Clinical Experience in the Management of a Series of Fetal–Neonatal Ovarian Cysts
Abstract
1. Introduction
2. Materials and Methods
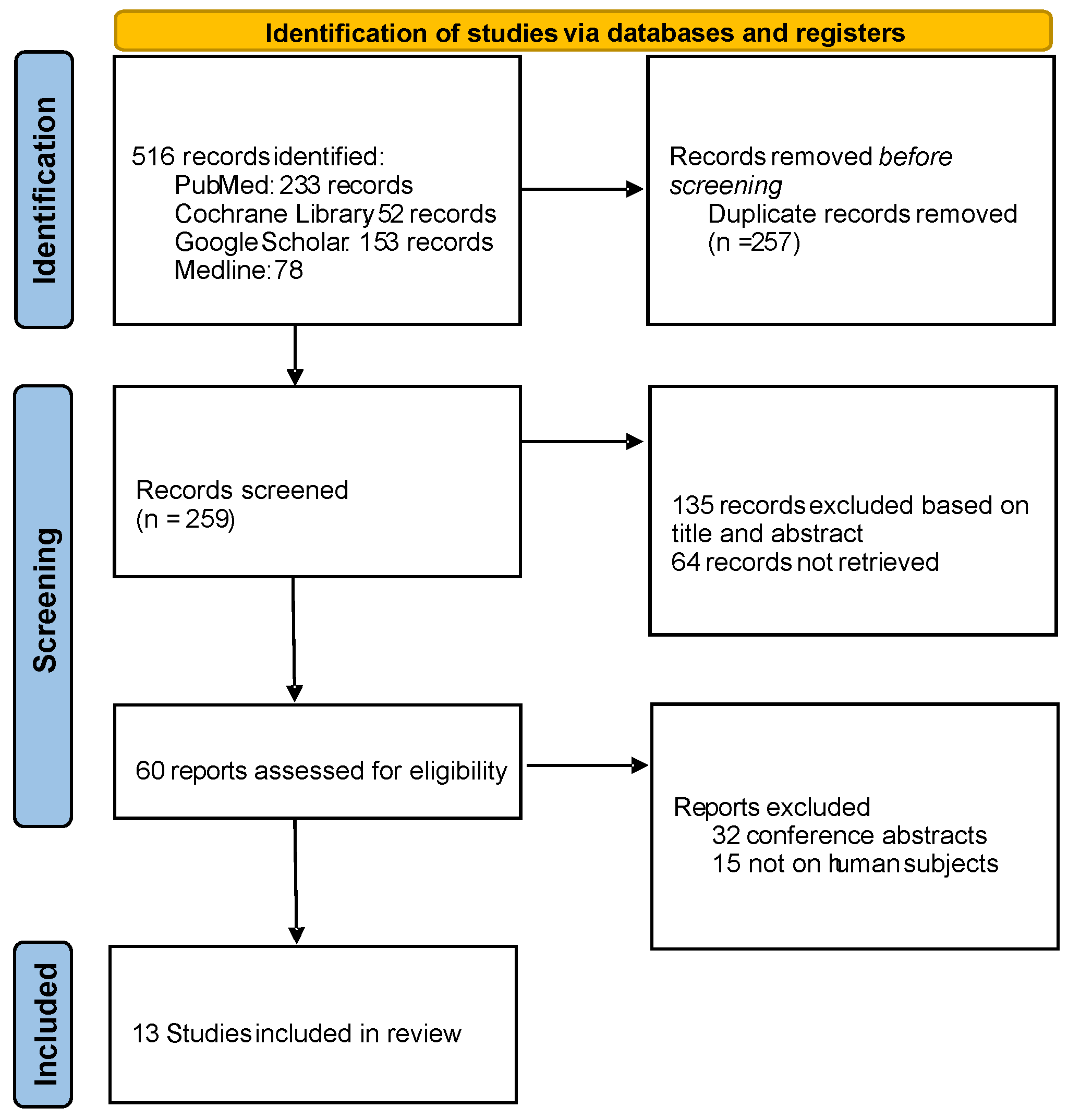
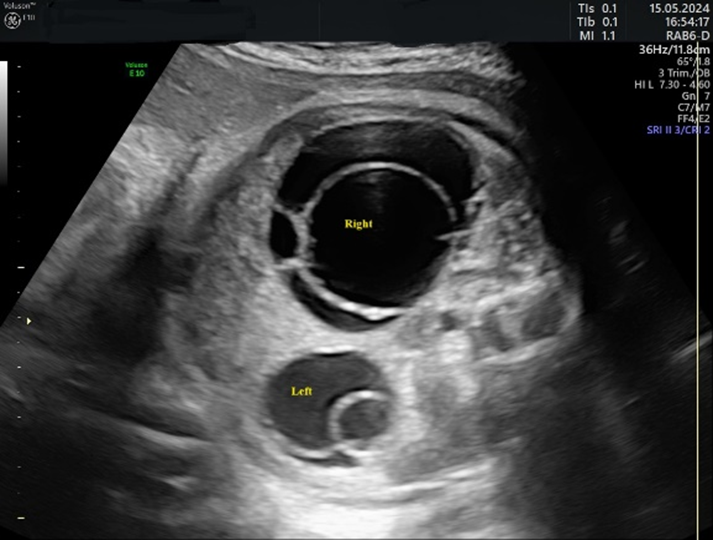
3. Results
4. Discussion
- -
- If a fetal ovarian cyst is <4 cm and appears simple, manage expectantly with periodic ultrasound surveillance. Plan for a normal delivery. Postnatal ultrasound in the first week of life is recommended to check the status. If the cyst is regressing or <1–2 cm, continue to observe; if it persists >~2–3 cm within a few months, consider surgery to rule out other pathology.
- -
- If a cyst is ≥4–5 cm or enlarging and is simple (anechoic), consider referral to a fetal therapy center for possible in utero aspiration around 32–34 weeks. If fetal intervention is not available or not preferred, then plan for early delivery at ~37 weeks and immediate postnatal evaluation/treatment.
- -
- If a cyst is complex, the benefit of prenatal aspiration is less clear (since complexity often means blood contents). These cases should be managed by observation or early delivery for postnatal surgical management. Complex cysts usually warrant neonatal surgery, as they often signify torsion/necrosis that will not self-resolve.
- -
- Any signs of fetal compromise (very rare, e.g., a cyst causing hydrops or severe polyhydramnios) would push toward intervention (either aspirate the cyst or amnioreduction plus early delivery).
- -
- Multidisciplinary consultation (maternal–fetal medicine, pediatric surgery, neonatology) is ideal in planning the timing and mode of delivery and immediate postnatal care for fetuses with large ovarian cysts.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalil, A.; Morlando, M.; Papageorghiou, A.T.; Jurkovic, D.; Bower, S.; Pandya, P.P. The Outcome of Simple Fetal Ovarian Cysts Diagnosed Antenatally. J. Pediatr. Surg. 2012, 47, 1757–1762. [Google Scholar] [CrossRef]
- Acharya, K.; Poudel, D.; Dahal, S.; kuikel, S.; Adhikari, A. A Case of Huge Ovarian Cyst in Second Trimester: A Rare Case Report. Ann. Med. Surg. 2022, 82, 104765. [Google Scholar] [CrossRef]
- Patru, C.L.; Iliescu, D.G.; Tanase, F.; Dragusin, R.C.; Sorop Florea, M.; Capitanescu, R.G.; Comanescu, A.; Pana, R.C.; Zorila, L.G.; Marinas, M.C.; et al. Fetal Heart in the First Trimester. The Added Value of the Outflow Tracts Evaluation. In Proceedings of the Al V lea Congres de Ecografie in OG 2017, Targu Mures, Romania, 20–22 April 2017; Filodiritto Editore—Proceedings. p. 471. [Google Scholar]
- Lee, H.J.; Lee, J.; Kim, M.-S.; Jung, H.J.; Kim, J.S. Antenatal Natural History and Postnatal Outcomes of Fetal Ovarian Cysts. Ultrasound Obstet. Gynecol. 2017, 50, 47–52. [Google Scholar] [CrossRef]
- Tyraskis, A.; Bakalis, S.; David, A.L.; Eaton, S.; De Coppi, P. A Systematic Review and Meta-Analysis on Fetal Ovarian Cysts: Impact of Size, Appearance and Prenatal Aspiration. Prenat. Diagn. 2017, 37, 951–958. [Google Scholar] [CrossRef]
- Abbott, D.H.; Padmanabhan, V.; Dumesic, D.A. Contributions of Androgen and Estrogen to Fetal Programming of Ovarian Dysfunction. Reprod. Biol. Endocrinol. 2006, 4, 17. [Google Scholar] [CrossRef]
- Brandt, M.L.; Helmrath, M.A. Ovarian Cysts in Infants and Children. Semin. Pediatr. Surg. 2005, 14, 78–85. [Google Scholar] [CrossRef]
- Galinier, P.; Carfagna, L.; Juricic, M.; Lemasson, F.; Moscovici, J.; Guitard, J.; Baunin, C.; Menendez, M.; Cartault, A.; Pienkowski, C.; et al. Fetal ovarian cysts management and ovarian prognosis: A report of 82 cases. J. Pediatr. Surg. 2008, 43, 2004–2009. [Google Scholar] [CrossRef] [PubMed]
- Montaguti, E.; Petrachi, B.; Fiorentini, M.; Arosio, V.; Doroldi, S.; Dionisi, C.; Bernardi, V.; Pilu, G. The Role of Prenatal Ultrasound Examination in Predicting the Outcomes of Ovarian Fetal Cysts: A Pictorial Essay. Diagnostics 2024, 14, 2726. [Google Scholar] [CrossRef]
- Vaduva, C.-C.; Sandulescu, M.S.; Tenovici, M.; Siminel, M.A.; Novac, M.B. Results of in Vitro Fertilization after Diagnosis and Treatment of Chronic Endometritis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1069–1076. [Google Scholar] [CrossRef]
- Bagolan, P.; Giorlandino, C.; Nahom, A.; Bilancioni, E.; Trucchi, A.; Gatti, C.; Aleandri, V.; Spina, V. The Management of Fetal Ovarian Cysts. J. Pediatr. Surg. 2002, 37, 25–30. [Google Scholar] [CrossRef]
- Mittermayer, C.; Blaicher, W.; Grassauer, D.; Horcher, E.; Deutinger, J.; Bernaschek, G.; Ulm, B. Fetal Ovarian Cysts: Development and Neonatal Outcome. Ultraschall Med. 2003, 24, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.W.; Sohn, Y.S.; Kim, S.K.; Kim, I.K.; Park, Y.W.; Kim, Y.H. Clinical Experiences of Fetal Ovarian Cyst: Diagnosis and Consequence. J. Korean Med. Sci. 2006, 21, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Mimura, K.; Endo, M.; Fujii, M.; Matsuyama, T.; Yagi, K.; Kawanishi, Y.; Tomimatsu, T.; Kimura, T. Diagnosis, Management, and Therapy of Fetal Ovarian Cysts Detected by Prenatal Ultrasonography: A Report of 36 Cases and Literature Review. Diagnostics 2021, 11, 2224. [Google Scholar] [CrossRef] [PubMed]
- Melinte-Popescu, A.-S.; Popa, R.-F.; Harabor, V.; Nechita, A.; Harabor, A.; Adam, A.-M.; Vasilache, I.-A.; Melinte-Popescu, M.; Vaduva, C.; Socolov, D. Managing Fetal Ovarian Cysts: Clinical Experience with a Rare Disorder. Medicina 2023, 59, 715. [Google Scholar] [CrossRef]
- Chen, L.; Hu, Y.; Hu, C.; Wen, H. Prenatal Evaluation and Postnatal Outcomes of Fetal Ovarian Cysts. Prenat. Diagn. 2020, 40, 1258–1264. [Google Scholar] [CrossRef]
- Nakamura, M.; Ishii, K.; Murata, M.; Sasahara, J.; Mitsuda, N. Postnatal Outcome in Cases of Prenatally Diagnosed Fetal Ovarian Cysts under Conservative Prenatal Management. Fetal Diagn. Ther. 2015, 37, 129–134. [Google Scholar] [CrossRef]
- Bascietto, F.; Liberati, M.; Marrone, L.; Khalil, A.; Pagani, G.; Gustapane, S.; Leombroni, M.; Buca, D.; Flacco, M.E.; Rizzo, G.; et al. Outcome of Fetal Ovarian Cysts Diagnosed on Prenatal Ultrasound Examination: Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2017, 50, 20–31. [Google Scholar] [CrossRef]
- Słodki, M.; Respondek-Liberska, M. [Fetal ovarian cysts—420 cases from literature—Metaanalysis 1984–2005]. Ginekol. Pol. 2007, 78, 324–328. [Google Scholar]
- Bucuri, C.; Mihu, D.; Malutan, A.; Oprea, V.; Berceanu, C.; Nati, I.; Rada, M.; Ormindean, C.; Blaga, L.; Ciortea, R. Fetal Ovarian Cyst—A Scoping Review of the Data from the Last 10 Years. Medicina 2023, 59, 186. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine (SMFM); Cheng, Y. Ovarian Cysts. Am. J. Obstet. Gynecol. 2021, 225, B23–B25. [Google Scholar] [CrossRef] [PubMed]
- Diguisto, C.; Winer, N.; Benoist, G.; Laurichesse-Delmas, H.; Potin, J.; Binet, A.; Lardy, H.; Morel, B.; Perrotin, F. In-Utero Aspiration vs Expectant Management of Anechoic Fetal Ovarian Cysts: Open Randomized Controlled Trial. Ultrasound Obstet. Gynecol. 2018, 52, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Jwa, S.C.; Aoki, H.; Anami, A.; Umehara, N.; Sumie, M.; Wada, S.; Sago, H. Management of Fetal Ovarian Cyst Using in Utero Aspiration. Case Rep. Perinat. Med. 2015, 4, 125–129. [Google Scholar] [CrossRef]
- Cho, M.J.; Kim, D.Y.; Kim, S.C. Ovarian Cyst Aspiration in the Neonate: Minimally Invasive Surgery. J. Pediatr. Adolesc. Gynecol. 2015, 28, 348–353. [Google Scholar] [CrossRef]
- Jafri, S.Z.; Bree, R.L.; Silver, T.M.; Ouimette, M. Fetal Ovarian Cysts: Sonographic Detection and Association with Hypothyroidism. Radiology 1984, 150, 809–812. [Google Scholar] [CrossRef]
- Rotar, I.C.; Tudorache, S.; Staicu, A.; Popa-Stanila, R.; Constantin, R.; Surcel, M.; Zaharie, G.C.; Mureşan, D. Fetal Ovarian Cysts: Prenatal Diagnosis Using Ultrasound and MRI, Management and Postnatal Outcome—Our Centers Experience. Diagnostics 2022, 12, 89. [Google Scholar] [CrossRef]
- Bromley, B. Diagnostic Imaging of Fetal Anomalies. J. Ultrasound Med. 2003, 22, 850. [Google Scholar] [CrossRef]
- Bower, R.; Dehner, L.P.; Ternberg, J.L. Bilateral Ovarian Cysts in the Newborn: A Triad of Neonatal Abdominal Masses, Polyhydramnios, and Maternal Diabetes Mellitus. Am. J. Dis. Child. 1974, 128, 731–733. [Google Scholar] [CrossRef]
- deSa, D.J. Follicular Ovarian Cysts in Stillbirths and Neonates. Arch. Dis. Child. 1975, 50, 45–50. [Google Scholar] [CrossRef][Green Version]
- Woodward, P. Diagnostic Imaging: Obstetrics, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-323-79396-4. [Google Scholar]
- Meizner, I.; Levy, A.; Katz, M.; Maresh, A.J.; Glezerman, M. Fetal ovarian cysts: Prenatal ultrasonographic detection and postnatal evaluation and treatment. Am. J. Obstet. Gynecol. 1991, 164, 874–878. [Google Scholar] [CrossRef]
- Garcia-Aguilar, P.; Maiz, N.; Rodó, C.; Garcia-Manau, P.; Arévalo, S.; Molino, J.A.; Guillen, G.; Carreras, E. Fetal Abdominal Cysts: Predicting Adverse Outcomes. Acta Obstet. Gynecol. Scand. 2023, 102, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.T.; Franco, A.; Hoossainy, S.; Lewis, K.N. Daughter Cyst Sign. J. Radiol. Case Rep. 2012, 6, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Woo, S.K.; Kim, J.S.; Suh, S.J. “Daughter Cyst” Sign: A Sonographic Finding of Ovarian Cyst in Neonates, Infants, and Young Children. AJR Am. J. Roentgenol. 2000, 174, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, L.; Paris, F.; Nicolino, M.; Hameury, F.; Bonnaure, H.; Pienkowski, C.; Servant, N.; Kalfa, N.; Sultan, C. Fetal Ovarian Cysts: An Early Manifestation of McCune-Albright Syndrome? Prenat. Diagn. 2012, 32, 859–863. [Google Scholar] [CrossRef]
- Heling, K.-S.; Chaoui, R.; Kirchmair, F.; Stadie, S.; Bollmann, R. Fetal Ovarian Cysts: Prenatal Diagnosis, Management and Postnatal Outcome. Ultrasound Obstet. Gynecol. 2002, 20, 47–50. [Google Scholar] [CrossRef]
- Abolmakarem, H.; Tharmaratnum, S.; Thilaganathan, B. Fetal Anemia as a Consequence of Hemorrhage into an Ovarian Cyst. Ultrasound Obstet. Gynecol. 2001, 17, 527–528. [Google Scholar] [CrossRef]
- Brans, Y.W.; Hayashi, R.H. Prenatal diagnosis of fetal abdominal masses by ultrasonography. Am. J. Perinatol. 1986, 3, 189–191. [Google Scholar] [CrossRef]
- Sherer, D.M.; Shah, Y.G.; Eggers, P.C.; Woods, J.R. Prenatal Sonographic Diagnosis and Subsequent Management of Fetal Adnexal Torsion. J. Ultrasound Med. 1990, 9, 161–163. [Google Scholar] [CrossRef]
- Bornstein, E.; Barnhard, Y.; Ferber, A.; Segarra, P.; Divon, M.Y. Acute Progression of a Unilateral Fetal Ovarian Cyst to Complex Bilateral Cysts Causing Acute Polyhydramnios. J. Ultrasound Med. 2006, 25, 523–526. [Google Scholar] [CrossRef]
- Foley, P.T.; Ford, W.D.A.; McEwing, R.; Furness, M. Is Conservative Management of Prenatal and Neonatal Ovarian Cysts Justifiable? Fetal Diagn. Ther. 2005, 20, 454–458. [Google Scholar] [CrossRef]
- Erol, O.; Erol, M.B.; İsenlik, B.S.; Özkiraz, S.; Karaca, M. Prenatal Diagnosis of Fetal Ovarian Cyst: Case Report and Review of the Literature. J. Turk. Ger. Gynecol. Assoc. 2013, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- David, Y.B.; Sela, N.; David, C.B.; Dujovni, T. Case of Fetal Ovarian Juvenile Granulosa Cell Tumor: Complications and Management. J. Obstet. Gynaecol. Res. 2021, 47, 2220–2224. [Google Scholar] [CrossRef] [PubMed]
- Capito, C.; Flechtner, I.; Thibaud, E.; Emond, S.; Kalfa, N.; Jaubert, F.; Sarnacki, S. Neonatal Bilateral Ovarian Sex Cord Stromal Tumors. Pediatr. Blood Cancer 2009, 52, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, E.E. Bilateral Ovarian Carcinoma in a 30 Week Fetus. Arch. Pathol. 1945, 40, 279–282. [Google Scholar]
- Dinu, M.; Stancioi-Cismaru, A.F.; Gheonea, M.; Luciu, E.D.; Aron, R.M.; Pana, R.C.; Marinas, C.M.; Degeratu, S.; Sorop-Florea, M.; Carp-Veliscu, A.; et al. Intrauterine Growth Restriction—Prediction and Peripartum Data on Hospital Care. Medicina 2023, 59, 773. [Google Scholar] [CrossRef]
- Safa, N.; Yanchar, N.; Puligandla, P.; Sewitch, M.; Baird, R.; Beaunoyer, M.; Campbell, N.; Chadha, R.; Griffiths, C.; Jones, S.; et al. Treatment and Outcomes of Congenital Ovarian Cysts A Study by the Canadian Consortium for Research in Pediatric Surgery (CanCORPS). Ann. Surg. 2023, 277, e1130–e1137. [Google Scholar] [CrossRef]
- Han, L.; Shi, G.; Zheng, A.; Ruan, J. Laparoscopic Management of Neonatal Ovarian Torsion. J. Minim. Invasive Gynecol. 2024. [Google Scholar] [CrossRef]
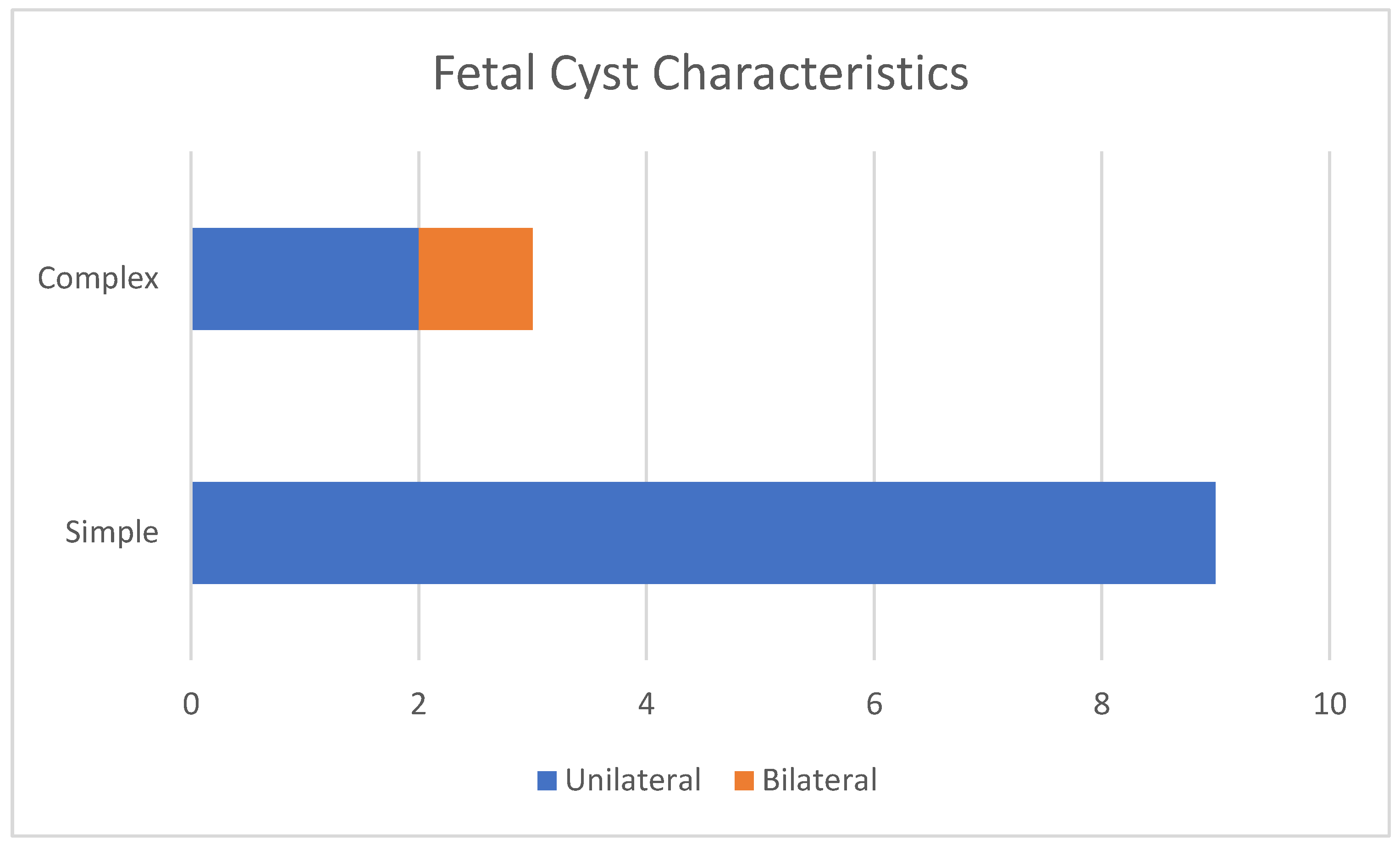
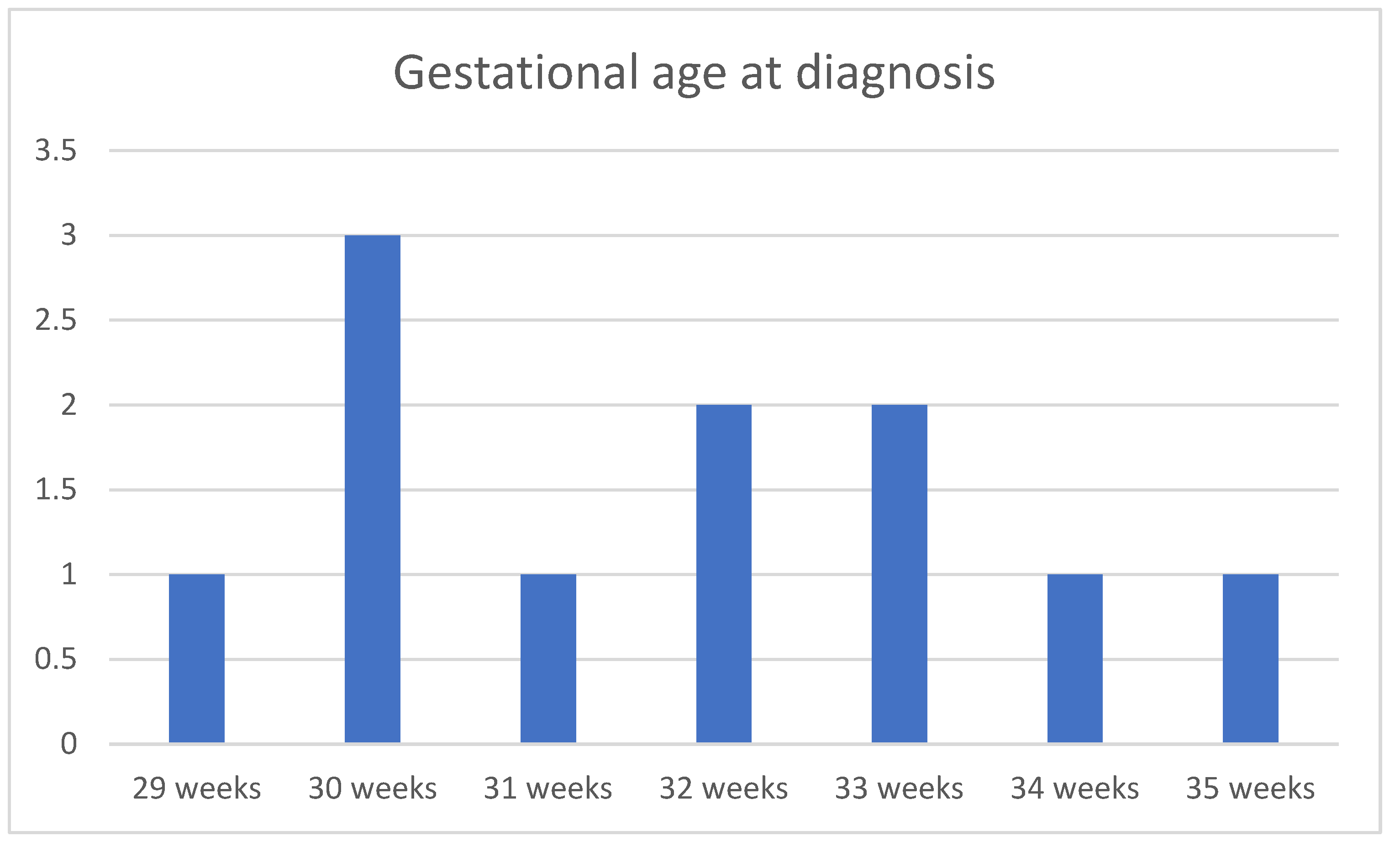
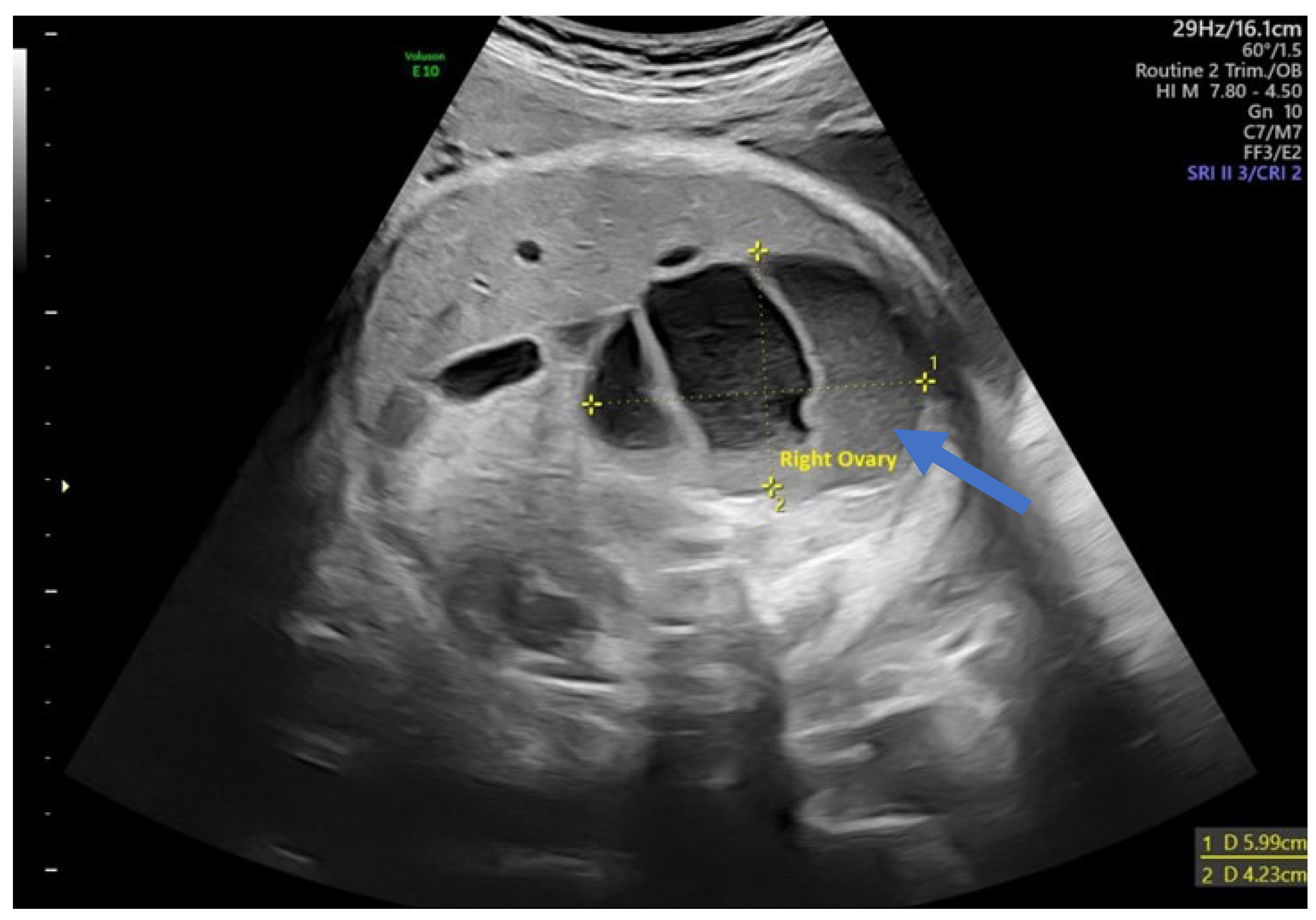

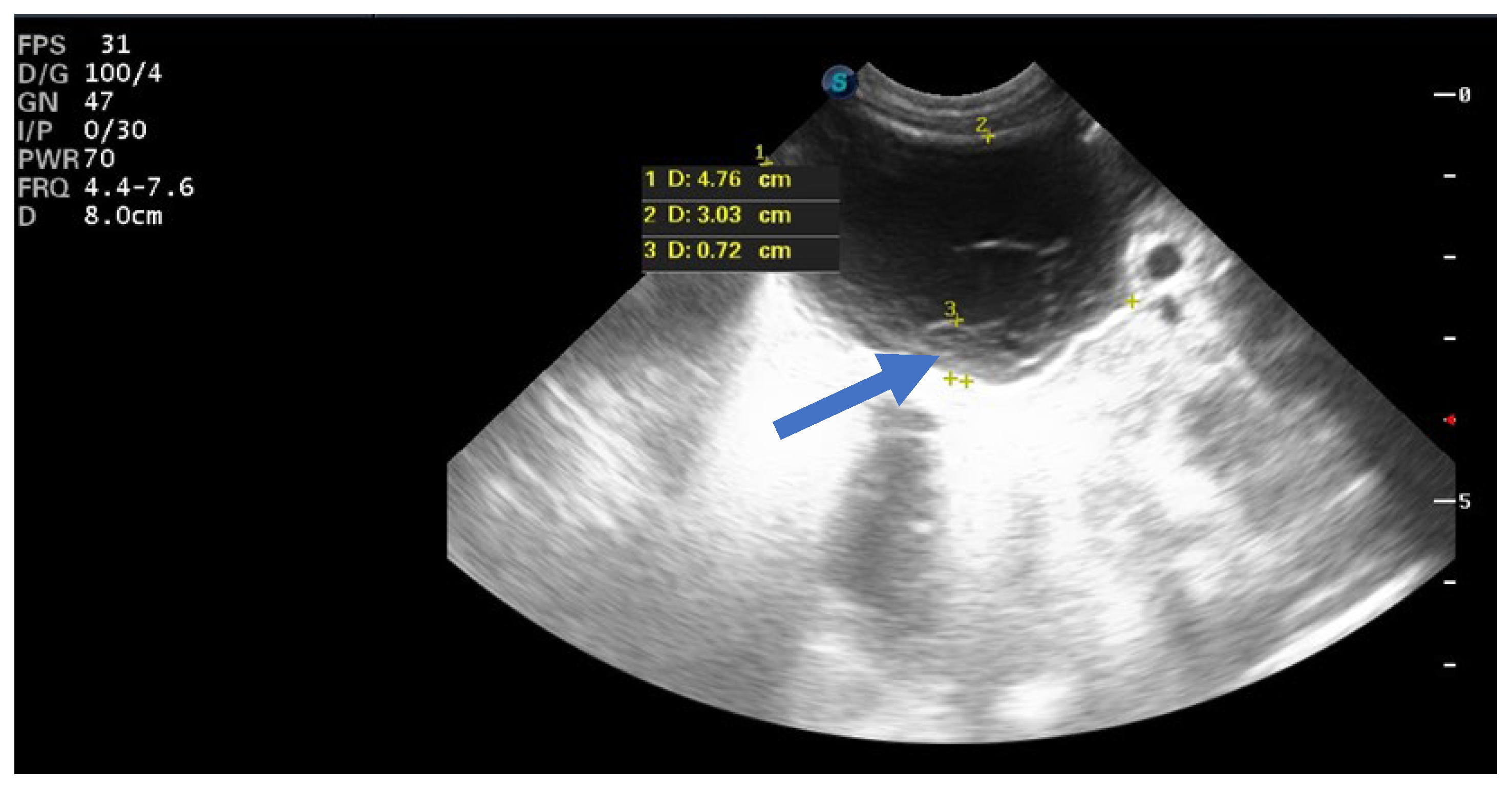
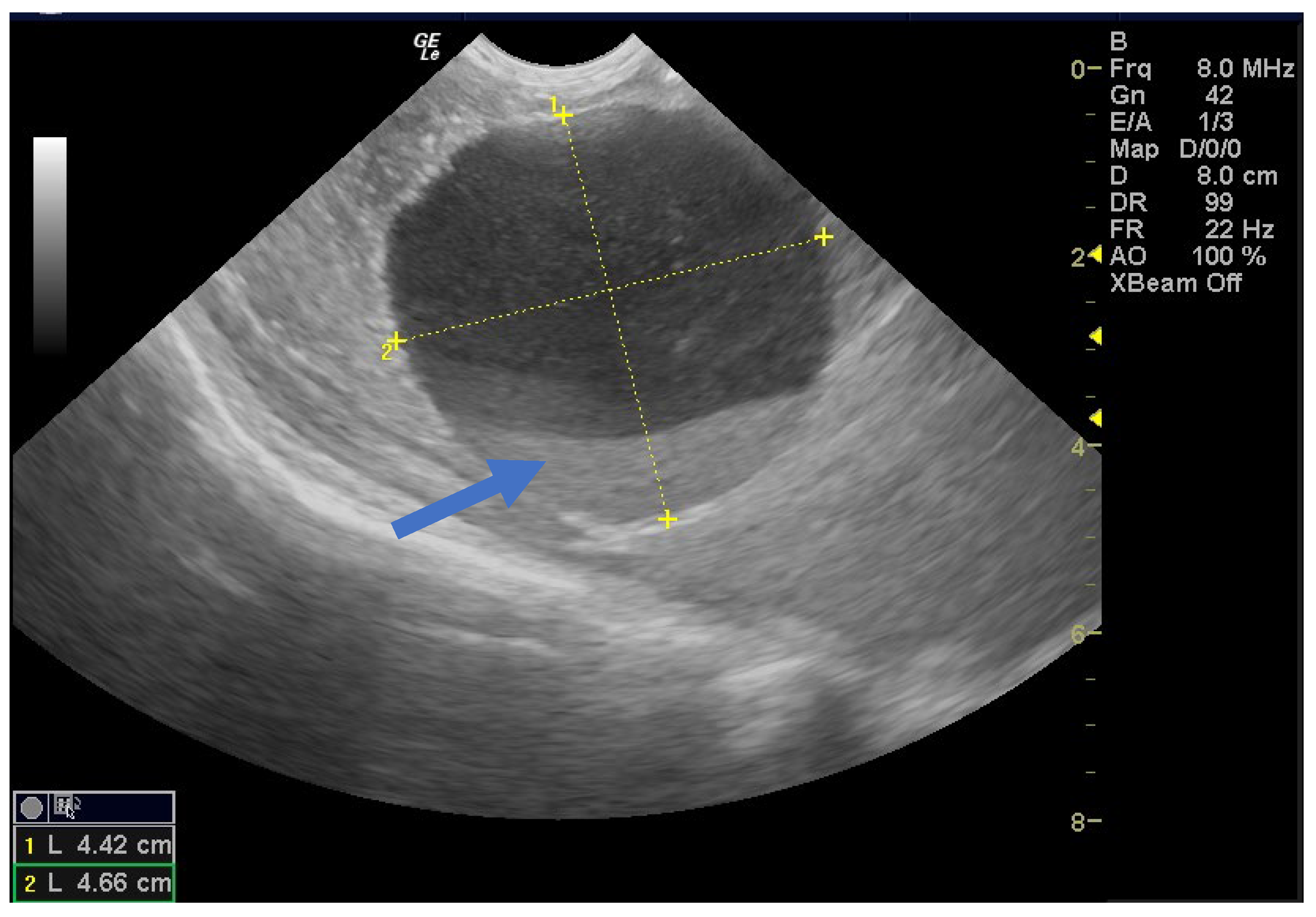


| Case No. | Maternal Age | Gestational Age of Diagnosis | Characteristics of the Cysts | Unilateral or Bilateral Ovarian Cysts | Gestational Age at Delivery (weeks) | Birth Weight (g) |
|---|---|---|---|---|---|---|
| 1 | 30 | 33 weeks | Simple | Unilateral | 38 | 3030 |
| 2 | 27 | 29 weeks | Simple | Unilateral | 39 | 3340 |
| 3 | 26 | 31 weeks | Complex | Bilateral | 36 | 2780 |
| 4 | 35 | 35 weeks | Complex | Unilateral | 39 | 3600 |
| 5 | 27 | 30 weeks | Simple | Unilateral | 38 | 3200 |
| 6 | 29 | 30 weeks | Simple | Unilateral | 39 | 3150 |
| 7 | 31 | 32 weeks | Simple | Unilateral | 38 | 3000 |
| 8 | 35 | 30 weeks | Simple | Unilateral | 39 | 3540 |
| 9 | 26 | 33 weeks | Simple | Unilateral | 39 | 2780 |
| 10 | 30 | 32 weeks | Simple | Unilateral | 39 | 2870 |
| 11 | 34 | 34 weeks | Complex | Unilateral | 38 | 3160 |
| 12 | 28 | 33 weeks | Simple | Unilateral | 37 | 2980 |
| Case No. | Characteristics of the Cysts | Unilateral or Bilateral Ovarian Cysts | Size at Diagnosis (cm) | Cyst Evolution 2 Months Postpartum |
|---|---|---|---|---|
| 1 | Simple | Unilateral | 1.5 | Disappeared |
| 2 | Simple | Unilateral | 3.4 | Disappeared |
| 3 | Complex | Bilateral | 5 | Torsion of the right ovary. Emergency laparoscopy |
| 4 | Complex | Unilateral | 2.9 | Lost to follow-up |
| 5 | Simple | Unilateral | 4.2 | Expectative |
| 6 | Simple | Unilateral | 3.8 | Disappeared |
| 7 | Simple | Unilateral | 3.1 | Disappeared |
| 8 | Simple | Unilateral | 3.5 | Disappeared |
| 9 | Simple | Unilateral | 1 | Disappeared |
| 10 | Simple | Unilateral | 4.5 | Expectative |
| 11 | Complex | Unilateral | 4 | Expectative |
| 12 | Simple | Unilateral | 3.3 | Disappeared |
| Study (Year) | No. of Patients | Mean GA at Diagnosis | Prenatal Treatment | Outcomes |
|---|---|---|---|---|
| Bagolan et al. (2002) [11] | 73 (single-center study) | ~33–34 weeks (range 23–39) | Ultrasound-guided in utero aspiration (IUA) for cysts >5 cm or rapidly growing; otherwise, observation | ~60% of cysts spontaneously resolved (no intervention); ~40% required postnatal surgery (for persistence or complications); high torsion rate (~55%) noted, mostly in larger cysts; and all surgically treated cases resulted in oophorectomy (no ovarian tissue could be saved). |
| Mittermayer et al. (2003) [12] | 61 (single-center study) | ~32 weeks (late 2nd to 3rd trimester, 1991–2000) | Expectant management for all; IUA attempted in 2 cases (large cysts) | 8/61 cysts regressed in utero, and an additional 35 cysts (in 40 fetuses) resolved postnatally with observation; 17 cysts required intervention: 14 underwent postnatal surgery due to persistence/enlargement (12 were benign follicular/theca lutein cysts, 1 lymphangioma, and 1 teratoma); 2 were aspirated in utero, and 1 after birth, resulting in cyst resolution. |
| Kwak et al. (2006) [13] | 17 (single-center study) | 34 weeks (range 30–38) | Expectant management prenatally (no aspirations performed) | 1 cyst (6%) regressed antenatally; 9 (53%) resolved spontaneously after birth (including 2 initially complex cysts); 7 infants (41%) underwent postnatal surgery due to large, persistent cysts or symptoms; ovarian torsion was confirmed in 4 of these 7. |
| Hara et al. (2021) [14] | 36 (single-center study) | 32 weeks (median; range 27–37) | Expectant management prior to 2018; after 2018, IUA for simple cysts ≥40 mm before 37 weeks; if the cyst ≥40 mm persisted at ≥37 weeks, labor was induced to facilitate early neonatal surgery | 29 simple and 7 complex cysts were diagnosed; among simple cysts <40 mm (n = 14), 12 remained simple and 2 (14%) became complex during follow-up evaluation—no torsion occurred in cysts <35 mm; Simple cysts ≥40 mm (n = 15): 3 (20%) progressed to complex (2 of which were confirmed ovarian necrosis at surgery); IUA was performed in 4 cases of large simple cysts, and ovaries were preserved in all 4 (no torsion after aspiration); overall, this strategy led to ovarian preservation in all cases that underwent prenatal aspiration, whereas two large cysts not aspirated resulted in torsion and ovarian loss. |
| Melinte-Popescu et al. (2023) [15] | 20 (single-center study) | ~34–35 weeks (median ~35) | No prenatal interventions (serial ultrasound follow-up evaluation for all) | Simple cysts ≤4 cm (n = 10): 7 (70%) completely resorbed and 3 (30%) reduced in size without complications; Simple cysts >4 cm (n = 3): only 1 (33%) showed size reduction; 2 (67%) developed ovarian torsion (autoamputation) during follow-up evaluation; Complex cysts (n = 4) detected prenatally: 1 (25%) resorbed, 1 (25%) reduced, and 2 (50%) had ovarian torsion; an additional 3 cysts were first detected postnatally (2 simple ≤4 cm, 1 complex ~4 cm) and all three of those cysts regressed or reduced spontaneously over time. |
| Chen et al. (2020 ) [16] | 96 (single-center study) | 32 | No prenatal interventions | 83 resolved spontaneously; 13 required surgery (69% ovarian preservation). |
| Nakamura et al. (2015) [17] | 31 | 32 | No prenatal interventions | 17 resolved spontaneously; 14 required surgery (79% ovarian preservation). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Văduva, C.-C.; Dira, L.; Iliescu, D.; Ruican, D.; Siminel, A.-M.; Stoica, G.A.; Şerbănescu, M.-S.; Carp-Velișcu, A. Clinical Experience in the Management of a Series of Fetal–Neonatal Ovarian Cysts. Children 2025, 12, 934. https://doi.org/10.3390/children12070934
Văduva C-C, Dira L, Iliescu D, Ruican D, Siminel A-M, Stoica GA, Şerbănescu M-S, Carp-Velișcu A. Clinical Experience in the Management of a Series of Fetal–Neonatal Ovarian Cysts. Children. 2025; 12(7):934. https://doi.org/10.3390/children12070934
Chicago/Turabian StyleVăduva, Constantin-Cristian, Laurentiu Dira, Dominic Iliescu, Dan Ruican, Anișoara-Mirela Siminel, George Alin Stoica, Mircea-Sebastian Şerbănescu, and Andreea Carp-Velișcu. 2025. "Clinical Experience in the Management of a Series of Fetal–Neonatal Ovarian Cysts" Children 12, no. 7: 934. https://doi.org/10.3390/children12070934
APA StyleVăduva, C.-C., Dira, L., Iliescu, D., Ruican, D., Siminel, A.-M., Stoica, G. A., Şerbănescu, M.-S., & Carp-Velișcu, A. (2025). Clinical Experience in the Management of a Series of Fetal–Neonatal Ovarian Cysts. Children, 12(7), 934. https://doi.org/10.3390/children12070934







