Three Decades of Managing Pediatric Obstructive Sleep Apnea Syndrome: What’s Old, What’s New
Abstract
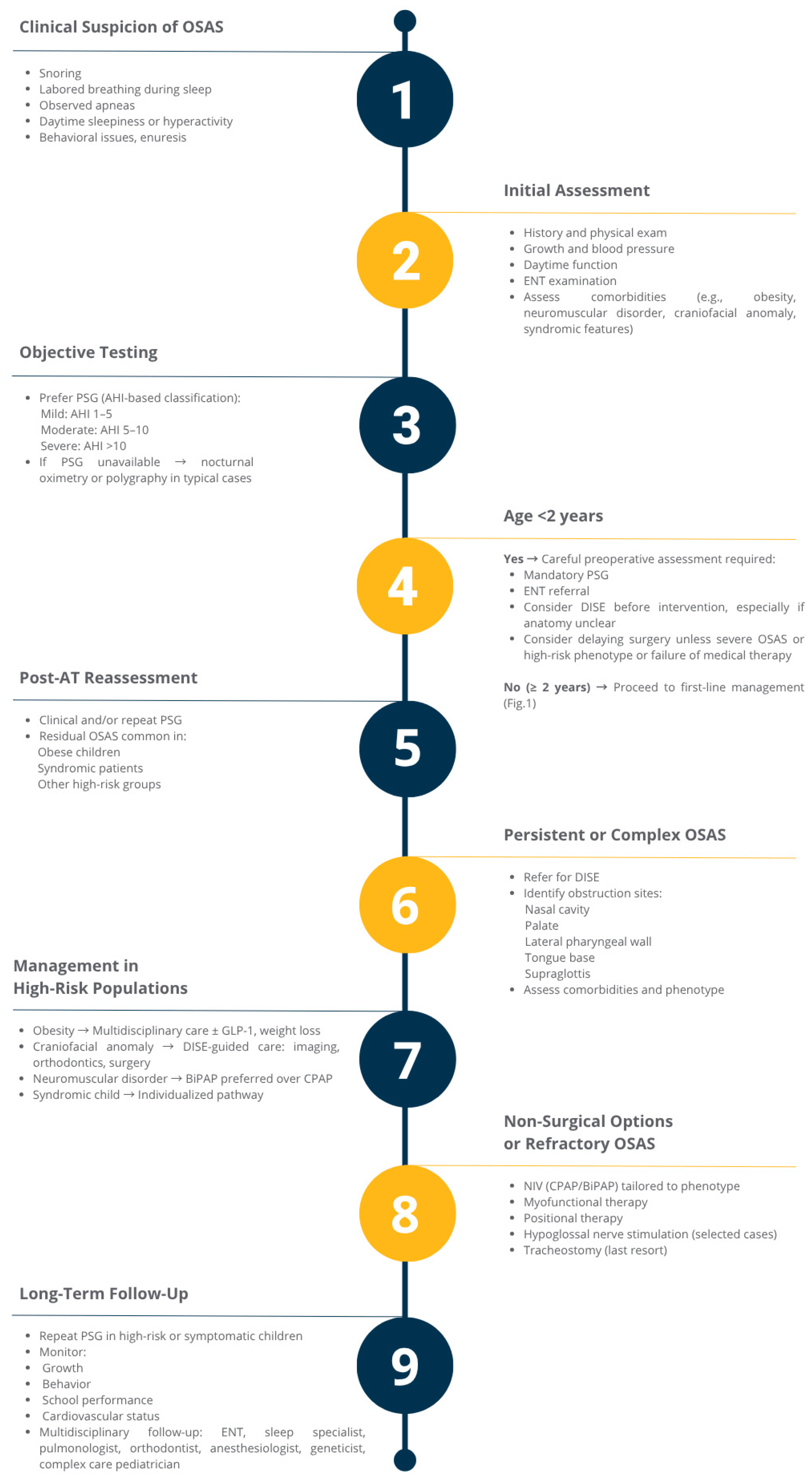
1. Introduction
2. Materials and Methods
3. Surgical Approaches
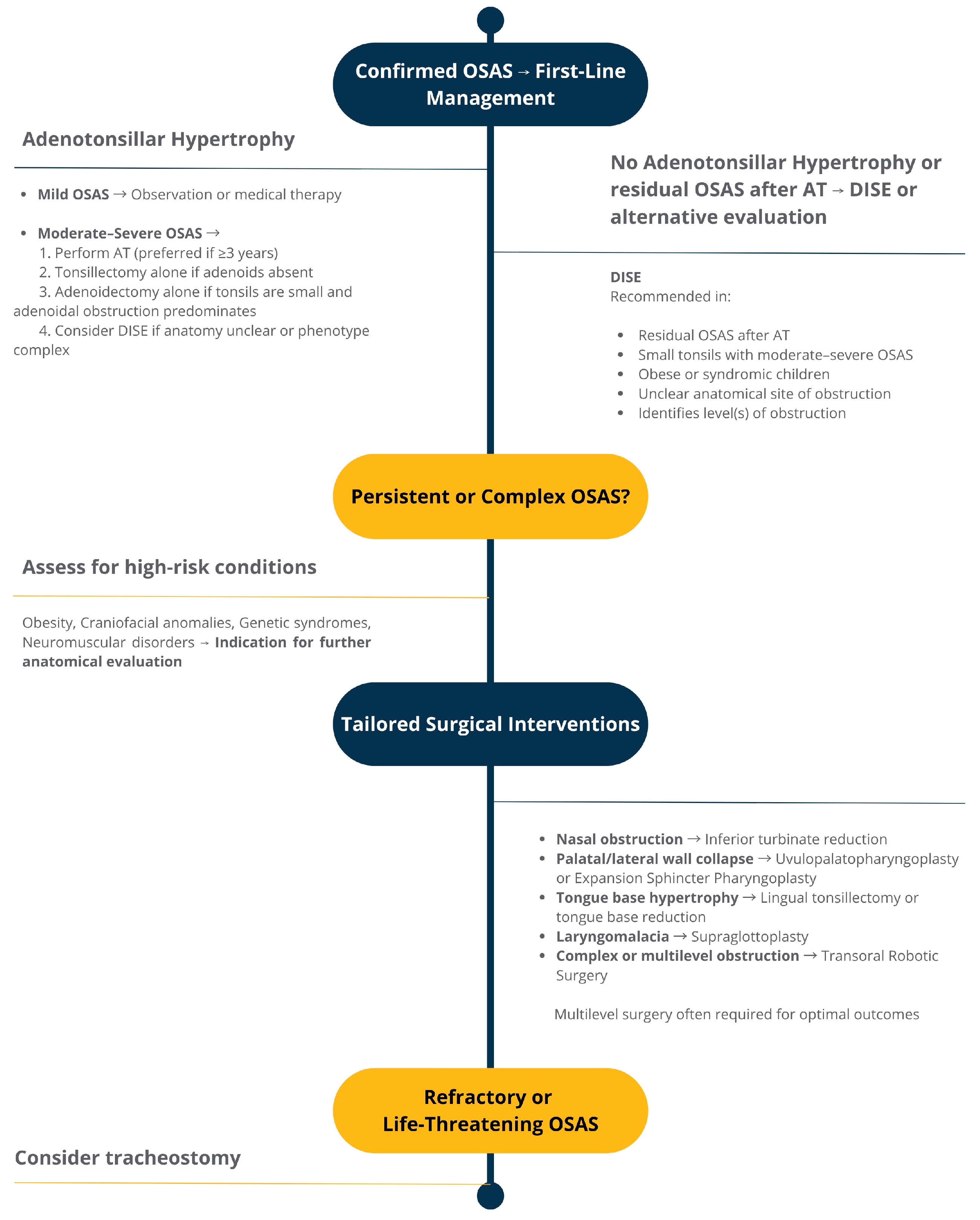
3.1. Adenotonsillectomy
3.2. Tonsillectomy
3.3. Adjunctive and Alternative Surgical Procedures for Residual or Complex OSAS
4. Non-Surgical Therapies for Pediatric Obstructive Sleep Apnea
4.1. Pharmacological Management
4.1.1. Intranasal Corticosteroids (INS)
4.1.2. Leukotriene Receptor Antagonists (Montelukast)
4.1.3. Systemic Corticosteroids and Preoperative Medical Therapy
4.2. Respiratory Support: CPAP and Beyond
4.2.1. Non-Invasive Ventilation
4.2.2. High-Flow Nasal Cannula
4.3. Orthodontic and Myofunctional Interventions
5. Innovative and Investigational Treatments for Pediatric Obstructive Sleep Apnea
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHI | Apnea–Hypopnea Index |
| APAP | Auto-adjusting Positive Airway Pressure |
| AT | Adenotonsillectomy |
| BPAP | Bilevel Positive Airway Pressure |
| CPAP | Continuous Positive Airway Pressure |
| DS | Down Syndrome |
| ENT | Ear, Nose, and Throat |
| ESP | Expansion Sphincter Pharyngoplasty |
| HFNC | High-Flow Nasal Cannula |
| HNS | Hypoglossal Nerve Stimulation |
| INS | Intranasal Corticosteroids |
| LTRA | Leukotriene Receptor Antagonists |
| MAA | Mandibular Advancement Appliances |
| MT | Myofunctional Therapy |
| OSAS | Obstructive Sleep Apnea Syndrome |
| PAP | Positive Airway Pressure |
| PMFT | Passive Myofunctional Therapy |
| PSG | Polysomnography |
| RCT | Randomized Controlled Trial |
| RFA | Radiofrequency Ablation |
| RME | Rapid Maxillary Expansion |
| ST | Spontaneous-Timed mode |
| TORS | Transoral Robotic Surgery |
| UPPP | Uvulopalatopharyngoplasty |
Appendix A
| Therapeutic Area | Search Terms |
|---|---|
| Surgery | “surgery” OR “adenotonsillectomy” OR “tonsillectomy” OR “surgical outcomes in pediatric OSAS” |
| Pharmacological Therapy | “drug therapy pediatric OSAS” OR “intranasal corticosteroids and OSAS” OR “montelukast pediatric OSAS” OR “anti-inflammatory therapy for sleep apnea” |
| Non-Invasive Ventilation | “pediatric CPAP” OR “paediatric CPAP” OR “CPAP adherence children” OR “CPAP efficacy in pediatric OSAS” OR “long-term CPAP outcomes” OR “non- invasive ventilation pediatric OSAS” OR “BiPAP children OSAS” OR “high-flow nasal cannula pediatric OSAS” OR “HFNC children sleep apnea” OR “high flow therapy pediatric sleep-disordered breathing” |
| Orthodontic Interventions | “rapid maxillary expansion OSAS” OR “mandibular advancement devices pediatric OSAS” OR “myofunctional therapy sleep-disordered breathing” |
| Emerging Treatments | “alternative pharmacological treatments for OSAS” OR “novel treatments pediatric OSAS” OR “hypoglossal nerve stimulation children” |
References
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Ward, S.D.; Sheldon, S.H.; Shiffman, R.N.; et al. Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome. Pediatrics 2012, 130, e714–e755. [Google Scholar] [CrossRef] [PubMed]
- Bitners, A.C.; Arens, R. Evaluation and Management of Children with Obstructive Sleep Apnea Syndrome. Lung 2020, 198, 257–270. [Google Scholar] [CrossRef]
- Lein, A.; Altumbabic, H.; Đešević, M.; Baumgartner, W.-D.; Salkic, A.; Umihanic, S.; Ramaš, A.; Harčinović, A.; Kosec, A.; Brkic, F.F. Association of Adenoid Hypertrophy and Clinical Parameters with Preoperative Polygraphy in Pediatric Patients Undergoing Adenoidectomy. Eur. Arch. Oto-Rhino-Laryngol. 2025, 282, 1075–1084. [Google Scholar] [CrossRef]
- Lumeng, J.C.; Chervin, R.D. Epidemiology of Pediatric Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mendoza, J.; He, F.; Calhoun, S.L.; Vgontzas, A.N.; Liao, D.; Bixler, E.O. Association of Pediatric Obstructive Sleep Apnea With Elevated Blood Pressure and Orthostatic Hypertension in Adolescence. JAMA Cardiol. 2021, 6, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Halbower, A.C.; Degaonkar, M.; Barker, P.B.; Earley, C.J.; Marcus, C.L.; Smith, P.L.; Prahme, M.C.; Mahone, E.M. Childhood Obstructive Sleep Apnea Associates with Neuropsychological Deficits and Neuronal Brain Injury. PLoS Med. 2006, 3, e301. [Google Scholar] [CrossRef]
- Smith, D.F.; Amin, R.S. OSA and Cardiovascular Risk in Pediatrics. Chest 2019, 156, 402–413. [Google Scholar] [CrossRef]
- Katz, E.S.; D’Ambrosio, C.M. Pathophysiology of Pediatric Obstructive Sleep Apnea. Proc Am Thorac Soc 2008, 5, 253–262. [Google Scholar] [CrossRef]
- Reuveni, H.; Simon, T.; Tal, A.; Elhayany, A.; Tarasiuk, A. Health Care Services Utilization in Children with Obstructive Sleep Apnea Syndrome. Pediatrics 2002, 110, 68–72. [Google Scholar] [CrossRef]
- Petrongari, D.; Ciarelli, F.; Di Filippo, P.; Di Ludovico, A.; Di Pillo, S.; Chiarelli, F.; Pellegrino, G.M.; Sferrazza Papa, G.F.; Nosetti, L.; Attanasi, M. Risk and Protective Factors for Obstructive Sleep Apnea Syndrome Throughout Lifespan: From Pregnancy to Adolescence. Children 2025, 12, 216. [Google Scholar] [CrossRef]
- Oboleviciene, G.; Vaideliene, L.; Miseviciene, V. Exploring Sleep-Related Breathing Disorders in Pediatric Obesity and Prader-Willi Syndrome. Respir. Med. 2024, 234, 107855. [Google Scholar] [CrossRef]
- Del-Río Camacho, G.; Medina Castillo, L.; Rodríguez-Catalán, J.; Soto Insuga, V.; Gómez García, T. Central Sleep Apnea in Children with Obstructive Sleep Apnea Syndrome and Improvement Following Adenotonsillectomy. Pediatr. Pulmonol. 2019, 54, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.; Carvalho, J.; Marinho, C.; Vanderpoorten, S.; Adónis, C.; Freire, F. Central and Mixed Apneas in Children with Obstructive Sleep Apnea: Effect of Adenotonsillectomy. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 3125–3130. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gong, X.; Gao, X. Age-Related Hypertrophy of Adenoid and Tonsil with Its Relationship with Craniofacial Morphology. BMC Pediatr. 2023, 23, 163. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, G.; Kambas, I.; Tsaoussoglou, M.; Panaghiotopoulou-Gartagani, P.; Chrousos, G.; Kaditis, A.G. Age-Dependent Changes in the Size of Adenotonsillar Tissue in Childhood: Implications for Sleep-Disordered Breathing. J. Pediatr. 2013, 162, 269–274.e4. [Google Scholar] [CrossRef]
- Brietzke, S.E.; Gallagher, D. The Effectiveness of Tonsillectomy and Adenoidectomy in the Treatment of Pediatric Obstructive Sleep Apnea/Hypopnea Syndrome: A Meta-Analysis. Otolaryngol. Head Neck Surg. 2006, 134, 979–984. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Kelly, J. Outcome of Adenotonsillectomy for Severe Obstructive Sleep Apnea in Children. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 1375–1379. [Google Scholar] [CrossRef]
- Mitchell, R.B. Adenotonsillectomy for Obstructive Sleep Apnea in Children: Outcome Evaluated by Pre- and Postoperative Polysomnography. Laryngoscope 2007, 117, 1844–1854. [Google Scholar] [CrossRef]
- Nguyen, B.K.; Quraishi, H.A. Tonsillectomy and Adenoidectomy—Pediatric Clinics of North America. Pediatr. Clin. North Am. 2022, 69, 247–259. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Archer, S.M.; Ishman, S.L.; Rosenfeld, R.M.; Coles, S.; Finestone, S.A.; Friedman, N.R.; Giordano, T.; Hildrew, D.M.; Kim, T.W.; et al. Clinical Practice Guideline: Tonsillectomy in Children (Update). Otolaryngol. Head Neck Surg. 2019, 160, S1–S42. [Google Scholar] [CrossRef]
- Venekamp, R.P.; Hearne, B.J.; Chandrasekharan, D.; Blackshaw, H.; Lim, J.; Schilder, A.G.M. Tonsillectomy or Adenotonsillectomy versus Non-Surgical Management for Obstructive Sleep-Disordered Breathing in Children. Cochrane Database Syst. Rev. 2015, 10, CD011165. [Google Scholar] [CrossRef] [PubMed]
- Redline, S.; Amin, R.; Beebe, D.; Chervin, R.D.; Garetz, S.L.; Giordani, B.; Marcus, C.L.; Moore, R.H.; Rosen, C.L.; Arens, R.; et al. The Childhood Adenotonsillectomy Trial (CHAT): Rationale, Design, and Challenges of a Randomized Controlled Trial Evaluating a Standard Surgical Procedure in a Pediatric Population. Sleep 2011, 34, 1509–1517. [Google Scholar] [CrossRef]
- Marcus, C.L.; Moore, R.H.; Rosen, C.L.; Giordani, B.; Garetz, S.L.; Taylor, H.G.; Mitchell, R.B.; Amin, R.; Katz, E.S.; Arens, R.; et al. A Randomized Trial of Adenotonsillectomy for Childhood Sleep Apnea. N. Engl. J. Med. 2013, 368, 2366–2376. [Google Scholar] [CrossRef] [PubMed]
- Locci, C.; Cenere, C.; Sotgiu, G.; Puci, M.V.; Saderi, L.; Rizzo, D.; Bussu, F.; Antonucci, R. Adenotonsillectomy in Children with Obstructive Sleep Apnea Syndrome: Clinical and Functional Outcomes. J. Clin. Med. 2023, 12, 5826. [Google Scholar] [CrossRef]
- Tsikopoulos, A.; Tsikopoulos, K.; Dilmperis, F.; Anastasiadou, S.; Garefis, K.; Fountarlis, A.; Triaridis, S. Adenotonsillectomy Versus Watchful Waiting for Children with Obstructive Sleep Apnea Syndrome: A Systematic Review with Meta-Analysis. Indian J. Otolaryngol. Head Neck Surg. 2024, 76, 4910–4922. [Google Scholar] [CrossRef] [PubMed]
- Waters, K.A.; Chawla, J.; Harris, M.-A.; Heussler, H.; Cheng, A.T.; Black, R.J. Sleep and Behavior 24 Months After Early Tonsillectomy for Mild OSA: An RCT. Pediatrics 2021, 148, e2020038588. [Google Scholar] [CrossRef]
- Redline, S.; Cook, K.; Chervin, R.D.; Ishman, S.; Baldassari, C.M.; Mitchell, R.B.; Tapia, I.E.; Amin, R.; Hassan, F.; Ibrahim, S.; et al. Adenotonsillectomy for Snoring and Mild Sleep Apnea in Children: A Randomized Clinical Trial. JAMA 2023, 330, 2084–2095. [Google Scholar] [CrossRef]
- Borgström, A.; Nerfeldt, P.; Friberg, D. Adenotonsillotomy Versus Adenotonsillectomy in Pediatric Obstructive Sleep Apnea: An RCT. Pediatrics 2017, 139, e20163314. [Google Scholar] [CrossRef]
- Sjölander, I.; Borgström, A.; Nerfeldt, P.; Friberg, D. Adenotonsillotomy versus Adenotonsillectomy in Pediatric Obstructive Sleep Apnea: A 5-Year RCT. Sleep Med. X 2022, 4, 100055. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Guilleminault, C.; Lee, L.-A.; Lin, C.-H.; Hwang, F.-M. Treatment Outcomes of Adenotonsillectomy for Children with Obstructive Sleep Apnea: A Prospective Longitudinal Study. Sleep 2014, 37, 71–76. [Google Scholar] [CrossRef]
- Burstein, D.H.; Jackson, A.; Weedon, J.; Graw-Panzer, K.D.; Fahmy, S.; Goldstein, N.A. Adenotonsillectomy for Sleep-Disordered Breathing in a Predominantly Obese Pediatric Population. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Sudarsan, S.S.; Paramasivan, V.K.; Arumugam, S.V.; Murali, S.; Kameswaran, M. Comparison of Treatment Modalities in Syndromic Children with Obstructive Sleep Apnea—A Randomized Cohort Study. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.J.; Mitchell, R. Adenotonsillectomy for Obstructive Sleep Apnea in Obese Children: A Meta-Analysis. Otolaryngol. Head Neck Surg. 2009, 140, 455–460. [Google Scholar] [CrossRef]
- Di Filippo, P.; Orlandi, G.; Neri, G.; Di Pillo, S.; Chiarelli, F.; Rossi, N.; Attanasi, M. Effect of Tonsillectomy in a Child with Obesity and Obstructive Sleep Apnea: A Case Report and Review of the Literature. Front. Pediatr. 2023, 10, 1101267. [Google Scholar] [CrossRef]
- Imanguli, M.; Ulualp, S.O. Risk Factors for Residual Obstructive Sleep Apnea after Adenotonsillectomy in Children. Laryngoscope 2016, 126, 2624–2629. [Google Scholar] [CrossRef] [PubMed]
- Kirk, V.; Baughn, J.; D’Andrea, L.; Friedman, N.; Galion, A.; Garetz, S.; Hassan, F.; Wrede, J.; Harrod, C.G.; Malhotra, R.K. American Academy of Sleep Medicine Position Paper for the Use of a Home Sleep Apnea Test for the Diagnosis of OSA in Children. J. Clin. Sleep Med. 2017, 13, 1199–1203. [Google Scholar] [CrossRef]
- Windfuhr, J.P.; Werner, J.A. Tonsillotomy: It’s Time to Clarify the Facts. Eur. Arch. Otorhinolaryngol. 2013, 270, 2985–2996. [Google Scholar] [CrossRef]
- Cameron, R.; Haymes, A.; Pepper, C.; Possamai, V.; Blaney, S.; Morrison, G.; Jonas, N.; Jablenska, L.; Ferguson, L.; Lilly, I.; et al. Coblation Intracapsular Tonsillectomy in a Paediatric Tertiary Centre: Revision Surgery Rates over a Nine-Year Period. Int. J. Pediatr. Otorhinolaryngol. 2024, 181, 111942. [Google Scholar] [CrossRef]
- Sagheer, S.H.; Kolb, C.M.; Crippen, M.M.; Tawfik, A.; Vandjelovic, N.D.; Nardone, H.C.; Schmidt, R.J. Predictive Pediatric Characteristics for Revision Tonsillectomy After Intracapsular Tonsillectomy. Otolaryngol. Head Neck Surg. 2022, 166, 772–778. [Google Scholar] [CrossRef]
- Mesolella, M.; Allosso, S.; Coronella, V.; Massimilla, E.A.; Mansi, N.; Motta, G.; Salerno, G.; Motta, G. Extracapsular Tonsillectomy versus Intracapsular Tonsillotomy in Paediatric Patients with OSAS. J. Pers. Med. 2023, 13, 806. [Google Scholar] [CrossRef]
- Lao, J.; Jian, F.; Ge, R.; Wu, S. Tonsillectomy Versus Tonsillotomy in Pediatric Sleep-Disordered Breathing: A Systematic Review and Multi-Subgroup Meta-Analysis. Laryngoscope 2025, 135, 529–539. [Google Scholar] [CrossRef]
- Virkkunen, J.; Nokso-Koivisto, J.; Sakki, A.J. Long-Term Effectiveness of Tonsillotomy versus Tonsillectomy: A 12-Year Follow-up Study. Eur. Arch. Otorhinolaryngol. 2025, 282, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, M.; De Luca, P.; De Bonis, E.; Maurizi, R.; Cassandro, C.; Ralli, M.; Cassandro, E.; Scarpa, A. Partial Intracapsular Tonsillectomy in the Treatment of Pediatric Obstructive Sleep Apnea/Hypopnea Syndrome: A Prospective Study with 5-Year Follow-Up. Eur. Arch. Otorhinolaryngol. 2022, 279, 3089–3093. [Google Scholar] [CrossRef]
- Bluher, A.E.; Ishman, S.L.; Baldassari, C.M. Managing the Child with Persistent Sleep Apnea. Otolaryngol. Clin. North Am. 2019, 52, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.; McArdle, E.H.; Morrow, V.R.; Zalzal, H.G.; Carr, M.M.; Coutras, S.W. Base of Tongue Surgery and Pediatric Obstructive Sleep Apnea. Otolaryngol. Head Neck Surg. 2023, 168, 839–847. [Google Scholar] [CrossRef]
- Verkest, V.; Verhulst, S.; Van Hoorenbeeck, K.; Vanderveken, O.; Saldien, V.; Boudewyns, A. Prevalence of Obstructive Sleep Apnea in Children with Laryngomalacia and Value of Polysomnography in Treatment Decisions. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110255. [Google Scholar] [CrossRef]
- Children with Laryngomalacia Requiring Supraglottoplasty: An Association with Future Development of Sleep Disordered Breathing—Sarkissian—Australian Journal of Otolaryngology. Available online: https://www.theajo.com/article/view/4867/html (accessed on 5 June 2025).
- Velu, P.S.; Kariveda, R.R.; Palmer, W.J.; Levi, J.R. A Review of Uvulopalatopharyngoplasty for Pediatric Obstructive Sleep Apnea. Int. J. Pediatr. Otorhinolaryngol. 2024, 176, 111819. [Google Scholar] [CrossRef]
- Ulualp, S.O. Modified Expansion Sphincter Pharyngoplasty for Treatment of Children With Obstructive Sleep Apnea. JAMA Otolaryngol. –Head Neck Surg. 2014, 140, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Com, G.; Carroll, J.L.; Tang, X.; Melguizo, M.S.; Bower, C.; Jambhekar, S. Characteristics and Surgical and Clinical Outcomes of Severely Obese Children with Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 467–474. [Google Scholar] [CrossRef]
- Cheng, P.-W.; Fang, K.-M.; Su, H.-W.; Huang, T.-W. Improved Objective Outcomes and Quality of Life after Adenotonsillectomy with Inferior Turbinate Reduction in Pediatric Obstructive Sleep Apnea with Inferior Turbinate Hypertrophy. Laryngoscope 2012, 122, 2850–2854. [Google Scholar] [CrossRef]
- Alves de Sousa, F.; Santos, M.; Casanova, M.; Nóbrega Pinto, A.; Gonçalves Ferreira, M.; Meireles, L.; Coutinho, M.B. Pediatric Inferior Turbinate Surgery: A Review and Meta-Analysis of Midterm Nasal Patency. Int. J. Pediatr. Otorhinolaryngol. 2023, 172, 111661. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, C.J.; Amin, J.D.; Isaiah, A.; Valdez, T.A.; Jeyakumar, A.; Smart, S.E.; Pereira, K.D. Tracheostomy for Severe Pediatric Obstructive Sleep Apnea: Indications and Outcomes. Otolaryngol. Head Neck Surg. 2017, 157, 309–313. [Google Scholar] [CrossRef]
- Fuller, C.; Wineland, A.M.; Richter, G.T. Update on Pediatric Tracheostomy: Indications, Technique, Education, and Decannulation. Curr. Otorhinolaryngol. Rep. 2021, 9, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Baldassari, C.M.; Lam, D.J.; Ishman, S.L.; Chernobilsky, B.; Friedman, N.R.; Giordano, T.; Lawlor, C.; Mitchell, R.B.; Nardone, H.; Ruda, J.; et al. Expert Consensus Statement: Pediatric Drug-Induced Sleep Endoscopy. Otolaryngol. Head Neck Surg. 2021, 165, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Ishman, S.L.; Maturo, S.; Schwartz, S.; McKenna, M.; Baldassari, C.M.; Bergeron, M.; Chernobilsky, B.; Ehsan, Z.; Gagnon, L.; Liu, Y.-C.C.; et al. Expert Consensus Statement: Management of Pediatric Persistent Obstructive Sleep Apnea After Adenotonsillectomy. Otolaryngol. Head Neck Surg. 2023, 168, 115–130. [Google Scholar] [CrossRef]
- Kuhle, S.; Hoffmann, D.U.; Mitra, S.; Urschitz, M.S. Anti-inflammatory Medications for Obstructive Sleep Apnoea in Children. In Cochrane Database of Systematic Reviews; Cochrane Library: London, UK, 2020. [Google Scholar]
- Mussi, N.; Forestiero, R.; Zambelli, G.; Rossi, L.; Caramia, M.R.; Fainardi, V.; Esposito, S. The First-Line Approach in Children with Obstructive Sleep Apnea Syndrome (OSA). J. Clin. Med. 2023, 12, 7092. [Google Scholar] [CrossRef]
- Scaparrotta, A.; Di Pillo, S.; Attanasi, M.; Rapino, D.; Cingolani, A.; Consilvio, N.P.; Verini, M.; Chiarelli, F. Montelukast versus Inhaled Corticosteroids in the Management of Pediatric Mild Persistent Asthma. Multidiscip. Respir. Med. 2012, 7, 13. [Google Scholar] [CrossRef]
- Goldbart, A.D.; Veling, M.C.; Goldman, J.L.; Li, R.C.; Brittian, K.R.; Gozal, D. Glucocorticoid Receptor Subunit Expression in Adenotonsillar Tissue of Children with Obstructive Sleep Apnea. Pediatr. Res. 2005, 57, 232–236. [Google Scholar] [CrossRef]
- Baker, A.; Grobler, A.; Davies, K.; Griffiths, A.; Hiscock, H.; Kubba, H.; Peters, R.L.; Ranganathan, S.; Rimmer, J.; Rose, E.; et al. Effectiveness of Intranasal Mometasone Furoate vs Saline for Sleep-Disordered Breathing in Children: A Randomized Clinical Trial. JAMA Pediatr. 2023, 177, 240–247. [Google Scholar] [CrossRef]
- Brouillette, R.T.; Manoukian, J.J.; Ducharme, F.M.; Oudjhane, K.; Earle, L.G.; Ladan, S.; Morielli, A. Efficacy of Fluticasone Nasal Spray for Pediatric Obstructive Sleep Apnea. J. Pediatr. 2001, 138, 838–844. [Google Scholar] [CrossRef]
- Chan, C.C.K.; Au, C.T.; Lam, H.S.; Lee, D.L.Y.; Wing, Y.K.; Li, A.M. Intranasal Corticosteroids for Mild Childhood Obstructive Sleep Apnea--a Randomized, Placebo-Controlled Study. Sleep Med. 2015, 16, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish-Gozal, L.; Gozal, D. Intranasal Budesonide Treatment for Children With Mild Obstructive Sleep Apnea Syndrome. Pediatrics 2008, 122, e149–e155. [Google Scholar] [CrossRef] [PubMed]
- Tapia, I.E.; Shults, J.; Cielo, C.M.; Kelly, A.B.; Elden, L.M.; Spergel, J.M.; Bradford, R.M.; Cornaglia, M.A.; Sterni, L.M.; Radcliffe, J. A Trial of Intranasal Corticosteroids to Treat Childhood OSA Syndrome. CHEST 2022, 162, 899–919. [Google Scholar] [CrossRef]
- Goldbart, A.D.; Goldman, J.L.; Veling, M.C.; Gozal, D. Leukotriene Modifier Therapy for Mild Sleep-Disordered Breathing in Children. Am. J. Respir. Crit. Care Med. 2005, 172, 364–370. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Bandla, H.P.R.; Gozal, D. Montelukast for Children with Obstructive Sleep Apnea: Results of a Double-Blind, Randomized, Placebo-Controlled Trial. Ann. Am. Thorac. Soc. 2016, 13, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Lu, T.; Qiu, Y.; Li, X.; Liu, Y.; Tai, J.; Guo, Y.; Zhang, J.; Wang, S.; Zhao, J.; et al. The Efficacy and Safety of Montelukast in Children with Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Sleep Med. 2021, 78, 193–201. [Google Scholar] [CrossRef]
- Liming, B.J.; Ryan, M.; Mack, D.; Ahmad, I.; Camacho, M. Montelukast and Nasal Corticosteroids to Treat Pediatric Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Otolaryngol. Head Neck Surg. 2019, 160, 594–602. [Google Scholar] [CrossRef]
- Zhang, Y.; Leng, S.; Hu, Q.; Li, Y.; Wei, Y.; Lu, Y.; Qie, D.; Yang, F. Pharmacological Interventions for Pediatric Obstructive Sleep Apnea (OSA): Network Meta-Analysis. Sleep Med. 2024, 116, 129–137. [Google Scholar] [CrossRef]
- FDA Requires Boxed Warning about Serious Mental Health Side Effects for Asthma and Allergy Drug Montelukast (Singulair); Advises Restricting Use for Allergic Rhinitis. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-boxed-warning-about-serious-mental-health-side-effects-asthma-and-allergy-drug (accessed on 7 July 2025).
- Lo, C.W.H.; Pathadka, S.; Qin, S.X.; Fung, L.W.Y.; Yan, V.K.C.; Yiu, H.H.E.; Bloom, C.I.; Wong, I.C.K.; Chan, E.W.Y. Neuropsychiatric Events Associated with Montelukast in Patients with Asthma: A Systematic Review. Eur. Respir. Rev. 2023, 32, 230079. [Google Scholar] [CrossRef]
- Evangelisti, M.; Barreto, M.; Di Nardo, G.; Del Pozzo, M.; Parisi, P.; Villa, M.P. Systemic Corticosteroids Could Be Used as Bridge Treatment in Children with Obstructive Sleep Apnea Syndrome Waiting for Surgery. Sleep Breath 2022, 26, 879–885. [Google Scholar] [CrossRef]
- Kajiyama, T.; Komori, M.; Hiyama, M.; Kobayashi, T.; Hyodo, M. Changes during Medical Treatments before Adenotonsillectomy in Children with Obstructive Sleep Apnea. Auris Nasus Larynx 2022, 49, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Don, D.M.; Goldstein, N.A.; Crockett, D.M.; Ward, S.D. Antimicrobial Therapy for Children with Adenotonsillar Hypertrophy and Obstructive Sleep Apnea: A Prospective Randomized Trial Comparing Azithromycin vs Placebo. Otolaryngol. Head Neck Surg. 2005, 133, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Ergenekon, A.P.; Gokdemir, Y.; Ersu, R. Medical Treatment of Obstructive Sleep Apnea in Children. J. Clin. Med. 2023, 12, 5022. [Google Scholar] [CrossRef] [PubMed]
- Hady, K.K.; Okorie, C.U.A. Positive Airway Pressure Therapy for Pediatric Obstructive Sleep Apnea. Children 2021, 8, 979. [Google Scholar] [CrossRef]
- Malhotra, A.; Crocker, M.E.; Willes, L.; Kelly, C.; Lynch, S.; Benjafield, A.V. Patient Engagement Using New Technology to Improve Adherence to Positive Airway Pressure Therapy: A Retrospective Analysis. Chest 2018, 153, 843–850. [Google Scholar] [CrossRef]
- Aurora, R.N.; Zak, R.S.; Karippot, A.; Lamm, C.I.; Morgenthaler, T.I.; Auerbach, S.H.; Bista, S.R.; Casey, K.R.; Chowdhuri, S.; Kristo, D.A.; et al. Practice Parameters for the Respiratory Indications for Polysomnography in Children. Sleep 2011, 34, 379–388. [Google Scholar] [CrossRef]
- Marcus, C.L.; Radcliffe, J.; Konstantinopoulou, S.; Beck, S.E.; Cornaglia, M.A.; Traylor, J.; DiFeo, N.; Karamessinis, L.R.; Gallagher, P.R.; Meltzer, L.J. Effects of Positive Airway Pressure Therapy on Neurobehavioral Outcomes in Children with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2012, 185, 998. [Google Scholar] [CrossRef]
- Watach, A.J.; Xanthopoulos, M.S.; Afolabi-Brown, O.; Saconi, B.; Fox, K.A.; Qiu, M.; Sawyer, A.M. Positive Airway Pressure Adherence in Pediatric Obstructive Sleep Apnea: A Systematic Scoping Review. Sleep Med. Rev. 2020, 51, 101273. [Google Scholar] [CrossRef]
- Xanthopoulos, M.S.; Kim, J.Y.; Blechner, M.; Chang, M.; Menello, M.K.; Brown, C.; Matthews, E.; Weaver, T.E.; Shults, J.; Marcus, C.L. Self-Efficacy and Short-Term Adherence to Continuous Positive Airway Pressure Treatment in Children. Sleep: J. Sleep Sleep Disord. Res. 2017, 40, zsx096. [Google Scholar] [CrossRef]
- Levert, D.; Maniaci, J.; Tapia, I.E.; Xanthopoulos, M.S. An Overview and Application of Evidence-Based Interventions to Improve Adherence to Positive Airway Pressure for the Treatment of Pediatric Obstructive Sleep Apnea. Clin. Pract. Pediatr. Psychol. 2022, 10, 384–393. [Google Scholar] [CrossRef]
- Sunkonkit, K.; Selvadurai, S.; Voutsas, G.; Benzon, D.; Baker, A.; Trinh, M.; Narang, I. The Impact of the COVID-19 Pandemic on Positive Airway Pressure Usage in Children with Sleep-Disordered Breathing. Sleep Breath 2022, 26, 533–540. [Google Scholar] [CrossRef]
- Sansone, F.; Pellegrino, G.M.; Caronni, A.; Bonazza, F.; Vegni, E.; Lué, A.; Bocci, T.; Pipolo, C.; Giusti, G.; Di Filippo, P.; et al. Long COVID in Children: A Multidisciplinary Review. Diagnostics 2023, 13, 1990. [Google Scholar] [CrossRef]
- Tovichien, P.; Kulbun, A.; Udomittipong, K. Comparing Adherence of Continuous and Automatic Positive Airway Pressure (CPAP and APAP) in Obstructive Sleep Apnea (OSA) Children. Front. Pediatr. 2022, 10, 841705. [Google Scholar] [CrossRef] [PubMed]
- Khaytin, I.; Tapia, I.E.; Xanthopoulos, M.S.; Cielo, C.; Kim, J.Y.; Smith, J.; Matthews, E.C.; Beck, S.E. Auto-Titrating CPAP for the Treatment of Obstructive Sleep Apnea in Children. J. Clin. Sleep Med. 2020, 16, 871–878. [Google Scholar] [CrossRef]
- Fuchs, F.S.; Wiest, G.H.; Frank, M.; Harsch, I.A.; Schahin, S.P.; Hahn, E.G.; Ficker, J.H. Auto-CPAP Therapy for Obstructive Sleep Apnea: Induction of Microarousals by Automatic Variations of CPAP Pressure? Sleep 2002, 25, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Arens, R.; Muzumdar, H. Sleep, Sleep Disordered Breathing, and Nocturnal Hypoventilation in Children with Neuromuscular Diseases. Paediatr. Respir. Rev. 2010, 11, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Baker, A.; Narang, I. Positive Airway Pressure in Pediatric Obstructive Sleep Apnea. Paediatr. Respir. Rev. 2019, 31, 43–51. [Google Scholar] [CrossRef]
- McGinley, B.; Halbower, A.; Schwartz, A.R.; Smith, P.L.; Patil, S.P.; Schneider, H. Effect of a High-Flow Open Nasal Cannula System on Obstructive Sleep Apnea in Children. Pediatrics 2009, 124, 179. [Google Scholar] [CrossRef]
- Joseph, L.; Goldberg, S.; Shitrit, M.; Picard, E. High-Flow Nasal Cannula Therapy for Obstructive Sleep Apnea in Children. J. Clin. Sleep Med. 2015, 11, 1007–1010. [Google Scholar] [CrossRef]
- Fishman, H.; Al-Shamli, N.; Sunkonkit, K.; Maguire, B.; Selvadurai, S.; Baker, A.; Amin, R.; Propst, E.J.; Wolter, N.E.; Eckert, D.J.; et al. Heated Humidified High Flow Nasal Cannula Therapy in Children with Obstructive Sleep Apnea: A Randomized Cross-over Trial. Sleep Med. 2023, 107, 81–88. [Google Scholar] [CrossRef]
- Polytarchou, A.; Moudaki, A.; Van de Perck, E.; Boudewyns, A.; Kaditis, A.G.; Verhulst, S.; Ersu, R. An Update on Diagnosis and Management of Obstructive Sleep Apnoea in the First 2 Years of Life. Eur. Respir. Rev. 2024, 33, 230121. [Google Scholar] [CrossRef] [PubMed]
- Kaditis, A.G.; Alvarez, M.L.A.; Boudewyns, A.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Larramona, H.; Miano, S.; Narang, I.; Trang, H.; et al. Obstructive Sleep Disordered Breathing in 2- to 18-Year-Old Children: Diagnosis and Management. Eur. Respir. J. 2015, 47, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Fauroux, B.; Abel, F.; Amaddeo, A.; Bignamini, E.; Chan, E.; Corel, L.; Cutrera, R.; Ersu, R.; Installe, S.; Khirani, S.; et al. ERS Statement on Paediatric Long-Term Noninvasive Respiratory Support. Eur. Respir. J. 2022, 59, 2101404. [Google Scholar] [CrossRef]
- Dental Appliances for the Treatment of Obstructive Sleep Apnea in Children: A Systematic Review and Meta-Analysis. Available online: https://www.mdpi.com/1648-9144/59/8/1447 (accessed on 5 June 2025).
- Bucci, R.; Rongo, R.; Zunino, B.; Michelotti, A.; Bucci, P.; Alessandri-Bonetti, G.; Incerti-Parenti, S.; D’Antò, V. Effect of Orthopedic and Functional Orthodontic Treatment in Children with Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2023, 67, 101730. [Google Scholar] [CrossRef] [PubMed]
- Monini, S.; Malagola, C.; Villa, M.P.; Tripodi, C.; Tarentini, S.; Malagnino, I.; Marrone, V.; Lazzarino, A.I.; Barbara, M. Rapid Maxillary Expansion for the Treatment of Nasal Obstruction in Children Younger Than 12 Years. Arch. Otolaryngol. –Head Neck Surg. 2009, 135, 22–27. [Google Scholar] [CrossRef]
- Sun, Y.; Jia, Y.; Wang, S.; Xu, C.; Qu, Y.; Hu, M.; Jiang, H. Effectiveness of Mandibular Advancement Orthodontic Appliances with Maxillary Expansion Device in Children with Obstructive Sleep Apnea: A Systematic Review. BMC Oral Health 2024, 24, 1303. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, L.; Lu, Y. The Role of Rapid Maxillary Expansion in Pediatric Obstructive Sleep Apnea: Efficacy, Mechanism and Multidisciplinary Collaboration. Sleep Med. Rev. 2023, 67, 101733. [Google Scholar] [CrossRef]
- Galeotti, A.; Gatto, R.; Caruso, S.; Piga, S.; Maldonato, W.; Sitzia, E.; Viarani, V.; Bompiani, G.; Aristei, F.; Marzo, G.; et al. Effects of Rapid Palatal Expansion on the Upper Airway Space in Children with Obstructive Sleep Apnea (OSA): A Case-Control Study. Children 2023, 10, 244. [Google Scholar] [CrossRef]
- Yoon, A.; Abdelwahab, M.; Bockow, R.; Vakili, A.; Lovell, K.; Chang, I.; Ganguly, R.; Liu, S.Y.-C.; Kushida, C.; Hong, C. Impact of Rapid Palatal Expansion on the Size of Adenoids and Tonsils in Children. Sleep Med. 2022, 92, 96–102. [Google Scholar] [CrossRef]
- Lee, C.-H.; Kang, K.-T.; Weng, W.-C.; Lee, P.-L.; Hsu, W.-C. Quality of Life after Adenotonsillectomy in Children with Obstructive Sleep Apnea: Short-Term and Long-Term Results. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 210–215. [Google Scholar] [CrossRef]
- Manetta, I.P.; Ettlin, D.; Sanz, P.M.; Rocha, I.; Cruz, M.M. e Mandibular Advancement Devices in Obstructive Sleep Apnea: An Updated Review. Sleep Sci. 2022, 15, 398. [Google Scholar] [CrossRef]
- Cozzi-Machado, C.; Albertini, F.R.; Silveira, S.; Machado-Júnior, A.J. Mandibular Advancement Appliances in Pediatric Obstructive Sleep Apnea: An Umbrella Review. Sleep Sci. 2023, 16, e468–e475. [Google Scholar] [CrossRef]
- Zhang, C.; He, H.; Ngan, P. Effects of Twin Block Appliance on Obstructive Sleep Apnea in Children: A Preliminary Study. Sleep Breath 2013, 17, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Johal, A.; Haria, P.; Manek, S.; Joury, E.; Riha, R. Ready-Made Versus Custom-Made Mandibular Repositioning Devices in Sleep Apnea: A Randomized Clinical Trial. J. Clin. Sleep Med. 2017, 13, 175–182. [Google Scholar] [CrossRef]
- Huynh, N.T.; Desplats, E.; Almeida, F.R. Orthodontics Treatments for Managing Obstructive Sleep Apnea Syndrome in Children: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2016, 25, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.P.; Rizzoli, A.; Miano, S.; Malagola, C. Efficacy of Rapid Maxillary Expansion in Children with Obstructive Sleep Apnea Syndrome: 36 Months of Follow-Up. Sleep Breath 2011, 15, 179–184. [Google Scholar] [CrossRef]
- Yu, M.; Ma, Y.; Xu, Y.; Bai, J.; Lu, Y.; Han, F.; Gao, X. Orthodontic Appliances for the Treatment of Pediatric Obstructive Sleep Apnea: A Systematic Review and Network Meta-Analysis. Sleep Med. Rev. 2023, 72, 101855. [Google Scholar] [CrossRef] [PubMed]
- Koka, V.; De Vito, A.; Roisman, G.; Petitjean, M.; Filograna Pignatelli, G.R.; Padovani, D.; Randerath, W. Orofacial Myofunctional Therapy in Obstructive Sleep Apnea Syndrome: A Pathophysiological Perspective. Medicina 2021, 57, 323. [Google Scholar] [CrossRef]
- Poncin, W.; Willemsens, A.; Gely, L.; Contal, O. Assessment and Rehabilitation of Tongue Motor Skills with Myofunctional Therapy in Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. J. Clin. Sleep Med. 2024, 20, 1535–1549. [Google Scholar] [CrossRef]
- Li, W.-Y.; Masse, J.-F.; Sériès, F. Myofunctional Therapy for Obstructive Sleep Apnoea. Aust. Dent. J. 2024, 69, S63–S67. [Google Scholar] [CrossRef]
- Saba, E.S.; Kim, H.; Huynh, P.; Jiang, N. Orofacial Myofunctional Therapy for Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Laryngoscope 2024, 134, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-J.; Huang, Y.-S.; Lian, Y.-C.; Lee, Y.-H.; Hervy-Auboiron, M.; Li, C.-H.; Lin, C.-H.; Chuang, L.-C. Craniofacial Morphologic Predictors for Passive Myofunctional Therapy of Pediatric Obstructive Sleep Apnea Using an Oral Appliance with a Tongue Bead. Children 2022, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Huang, Y.S.; Monteyrol, P.J.; Sato, R.; Quo, S.; Lin, C.H. Critical Role of Myofascial Reeducation in Pediatric Sleep-Disordered Breathing. Sleep Med. 2013, 14, 518–525. [Google Scholar] [CrossRef]
- Tamura, A.; Yamaguchi, K.; Yanagida, R.; Miyata, R.; Tohara, H. At-Home Orthodontic Treatment for Severe Teeth Arch Malalignment and Severe Obstructive Sleep Apnea Syndrome in a Child with Cerebral Palsy. Int. J. Environ. Res. Public Health 2022, 19, 5333. [Google Scholar] [CrossRef]
- Skotko, B.G.; Macklin, E.A.; Muselli, M.; Voelz, L.; McDonough, M.E.; Davidson, E.; Allareddy, V.; Jayaratne, Y.S.N.; Bruun, R.; Ching, N.; et al. A Predictive Model for Obstructive Sleep Apnea and Down Syndrome. Am. J. Med. Genet. Part A 2017, 173, 889–896. [Google Scholar] [CrossRef]
- Olson, M.D.; Junna, M.R. Hypoglossal Nerve Stimulation Therapy for the Treatment of Obstructive Sleep Apnea. Neurotherapeutics 2021, 18, 91–99. [Google Scholar] [CrossRef]
- Liu, P.; Kong, W.; Fang, C.; Zhu, K.; Dai, X.; Meng, X. Hypoglossal Nerve Stimulation in Adolescents with down Syndrome and Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Front. Neurol. 2022, 13, 1037926. [Google Scholar] [CrossRef] [PubMed]
- Stenerson, M.E.; Yu, P.K.; Kinane, T.B.; Skotko, B.G.; Hartnick, C.J. Long-Term Stability of Hypoglossal Nerve Stimulation for the Treatment of Obstructive Sleep Apnea in Children with Down Syndrome. Int. J. Pediatr. Otorhinolaryngol. 2021, 149, 110868. [Google Scholar] [CrossRef]
- Rodriguez Lara, F.; Carnino, J.M.; Cohen, M.B.; Levi, J.R. Advances in the Use of Hypoglossal Nerve Stimulator in Adolescents With Down Syndrome and Persistent Obstructive Sleep Apnea-A Systematic Review. Ann. Otol. Rhinol. Laryngol. 2024, 133, 317–324. [Google Scholar] [CrossRef]
- Callans, K.; Carroll, D.L.; McDonough, A. Parental Experience of Hypoglossal Nerve Stimulator Implantation in Adolescents with Down Syndrome and Obstructive Sleep Apnea. J. Pediatr. Nurs. Nurs. Care Child. Fam. 2023, 68, 24–29. [Google Scholar] [CrossRef]
- Chieffe, D.; Liu, R.H.; Hartnick, C. Challenges and Adverse Events in Pediatric Hypoglossal Nerve Stimulation. Int. J. Pediatr. Otorhinolaryngol. 2024, 176, 111831. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Baker, A.; Massicotte, C.; Reyna, M.E.; Shi, J.; Wolter, N.E.; Propst, E.J.; Mahant, S.; Amin, R.; Parekh, R.S.; et al. Positional Therapy for the Treatment of Positional Obstructive Sleep Apnea in Children: A Randomized Controlled Crossover Trial. Sleep Med. 2025, 130, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kent, D.T.; Zealear, D.; Schwartz, A.R. Ansa Cervicalis and Hypoglossal Nerve Stimulation in a Patient With Obstructive Sleep Apnea. Otolaryngol. Head Neck Surg. 2021, 165, 602–604. [Google Scholar] [CrossRef]
- Combs, D.; Edgin, J.; Hsu, C.-H.; Bottrill, K.; Van Vorce, H.; Gerken, B.; Matloff, D.; La Rue, S.; Parthasarathy, S. The Combination of Atomoxetine and Oxybutynin for the Treatment of Obstructive Sleep Apnea in Children with Down Syndrome. J. Clin. Sleep Med. 2023, 19, 2065–2073. [Google Scholar] [CrossRef]
- Hedner, J.; Stenlöf, K.; Zou, D.; Hoff, E.; Hansen, C.; Kuhn, K.; Lennartz, P.; Grote, L. A Randomized Controlled Clinical Trial Exploring Safety and Tolerability of Sulthiame in Sleep Apnea. Am. J. Respir. Crit. Care Med. 2022, 205, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Smith, C.A.; Przybylowski, T.; Chenuel, B.; Xie, A.; Nakayama, H.; Skatrud, J.B. The Ventilatory Responsiveness to CO2 below Eupnoea as a Determinant of Ventilatory Stability in Sleep. J. Physiol. 2004, 560, 1–11. [Google Scholar] [CrossRef]
- Caravita, S.; Faini, A.; Lombardi, C.; Valentini, M.; Gregorini, F.; Rossi, J.; Meriggi, P.; Rienzo, M.D.; Bilo, G.; Agostoni, P.; et al. Sex and Acetazolamide Effects on Chemoreflex and Periodic Breathing During Sleep at Altitude. CHEST 2015, 147, 120–131. [Google Scholar] [CrossRef]
| Surgical Technique | Mechanism | Key Advantages | Limitations | Typical Indications |
|---|---|---|---|---|
| Cold Steel Dissection | Dissection of the tonsillar capsule using scalpel and scissors; exposes pharyngeal muscles | Cost-effective, traditional approach | Higher postoperative pain and hemorrhage risk | Standard pediatric OSAS without complicating factors |
| Electrocautery Tonsillectomy | Monopolar or bipolar cautery (temperatures up to 400 °C) enabling dissection and hemostasis | Reduced operative time, effective bleeding control | Greater thermal damage to adjacent tissues | Routine tonsillectomy when equipment is available |
| Harmonic Scalpel Tonsillectomy | Ultrasonic energy simultaneously cuts and coagulates at lower temperatures | Lower thermal spread, reduced postoperative pain | Costly, less widespread availability | Centers equipped with harmonic technology |
| Coblation Tonsillotomy | Uses a plasma field (40–70 °C) generated by radiofrequency and saline | Minimal tissue trauma, low intraoperative bleeding | Higher cost, possible regrowth in younger patients | Favorable in intracapsular tonsillectomy in children |
| Microdebrider-Assisted Intracapsular Tonsillotomy | Rotating blade preserves tonsillar capsule while removing hypertrophic tissue | Less pain, faster recovery, low bleeding | May be suboptimal in massive hypertrophy | Mild/moderate OSAS or patients needing rapid return to activity |
| Laser-Assisted Serial Tonsillectomy (LAST) | CO2 or diode laser vaporizes tonsillar tissue gradually | Lower intraoperative bleeding, shorter healing time | Risk of incomplete removal, recurrence | Selected cases, limited pediatric application |
| Radiofrequency Ablation (RFA) | Submucosal tissue reduction via low-energy RF waves | Immune tissue sparing, reduced pain | Limited evidence in pediatric OSAS | Younger children with immune considerations |
| Cryo-Tonsillectomy | Cryogenic tissue destruction using liquid nitrogen (−196 °C) | Minimal bleeding | Longer operative time, delayed healing | Rarely employed in modern practice |
| Cryogenic Plasma Tonsillectomy | Cold plasma ablation reduces thermal injury and preserves mucosa | Less trauma, good postoperative oxygenation | Specialized equipment, limited data | Promising in selected or recurrent cases |
| Transoral Robotic Surgery (TORS) | Robotic-assisted precision excision, typically for lingual or residual tonsillar tissue | Enhanced visualization, high precision in anatomically complex cases | High cost, specialized training, longer operative time | Refractory OSAS after AT, especially in syndromic patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panetti, B.; Federico, C.; Sferrazza Papa, G.F.; Di Filippo, P.; Di Ludovico, A.; Di Pillo, S.; Chiarelli, F.; Scaparrotta, A.; Attanasi, M. Three Decades of Managing Pediatric Obstructive Sleep Apnea Syndrome: What’s Old, What’s New. Children 2025, 12, 919. https://doi.org/10.3390/children12070919
Panetti B, Federico C, Sferrazza Papa GF, Di Filippo P, Di Ludovico A, Di Pillo S, Chiarelli F, Scaparrotta A, Attanasi M. Three Decades of Managing Pediatric Obstructive Sleep Apnea Syndrome: What’s Old, What’s New. Children. 2025; 12(7):919. https://doi.org/10.3390/children12070919
Chicago/Turabian StylePanetti, Beatrice, Claudia Federico, Giuseppe Francesco Sferrazza Papa, Paola Di Filippo, Armando Di Ludovico, Sabrina Di Pillo, Francesco Chiarelli, Alessandra Scaparrotta, and Marina Attanasi. 2025. "Three Decades of Managing Pediatric Obstructive Sleep Apnea Syndrome: What’s Old, What’s New" Children 12, no. 7: 919. https://doi.org/10.3390/children12070919
APA StylePanetti, B., Federico, C., Sferrazza Papa, G. F., Di Filippo, P., Di Ludovico, A., Di Pillo, S., Chiarelli, F., Scaparrotta, A., & Attanasi, M. (2025). Three Decades of Managing Pediatric Obstructive Sleep Apnea Syndrome: What’s Old, What’s New. Children, 12(7), 919. https://doi.org/10.3390/children12070919







