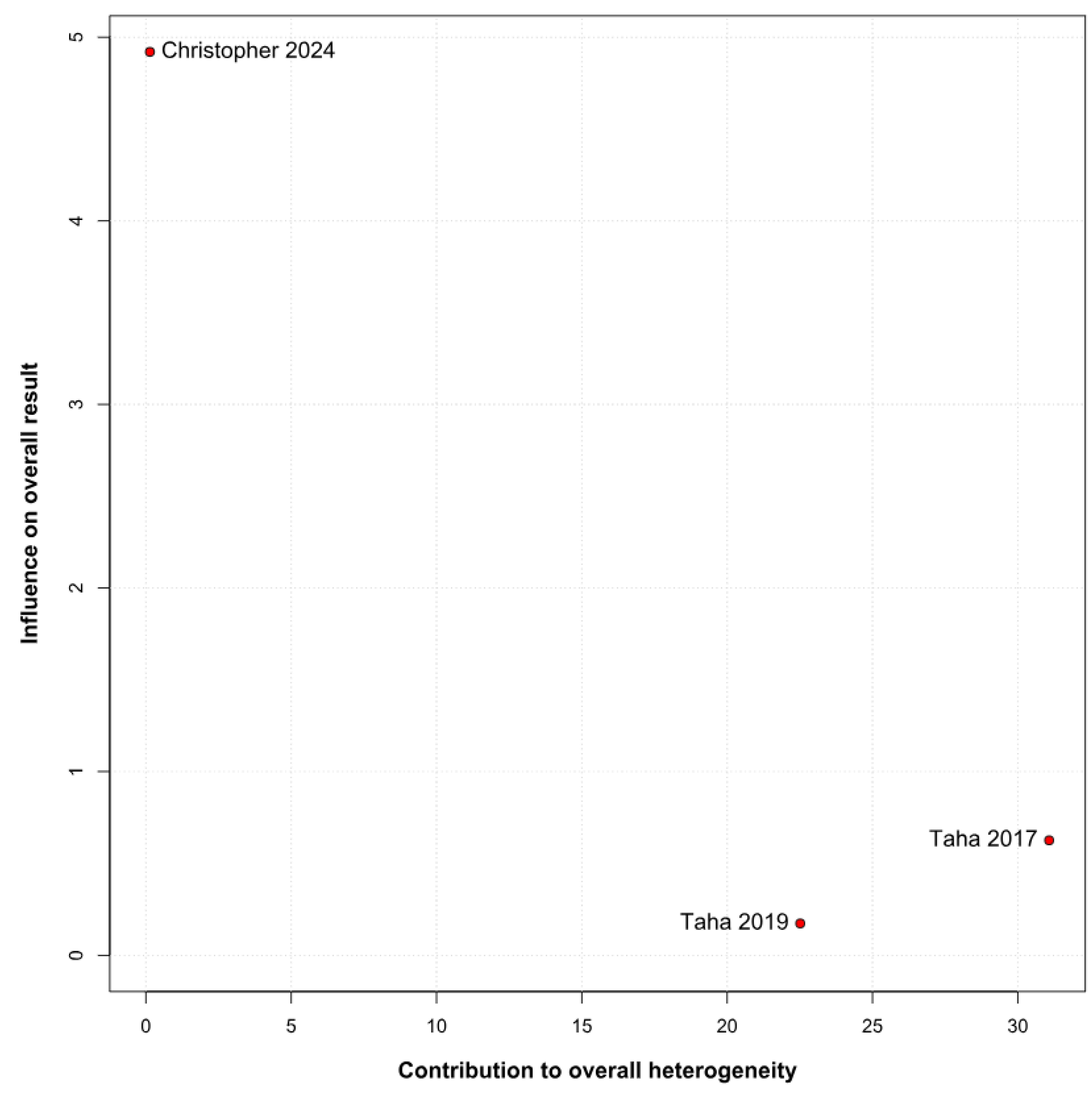
3.3.2. Memory Function Based on the Risk of Bias
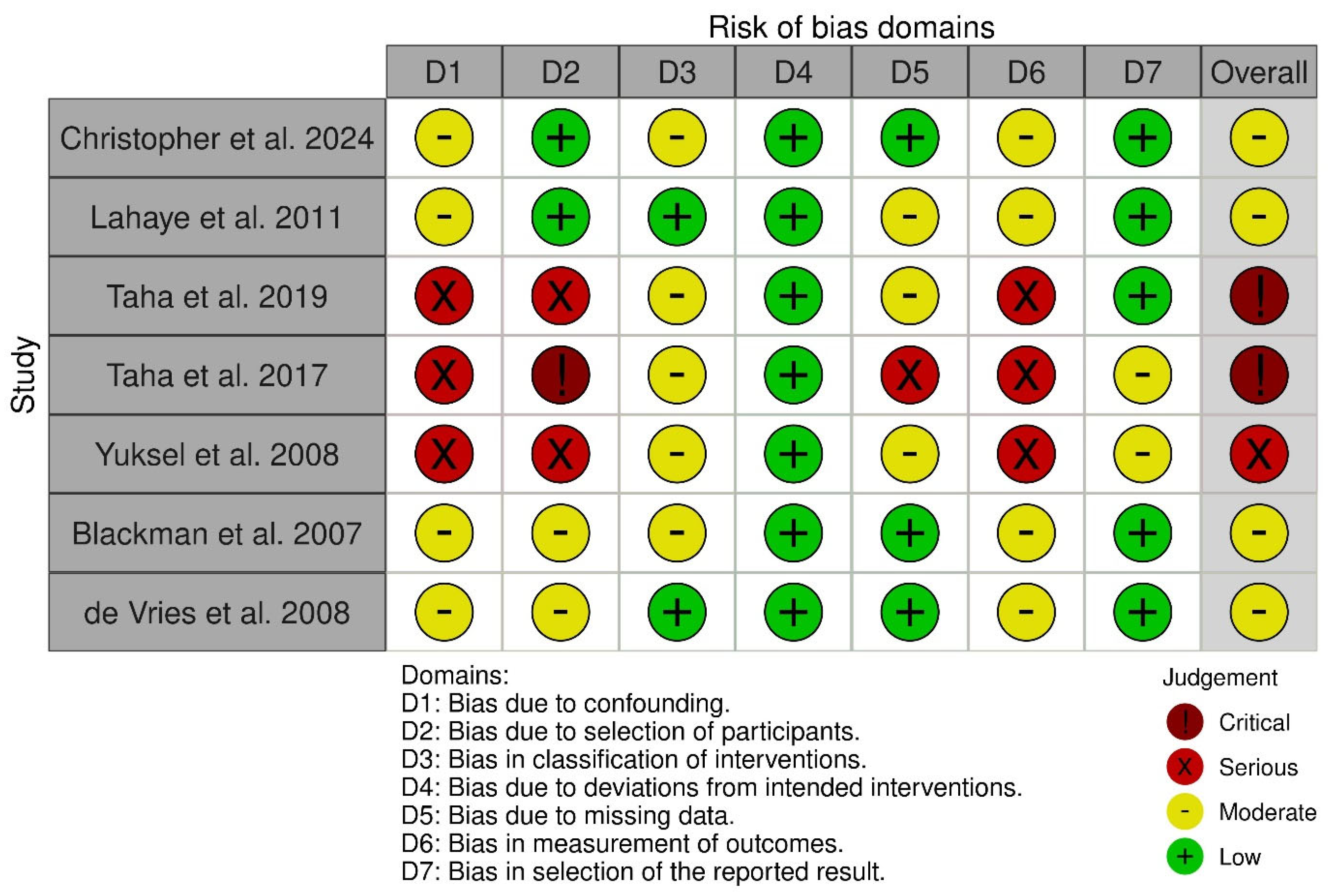
3.4. Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lahaye, M.; Luminet, O.; Van Broeck, N.; Bodart, E. Psychological, social and school implications of asthma: A comparison of Belgian French-speaking children having asthma with healthy children. Acta Clin. Belg. 2012, 67, 25–29. [Google Scholar] [PubMed]
- Yuksel, H.; Sogut, A.; Yilmaz, O. Attention deficit and hyperactivity symptoms in children with asthma. J. Asthma 2008, 45, 545–547. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, E.L.; Kopel, S.J.; Nassau, J.H. Behavioral adjustment in children with asthma: A meta-analysis. J. Dev. Behav. Pediatr. 2001, 22, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, C.; Koyama, M.; Ota, E.; Swa, T.; Mlunde, L.B.; Amiya, R.M.; Tachibana, Y.; Yamamoto-Hanada, K.; Mori, R. Allergic diseases in children with attention deficit hyperactivity disorder: A systematic review and meta-analysis. BMC Psychiatry 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, T.H.; Tai, Y.H.; Dai, Y.X.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Risk of Atopic Diseases among Siblings of Patients with Attention-Deficit Hyperactivity Disorder: A Nationwide Population-Based Cohort Study. Int. Arch. Allergy Immunol. 2019, 180, 37–43. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, Y.H.; Na, D.Y.; Jeong, S.W.; Lee, M.G.; Lee, J.H.; Yang, Y.N.; Kang, M.G.; Yeom, S.W.; Kim, J.S.; et al. Long-term bidirectional association between asthma and attention deficit hyperactivity disorder: A big data cohort study. Front. Psychiatry 2023, 13, 1044742. [Google Scholar] [CrossRef]
- Zhuoran, Z.; Xiaoman, W.; Rui, Y.; Qingjiu, C. Association Between ADHD and Pediatric Asthma: Results from a Large-Sample Cross-Sectional Study of National Surveys and Mendelian Randomization Analyses. J. Atten. Disord. 2025. [Google Scholar] [CrossRef] [PubMed]
- Bender, B.G.; Lerner, J.A.; Kollasch, E. Mood and memory changes in asthmatic children receiving corticosteroids. J. Am. Acad. Child Adolesc. Psychiatry 1988, 27, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Christopher-Hayes, N.J.; Haynes, S.C.; Kenyon, N.J.; Merchant, V.D.; Schweitzer, J.B.; Ghetti, S. Asthma and Memory Function in Children. JAMA Netw. Open 2024, 7, e2442803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hajek, C.A.; Yeates, K.O.; Anderson, V.; Mackay, M.; Greenham, M.; Gomes, A.; Lo, W. Cognitive outcomes following arterial ischemic stroke in infants and children. J. Child Neurol. 2014, 29, 887–894. [Google Scholar] [CrossRef]
- Jiang, K.; Zhu, L.; Ge, Y.; Shangguan, Q.; Chen, Y.; He, Y.; Wu, J.; Qin, C.; Xiong, J.; Zhao, J. Brain network study of attentional cognitive impairment in children with bronchial asthma. Res. Sq. 2021; preprint. [Google Scholar] [CrossRef]
- Taha, H.; Khalil, M. Verbal and visual memory performances of children with moderate-into-severe asthma. Pol. Psychol. Bull. 2019, 50, 13–17. [Google Scholar] [CrossRef]
- Schans, J.V.; Çiçek, R.; de Vries, T.W.; Hak, E.; Hoekstra, P.J. Association of atopic diseases and attention-deficit/hyperactivity disorder: A systematic review and meta-analyses. Neurosci. Biobehav. Rev. 2017, 74, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kaas, T.H.; Vinding, R.K.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Chawes, B.L. Association between childhood asthma and attention deficit hyperactivity or autism spectrum disorders: A systematic review with meta-analysis. Clin. Exp. Allergy 2021, 51, 228–252. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Sun, S.; Zhang, J.; Sharma, E.; Chang, Z.; Kuja-Halkola, R.; Almqvist, C.; Larsson, H.; Faraone, S.V. Association between attention deficit hyperactivity disorder and asthma: A systematic review and meta-analysis and a Swedish population-based study. Lancet Psychiatry 2018, 5, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023); Cochrane: London, UK, 2023; Available online: https://www.training.cochrane.org/handbook (accessed on 28 February 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Penchev, P.; Ivanov, K.; Milanova-Ilieva, D.; Gaydarski, L.; Kostov, K.; Boyadzhiev, N.; Petrov, P.-P.; Mehandzhiev, P.; Hyusein, R.; Velchev, V.; et al. Mental Health and Quality of Life in Patients with Untreated Unruptured Intracranial Aneurysms: A Systematic Review and Meta-Analysis of 417,152 Patients with Trial Sequential Analysis. Brain Sci. 2025, 15, 764. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Nakagawa, S.; Noble, D.W.A.; Senior, A.M.; Lagisz, M. Meta-evaluation of meta-analysis: Ten appraisal questions for biologists. BMC Biol. 2017, 15, 18. [Google Scholar] [CrossRef]
- Hartung, J.; Knapp, G. On tests of the overall treatment effect in meta-analysis with normally distributed responses. Stat. Med. 2001, 20, 1771–1782. [Google Scholar] [CrossRef]
- Knapp, G.; Hartung, J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003, 22, 2693–2710. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Trial Sequential Analysis (TSA) [Computer program] Version 0.9.5.10 Beta; The Copenhagen Trial Unit, Centre for Clinical Intervention Research, The Capital Region, Copenhagen University Hospital—Rigshospitalet: Copenhagen, Denmark, 2021.
- Taha, H. Poor Executive Functions among Children with Moderate-into-Severe Asthma: Evidence from WCST Performance. Front. Psychol. 2017, 8, 793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blackman, J.A.; Gurka, M.J. Developmental and behavioral comorbidities of asthma in children. J. Dev. Behav. Pediatr. 2007, 28, 92–99. [Google Scholar] [CrossRef] [PubMed]
- de Vries, T.W.; van Roon, E.N.; Duiverman, E.J. Inhaled corticosteroids do not affect behaviour. Acta Paediatr. 2008, 97, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Fryt, J.; Pilecka, W.; Smoleñ, T. Importance of symptom control: Self-regulation in children with diabetes type 1 and asthma. Stud. Psychol. 2013, 51, 5–18. [Google Scholar]
- Fuster, J.M. Frontal lobe and cognitive development. J. Neurocytol. 2002, 31, 373–385. [Google Scholar] [CrossRef] [PubMed]



| Study | No. Patients | Children with Asthma | Healthy Children | Age * | Female, % | Race, Caucasian, % | Severe Asthma, % | High SES, % |
|---|---|---|---|---|---|---|---|---|
| Blackman, 2007 [27] | 101,778 | 8689 | 93,089 | 7.8 | 66% | 8.1 | 5 | 8.8 |
| Christopher-Hayes, 2024 [9] | 474 | 237 | 237 | 9.9 | 49 | 62 | N/A | N/A |
| 2062 | 1031 | 1031 | 11.9 | 43 | 54 | |||
| Lahaye, 2011 [1] | 201 | 99 | 102 | 11.4 | 34 | N/A | 54 | 61 |
| Taha, 2019 [12] | 30 | 15 | 15 | 4.7 | 60 | N/A | N/A | N/A |
| Taha, 2017 [26] | 57 | 27 | 30 | 12.5 | 41 | N/A | N/A | N/A |
| Vries, 2008 [28] | 273 | 40 | 233 | 4.4 | 27 | N/A | N/A | 8 |
| Yuksel, 2008 [2] | 100 | 62 | 38 | 9.2 ± 1.5 | 40 | N/A | N/A | N/A |
| Study | Psychometric Tool for ADHD/Attention Deficit |
|---|---|
| Blackman, 2007 [27] | Parent-reported diagnosis (NSCH single-item question) |
| Christopher-Hayes, 2024 [9] | NIH Toolbox—Flanker Inhibitory Control and Attention Test |
| Lahaye, 2011 [1] | CBCL—Attention Problems Subscale (parent-reported) |
| Taha, 2017 [26] | WCST—Sustained attention and memory (via non-perseverative errors) |
| Vries, 2008 [28] | DSM-IV-based questionnaire (covering attention deficit, hyperactivity, impulsivity); CBCL (attention axis) |
| Yuksel, 2008 [2] | Conners’ Parent Rating Scale-48 (CPRS-48) |
| Study | Psychometric Tool for Memory Function |
|---|---|
| Christopher-Hayes, 2024 [9] | NIH Toolbox—Picture Sequence Memory Test |
| Taha, 2017 [26] | WCST—Sustained attention and memory (via non-perseverative errors) |
| Taha, 2019 [12] | Digit Span (forward), Backward Digit Span, Adapted Rey Auditory Verbal Learning Test (RAVLT), MVPT-3, RCFT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penchev, P.; Milanova-Ilieva, D.; Gaydarski, L.; Petrov, P.-P.; Ketev, K.; Stanchev, P.; Husain, N.; Ramadanov, N. Attention Deficit and Memory Function in Children with Bronchial Asthma: A Systematic Review and Meta-Analysis of 104,975 Patients with Trial Sequential Analysis. Children 2025, 12, 1013. https://doi.org/10.3390/children12081013
Penchev P, Milanova-Ilieva D, Gaydarski L, Petrov P-P, Ketev K, Stanchev P, Husain N, Ramadanov N. Attention Deficit and Memory Function in Children with Bronchial Asthma: A Systematic Review and Meta-Analysis of 104,975 Patients with Trial Sequential Analysis. Children. 2025; 12(8):1013. https://doi.org/10.3390/children12081013
Chicago/Turabian StylePenchev, Plamen, Daniela Milanova-Ilieva, Lyubomir Gaydarski, Petar-Preslav Petrov, Kostadin Ketev, Pavel Stanchev, Noor Husain, and Nikolai Ramadanov. 2025. "Attention Deficit and Memory Function in Children with Bronchial Asthma: A Systematic Review and Meta-Analysis of 104,975 Patients with Trial Sequential Analysis" Children 12, no. 8: 1013. https://doi.org/10.3390/children12081013
APA StylePenchev, P., Milanova-Ilieva, D., Gaydarski, L., Petrov, P.-P., Ketev, K., Stanchev, P., Husain, N., & Ramadanov, N. (2025). Attention Deficit and Memory Function in Children with Bronchial Asthma: A Systematic Review and Meta-Analysis of 104,975 Patients with Trial Sequential Analysis. Children, 12(8), 1013. https://doi.org/10.3390/children12081013







