Polyhydramnios at Term in Gestational Diabetes: Should We Be Concerned?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Diagnosis of Gestational Complications and Adverse Maternal Outcomes
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. Clinical and Anthropometric Characteristics
3.2. AFV and Their Association with Adverse Maternal and Perinatal Outcomes
3.3. Logistic Regression Analysis
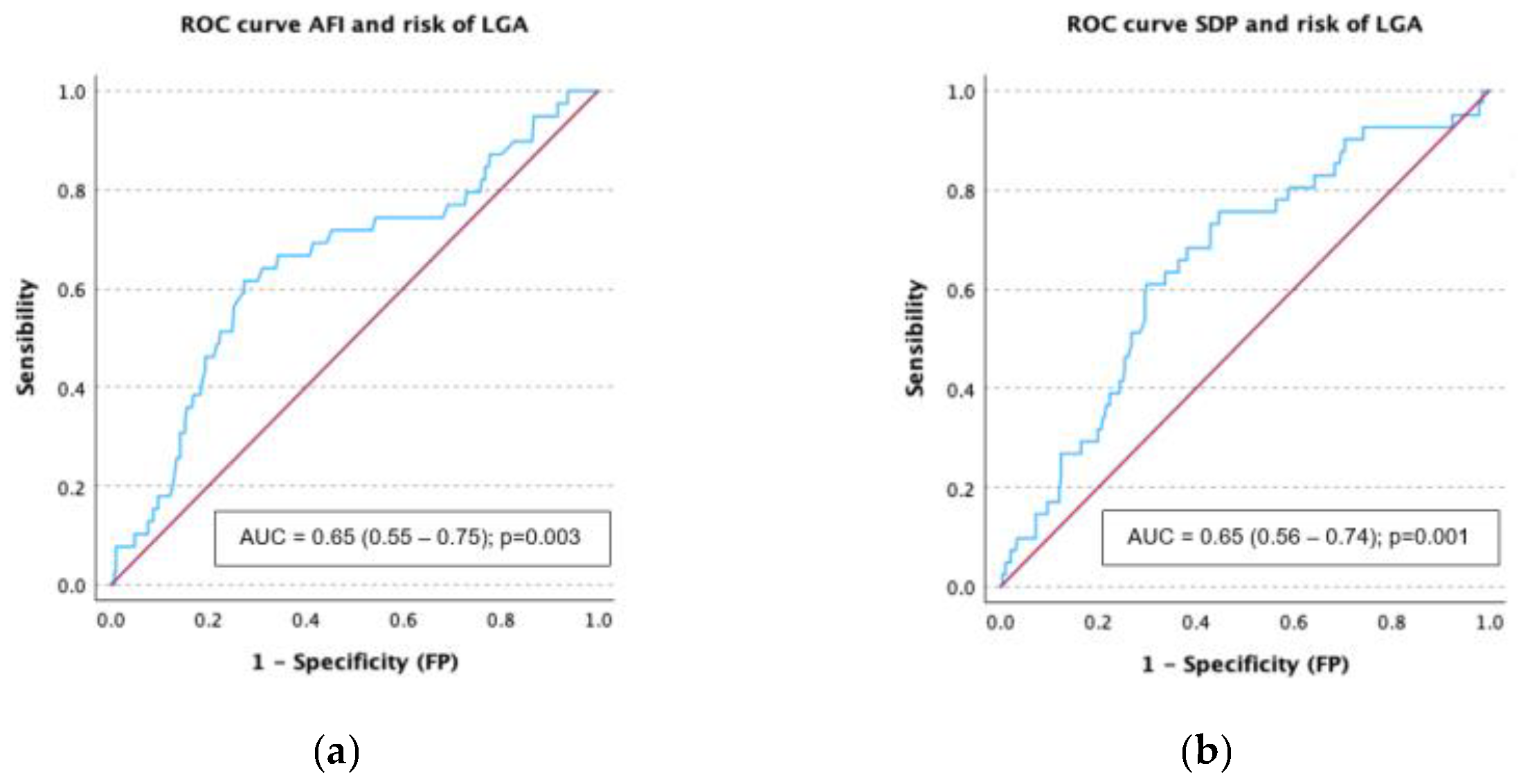
3.4. Receiver Operating Characteristic (ROC) Curves Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFV | Amniotic Fluid Volume |
| AFI | Amniotic Fluid Index |
| APO | Adverse Perinatal Outcomes |
| BMI | Body Mass Index (kg/m2) |
| GDM | Gestational Diabetes Mellitus |
| LGA | Large for Gestational Age fetus |
| SDP | Single Deepest Pocket |
| SGA | Small for Gestational Age fetus |
| IUFD | Intrauterine Fetal Demise |
| IUGR | Intrauterine Growth Restricted Fetus |
References
- Dashe, J.S.; Pressman, E.K.; Hibbard, J.U. SMFM Consult Series #46: Evaluation and management of polyhydramnios. Am. J. Obstet. Gynecol. 2018, 219, B2–B8. [Google Scholar]
- Reddy, U.M. Fetal imaging: Executive summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American institute of Ultrasound in Medicine, ACOG, American College of Radiology, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound Fetal imaging workshop. Am. J. Obstet. Gynecol. 2014, 123, 1070. [Google Scholar]
- Whittington, J.R.; Chauhan, S.P.; Wendel, M.P.; Ghahremani, T.L.; Pagan, M.E.; Carter, M.M.; Magann, E.F. Historical Assessment, Practical Management, and Future Recommendations for Abnormal Amniotic Fluid Volumes. J. Clin. Med. 2024, 13, 4702. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Alfirevic, Z.; Da Silva Costa, F.; Deter, R.L.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG Practice Guidelines: Ultrasound assessment of fetal biometry and growth. Ultrasound Obstet. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.S.; Magann, E.F.; Whittington, J.R.; Wendel, M.P.; Sandlin, A.T.; Ounpraseuth, S.T. Accuracy of the Ultrasound Estimate of the Amniotic Fluid Volume (Amniotic Fluid Index and Single Deepest Pocket) to Identify Actual Low, Normal, and High Amniotic Fluid Volumes as Determined by Quantile Regression. J. Ultrasound Med. 2020, 39, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Magann, E.F.; Whittington, J.R.; Morrison, J.C.; Chauhan, S.P. Amniotic Fluid Volume Assessment: Eight Lessons Learned. Int. J. Womens Health 2021, 13, 773–779. [Google Scholar] [CrossRef]
- Kechagias, K.S.; Triantafyllidis, K.K.; Zouridaki, G.; Savvidou, M. Obstetric and neonatal outcomes in pregnant women with idiopathic polyhydramnios: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 5296. [Google Scholar] [CrossRef]
- Pagan, M.; Magann, E.F.; Rabie, N.; Steelman, S.C.; Hu, Z.; Ounpraseuth, S. Idiopathic polyhydramnios and pregnancy outcome: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2023, 61, 302–309. [Google Scholar] [CrossRef]
- Karkia, R.; Giacchino, T.; Hii, F.; Bradshaw, C.; Ramadan, G.; Akolekar, R. Gestational diabetes mellitus: Relationship of adverse outcomes with severity of disease. J. Matern.-Fetal Neonatal Med. 2024, 37, 2356031. [Google Scholar] [CrossRef]
- Morris, R.; Meller, C.; Tamblyn, J.; Malin, G.; Riley, R.; Kilby, M.D.; Robson, C.C.; Khan, K.S. Association and prediction of amniotic fluid measurements for adverse pregnancy outcome: Systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 686–699. [Google Scholar] [CrossRef]
- Dashe, J.S.; Nathan, L.; McIntire, D.D.; Leveno, K.J. Correlation between amniotic fluid glucose concentration and amniotic fluid volume in pregnancy complicated by diabetes. Am. J. Obstet. Gynecol. 2000, 182, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Sotiriadis, A.; D’Antonio, F.; Da Silva Costa, F.; Odibo, A.; Prefumo, F.; Papageorghiou, A.T.; Salomon, L.J. ISUOG Practice Guidelines: Performance of third-trimester obstetric ultrasound scan. Ultrasound Obstet. Gynecol. 2024, 63, 131–147. [Google Scholar] [CrossRef] [PubMed]
- National Diabetes Data Group. Classification and Diagnosis of Diabetes Mellitus and Other Categories of Glucose Intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef]
- Goya, M.; Codina, M. Diabetes mellitus and pregnancy. Updated clinical practice guideline 2021. Executive summary. Endocrinol. Diabetes Y Nutr. 2023, 70, 1–6. [Google Scholar] [CrossRef]
- Figueras, F.; Meler, E.; Iraola, A.; Eixarch, E.; Coll, O.; Figueras, J.; Francis, A.; Gratacos, E.; Gardosi, J. Customized birthweight standards for a Spanish population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 20–24. [Google Scholar] [CrossRef]
- Lees, C.C.; Stampalija, T.; Baschat, A.A.; Da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Poon, L.C.; Salomon, L.J.; Unterscheider, J. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Barbouni, K.; Jotautis, V.; Metallinou, D.; Diamanti, A.; Orovou, E.; Liepinaitienė, A.; Nikolaidis, P.; Karampas, G.; Sarantaki, A. When Weight Matters: How Obesity Impacts Reproductive Health and Pregnancy-A Systematic Review. Curr. Obes. Rep. 2025, 14, 37. [Google Scholar] [CrossRef]
- Ramonienė, G.; Malakauskienė, L.; Savukynė, E.; Maleckienė, L.; Gruzdaitė, G. Pregnancy Complications and Outcomes in Obese Women with Gestational Diabetes. Med. Kaunas Lith. 2025, 61, 51. [Google Scholar] [CrossRef]
- Ramos, S.Z.; Lewkowitz, A.K.; Lord, M.G.; Has, P.; Danilack, V.A.; Savitz, D.A.; Werner, E.F. Predicting primary cesarean delivery in pregnancies complicated by gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2023, 229, 549.e1–549.e16. [Google Scholar] [CrossRef]
- Chen, D.; Gao, X.; Yang, T.; Xin, X.; Wang, G.; Wang, H.; He, R.; Liu, M. Independent risk factors for placental abruption: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2025, 25, 351. [Google Scholar] [CrossRef]
- Crimmins, S.; Mo, C.; Nassar, Y.; Kopelman, J.; Turan, O. Polyhydramnios or Excessive Fetal Growth Are Markers for Abnormal Perinatal Outcome in Euglycemic Pregnancies. Am. J. Perinatol. 2018, 35, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, Y.; Yin, Z.; Wei, L.; Lv, B.; Wu, Y. Establishment of a nomogram model to predict macrosomia in pregnant women with gestational diabetes mellitus. BMC Pregnancy Childbirth 2021, 21, 581. [Google Scholar] [CrossRef] [PubMed]
- Shulman, Y.; Shah, B.R.; Berger, H.; Yoon, E.W.; Helpaerin, I.; Mei-Dan, E.; Aviram, A.; Retnakaran, R.; Melamed, N. Prediction of birthweight and risk of macrosomia in pregnancies complicated by diabetes. Am. J. Obstet. Gynecol. MFM 2023, 5, 101042. [Google Scholar] [CrossRef]
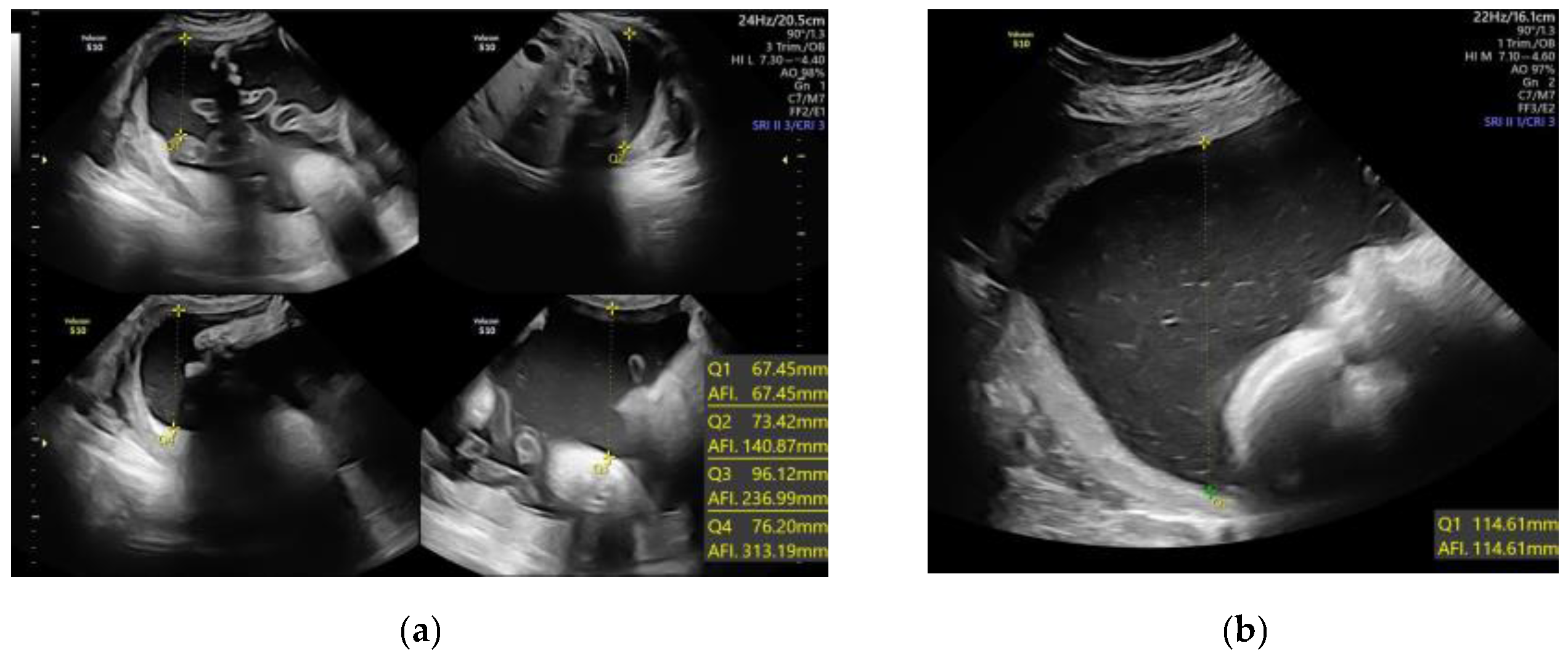


| AFI ≥ 24 cm or SDP ≥ 8 cm (Standard Definition) | AFI ≥ 18 cm or SDP ≥ 6.5 cm (>75th Centile) | |||||
|---|---|---|---|---|---|---|
| Normal AFV (n = 305) | Polyhydramnios (n = 35) | p Value | Normal AFV (n = 224) | Increased AFV (n = 116) | p Value | |
| Maternalcharacteristics | ||||||
| Maternal age (years) | 34 ± 5.1 | 35.4 ± 5.9 | p = 0.50 | 34.7 ± 5.1 | 35.2 ± 5.2 | p = 0.42 |
| Height (m) | 1.62 ± 0.06 | 1.62 ± 0.06 | p = 0.90 | 1.61 ± 0.06 | 1.62 ± 0.06 | p = 0.23 |
| Pregestational weight (Kg) | 73.6 ± 17.2 | 79.9 ± 21.3 | p = 0.04 * | 72.3 ± 16.0 | 77.9 ± 20.4 | p = 0.01 * |
| Pregestational BMI (Kg/m2) | 27.9 ± 6.0 | 30.3 ± 7.4 | p = 0.03 * | 27.5 ± 5.8 | 29.3 ± 6.9 | p = 0.01 * |
| Nulliparous (n, %) | 155 (50.8) | 18 (51.4) | p = 0.94 | 127 (56.7) | 46 (37.9) | p = 0.003 * |
| Weight gain (kg) | 7.9 ± 5.8 | 6.7 ± 6.3 | p = 0.26 | 7.7 ± 5.9 | 7.9 ± 5.9 | p = 0.80 |
| Smoking (n, %) | 42 (13.9) | 7 (20.6) | p = 0.26 | 27 (12.2) | 22 (19.3) | p = 0.08 |
| Insulin treatment (n, %) | 120 (39.5) | 14 (40) | p = 0.95 | 80 (35.7) | 54 (47) | p = 0.04 * |
| AFV measurements | ||||||
| Gestational age at US scan | 37 (37, 38) | 38 (37,38) | p = 0.53 | 37 (37, 38) | 37 (37, 38) | p = 0.28 |
| AFI (cm) | 14.5 ± 4.0 | 23.10 ± 4.4 | p = 0.001 * | 12.9 ± 3.0 | 20.1 ± 4.1 | p = 0.001 * |
| SDP (cm) | 5.3 ± 1.1 | 8.8 ± 7.9 | p = 0.001 * | 4.8 ± 0.9 | 7.3 ± 1.2 | p = 0.001 * |
| Newborn characteristics | ||||||
| Gestational age at birth (weeks) | 39 (38, 40) | 39 (39, 40) | p = 0.17 | 39 (38, 40) | 39 (39, 40) | p = 0.84 |
| Newborn male sex (n, %) | 174 (57) | 17 (48.6) | p = 0.33 | 123 (54.9) | 68 (58.6) | p = 0.51 |
| Newborn weight (g) | 3271 ± 466 | 3515 ± 420 | p = 0.02 * | 3242 ± 439 | 3538 ± 452 | p < 0.001 * |
| Newborn centile | 49 (20, 80) | 73 (36, 93) | p = 0.03 * | 39 (14, 64) | 75 (40, 94) | p < 0.001 * |
| AFI ≥ 24 cm or SDP ≥ 8 cm (Standard Definition) | AFI ≥ 18 cm or SDP ≥ 6.5 cm (75th Centile) | |||||
|---|---|---|---|---|---|---|
| Normal AFV (n = 305) | Polyhydramnios (n = 35) | p Value | Normal AFV (n = 224) | Increased AFV (n = 116) | p Value | |
| Gestationalcomplications (n, %) | ||||||
| Composite for Gestational complications | 84 (27.5) | 19 (54.3) | p < 0.001 * | 68 (30.4) | 35 (30.2) | p = 0.97 |
| Gestational hypertension | 7 (2.3) | 2 (5.7) | p = 0.23 | 6 (2.7) | 3 (2.6) | p = 0.96 |
| Preeclampsia | 4 (1.23) | 0 (0) | p = 0.98 | 4 (1.8) | 0 (0) | p = 0.30 |
| Hepatic cholestasis | 2 (0.7) | 0 (0) | p = 0.99 | 1 (0.4) | 1 (0.9) | p = 0.56 |
| Total cesarean deliveries | 78 (25.6) | 18 (51.4) | p = 0.001 * | 64 (28.6) | 32 (27.6) | p = 0.84 |
| Urgent cesarean deliveries | 62(20.3) | 12 (34.3) | p = 0.05 | 49 (21.9) | 25 (21.6) | p = 0.94 |
| Adverse perinatal outcomes (n, %) | ||||||
| Composite for APO | 141 (46.5) | 23 (65.7) | p = 0.03 * | 102 (45.9) | 62 (53.4) | p = 0.19 |
| SGA (centile < 10th) | 34 (11.1) | 3 (8.8) | p = 0.99 | 30 (13.6) | 7 (6.3) | p = 0.04 * |
| IUGR | 15 (4.9) | 1 (2.9) | p = 0.58 | 14 (6.3) | 2 (1.7) | p = 0.06 |
| LGA (centile > 90th) | 35 (11.5) | 7 (20) | p = 0.14 | 18 (8.2) | 24 (21.4) | p < 0.001 * |
| Macrosomia (>4000 g) | 30 (9.38) | 5 (14.3) | p = 0.41 | 12 (5.4) | 23 (19.8) | p < 0.001 * |
| Intrapartum fetal distress | 23 (7.6) | 4 (11.4) | p = 0.50 | 19 (8.5) | 8 (6.9) | p = 0.60 |
| Apgar < 7 at 5 min | 3 (1) | 0 (0) | p = 0.72 | 2 (0.9) | 1 (0) | p = 0.72 |
| Umbilical cord pH< 7.20 | 40 (7) | 7 (20) | p = 0.26 | 29 (13) | 18 (15.5) | p = 0.52 |
| NICU Admission | 25 (8.3) | 4 (8.6) | p = 0.52 | 18 (8.1) | 11 (9.5) | p = 0.65 |
| Shoulder dystocia | 2 (0.7) | 2 (5.7) | p = 0.05 | 1 (0.4) | 3 (2.6) | p = 0.11 |
| Respiratory distress | 5 (1.7) | 1 (2.9) | p = 0.48 | 2 (0.9) | 4 (3.4) | p = 0.10 |
| Hypoglycemia (<40 mg/dL) | 12 (3.9) | 1 (2.9) | p = 0.60 | 10 (4.5) | 3 (2.6) | p = 0.29 |
| Hyperbilirubinemia (phototherapy) | 13 (4.3) | 0 (0) | p = 0.23 | 11 (5) | 2 (1.7) | p = 0.11 |
| IUFD | 1 (0.3) | 0 (0) | p = 0.89 | 1 (0.4) | 0 (0) | p = 0.65 |
| Variable | AFI (cm) | SDP (cm) | ||
|---|---|---|---|---|
| aOR (95% IC) | p Value | aOR (95% IC) | p Value | |
| Composite of gestational complications | 1.032 (0.98–1.087) | p = 0.22 | 1.010 (0.995–1.026) | p = 0.19 |
| Gestational hypertension | 0.947 (0.814–1.101) | p = 0.47 | 1.004 (0.964–1.045) | p = 0.85 |
| Preeclampsia | 0.969 (0.779–1.206) | p = 0.78 | 0.961 (0.891–1.036) | p = 0.29 |
| Hepatic cholestasis | 0.955 (0.710–1.283) | p = 0.75 | 1.010 (0.929–1.097) | p = 0.82 |
| Cesarean delivery | 1.036 (0.982–1.093) | p = 0.17 | 1.012 (0.996–1.028) | p = 0.15 |
| Urgent cesarean delivery | 1.041 (0.982–1.105) | p = 0.18 | 1.008 (0.991–1.026) | p = 0.35 |
| Composite of APO | 1.016 (0.971–1.064) | p = 0.50 | 1.009 (0.995–1.024) | p = 0.19 |
| SGA (p < 10) | 0.893 (0.821–0.973) | p = 0.009 * | 0.975 (0.952–0.999) | p = 0.04 * |
| IUGR | 0.860 (0.748–0.990) | p = 0.03 * | 0.968 (0.932–1.005) | p = 0.08 |
| LGA (p > 90) | 1.110 (1.034–1.191) | p = 0.004 * | 1.029 (1.007–1.051) | p = 0.009 * |
| Macrosomia (>4000 g) | 1.127 (1.045–1.216) | p = 0.002 * | 1.024 (1.001–1.047) | p = 0.03 * |
| Fetal Distress | 0.974 (0.890–1.066) | p = 0.56 | 1.005 (0.980–1.031) | p = 0.68 |
| Apgar < 7 at 5 min | 1.087 (0.823–1.435) | p = 0.55 | 0.984 (0.912–1.062) | p = 0.67 |
| Umbilical cord pH < 7.20 | 1.035 (0.968–1.107) | p = 0.31 | 1.022 (1.003–1.042) | p = 0.02 * |
| NICU Admission | 0.998 (0.917–1.085) | p = 0.95 | 1.006 (0.981–1.031) | p = 0.64 |
| Shoulder dystocia | 1.122 (0.928–1.358) | p = 0.23 | 1.025 (0.968–1.085) | p = 0.40 |
| Respiratory distress | 1.121 (0.960–1.309) | p = 0.15 | 1.015 (0.971–1.062) | p = 0.51 |
| Hypoglycemia (<40 mg/dL) | 0.910 (0.799–1.038) | p = 0.16 | 0.983 (0.946–1.022) | p = 0.38 |
| Hyperbilirubinemia (phototherapy) | 0.959 (0.843–1.091) | p = 0.52 | 0.971 (0.931–1.012) | p = 0.16 |
| IUFD | 0.696 (0.363–1.335) | p = 0.27 | 0.931 (0.788–1.099) | p = 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horcas-Martín, M.; Luque-Patiño, T.; Usandizaga-Prat, C.; Díaz-Fernández, E.; Melero-Jiménez, V.; Vázquez-Fonseca, L.; Visiedo, F.; Broullón-Molanes, J.R.; Quintero-Prado, R.; Bugatto, F. Polyhydramnios at Term in Gestational Diabetes: Should We Be Concerned? Children 2025, 12, 920. https://doi.org/10.3390/children12070920
Horcas-Martín M, Luque-Patiño T, Usandizaga-Prat C, Díaz-Fernández E, Melero-Jiménez V, Vázquez-Fonseca L, Visiedo F, Broullón-Molanes JR, Quintero-Prado R, Bugatto F. Polyhydramnios at Term in Gestational Diabetes: Should We Be Concerned? Children. 2025; 12(7):920. https://doi.org/10.3390/children12070920
Chicago/Turabian StyleHorcas-Martín, Mercedes, Tania Luque-Patiño, Claudia Usandizaga-Prat, Elena Díaz-Fernández, Victoria Melero-Jiménez, Luis Vázquez-Fonseca, Francisco Visiedo, José Román Broullón-Molanes, Rocío Quintero-Prado, and Fernando Bugatto. 2025. "Polyhydramnios at Term in Gestational Diabetes: Should We Be Concerned?" Children 12, no. 7: 920. https://doi.org/10.3390/children12070920
APA StyleHorcas-Martín, M., Luque-Patiño, T., Usandizaga-Prat, C., Díaz-Fernández, E., Melero-Jiménez, V., Vázquez-Fonseca, L., Visiedo, F., Broullón-Molanes, J. R., Quintero-Prado, R., & Bugatto, F. (2025). Polyhydramnios at Term in Gestational Diabetes: Should We Be Concerned? Children, 12(7), 920. https://doi.org/10.3390/children12070920







