Integrating Radiomics and Lesion Mapping for Cerebellar Mutism Syndrome Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
- (1)
- Patients aged between 0 and 18 years;
- (2)
- Completion of presurgical MRI at our center;
- (3)
- Diagnosis of posterior fossa tumor confirmed at our center;
- (4)
- Received surgical resection for the tumor;
- (5)
- Definitive diagnosis of CMS or non-CMS determined by two senior neurosurgeons.
- (1)
- Incomplete clinical data;
- (2)
- Missing MRI data;
- (3)
- Unsatisfactory normalization upon visual inspection.
2.2. Imaging Procedures, Lesion Map Extraction, and Voxel Values Calculation
2.3. Radiomic Feature Extraction and Selection
2.4. CMS Definition and Risk Factors
2.5. Model Development and Validation
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Cohorts
3.2. Group Comparison of Non-CMS and CMS Cohorts
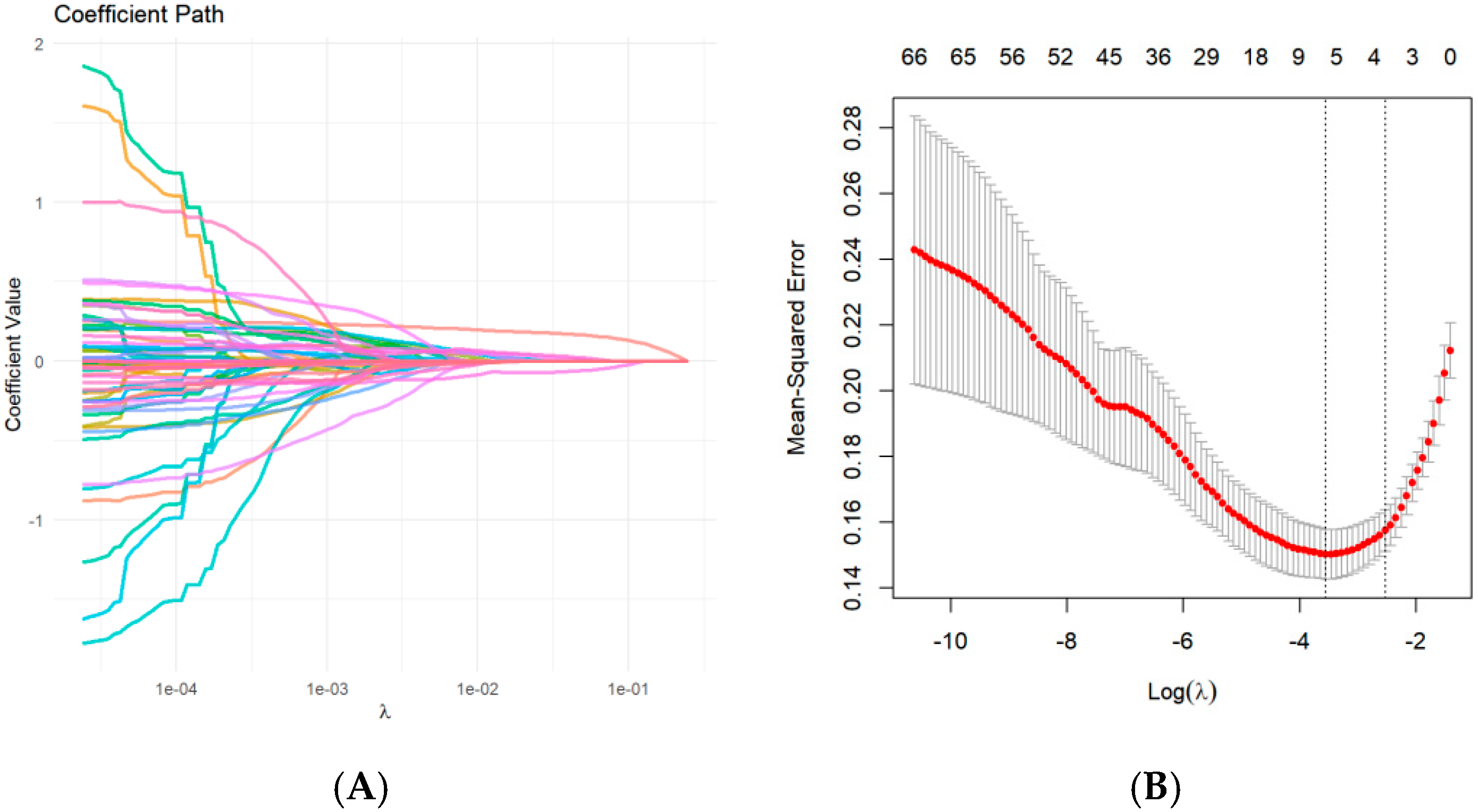
3.3. Feature Selection
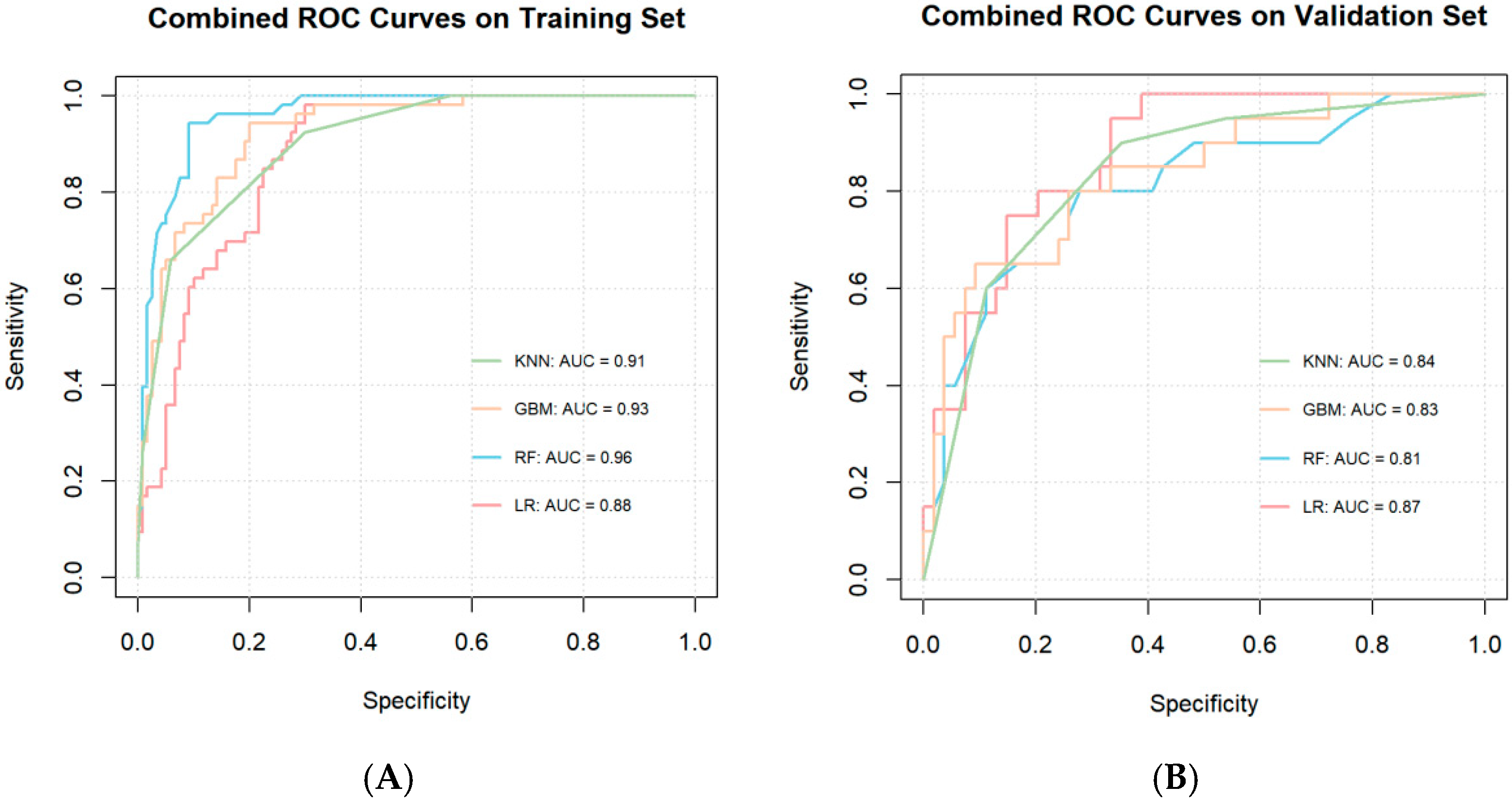
3.4. Model Construction and Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bale, T.A.; Rosenblum, M.K. The 2021 WHO Classification of Tumors of the Central Nervous System: An update on pediatric low-grade gliomas and glioneuronal tumors. Brain Pathol. 2022, 32, e13060. [Google Scholar] [CrossRef] [PubMed]
- Formentin, C.; Joaquim, A.F.; Ghizoni, E. Posterior fossa tumors in children: Current insights. Eur. J. Pediatr. 2023, 182, 4833–4850. [Google Scholar] [CrossRef] [PubMed]
- Catsman-Berrevoets, C.E. Cerebellar mutism syndrome: Cause and rehabilitation. Curr. Opin. Neurol. 2017, 30, 133–139. [Google Scholar] [CrossRef]
- Khan, R.B.; Patay, Z.; Klimo, P., Jr.; Huang, J.; Kumar, R.; Boop, F.A.; Raches, D.; Conklin, H.M.; Sharma, R.; Simmons, A.; et al. Clinical features, neurologic recovery, and risk factors of postoperative posterior fossa syndrome and delayed recovery: A prospective study. Neuro-Oncolology 2021, 23, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Fabozzi, F.; Margoni, S.; Andreozzi, B.; Musci, M.S.; Del Baldo, G.; Boccuto, L.; Mastronuzzi, A.; Carai, A. Cerebellar mutism syndrome: From pathophysiology to rehabilitation. Front. Cell Dev. Biol. 2022, 10, 1082947. [Google Scholar] [CrossRef]
- Jabarkheel, R.; Amayiri, N.; Yecies, D.; Huang, Y.; Toescu, S.; Nobre, L.; Mabbott, D.J.; Sudhakar, S.V.; Malik, P.; Laughlin, S.; et al. Molecular correlates of cerebellar mutism syndrome in medulloblastoma. Neuro-Oncology 2020, 22, 290–297. [Google Scholar] [CrossRef]
- Pettersson, S.D.; Kitlinski, M.; Miękisiak, G.; Ali, S.; Krakowiak, M.; Szmuda, T. Risk factors for postoperative cerebellar mutism syndrome in pediatric patients: A systematic review and meta-analysis. J. Neurosurg. Pediatr. 2022, 29, 467–475. [Google Scholar] [CrossRef]
- Gleichgerrcht, E.; Fridriksson, J.; Rorden, C.; Bonilha, L. Connectome-based lesion-symptom mapping (CLSM): A novel approach to map neurological function. NeuroImage Clin. 2017, 16, 461–467. [Google Scholar] [CrossRef]
- Yang, W.; Li, Y.; Ying, Z.; Cai, Y.; Peng, X.; Sun, H.; Chen, J.; Zhu, K.; Hu, G.; Peng, Y.; et al. A presurgical voxel-wise predictive model for cerebellar mutism syndrome in children with posterior fossa tumors. NeuroImage Clin. 2023, 37, 103291. [Google Scholar] [CrossRef]
- Yang, W.; Chai, X.; Zhang, N.; Zhi, Z.; Cai, Y.; Peng, X.; Wang, J.; Zhang, H.; Sun, H.; Ji, Y.; et al. Predicting cerebellar mutism syndrome in children using lesion map combined with clinical features. J. Neurooncol. 2024, 170, 591–599. [Google Scholar] [CrossRef]
- RGillies, J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, C.; Chen, X.; Feng, Y.; Feng, J.; Zhang, R.; Ouyang, F.; Li, X.; Tan, Z.; Deng, L.; et al. Noninvasive prediction of perineural invasion in intrahepatic cholangiocarcinoma by clinicoradiological features and computed tomography radiomics based on interpretable machine learning: A multicenter cohort study. Int. J. Surg. Lond. Engl. 2024, 110, 1039–1051. [Google Scholar] [CrossRef]
- Pitsika, M.; Tsitouras, V. Cerebellar mutism. J. Neurosurg. Pediatr. 2013, 12, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Charalambides, C.; Dinopoulos, A.; Sgouros, S. Neuropsychological sequelae and quality of life following treatment of posterior fossa ependymomas in children. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2009, 25, 1313–1320. [Google Scholar] [CrossRef]
- Catsman-Berrevoets, C.E.; Aarsen, F.K. The spectrum of neurobehavioural deficits in the Posterior Fossa Syndrome in children after cerebellar tumour surgery. Cortex J. Devoted Study Nerv. Syst. Behav. 2010, 46, 933–946. [Google Scholar] [CrossRef]
- Liu, J.-F.; Dineen, R.A.; Avula, S.; Chambers, T.; Dutta, M.; Jaspan, T.; MacArthur, D.C.; Howarth, S.; Soria, D.; Quinlan, P.; et al. Development of a pre-operative scoring system for predicting risk of post-operative paediatric cerebellar mutism syndrome. Br. J. Neurosurg. 2018, 32, 18–27. [Google Scholar] [CrossRef]
- Bae, D.; Mlc, V.V.; Catsman-Berrevoets, C.E. Preoperative prediction of postoperative cerebellar mutism syndrome. Validation of existing MRI models and proposal of the new Rotterdam pCMS prediction model. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2020, 36, 1471–1480. [Google Scholar] [CrossRef]
- Miller, N.G.; Reddick, W.E.; Kocak, M.; Glass, J.O.; Löbel, U.; Morris, B.; Gajjar, A.; Patay, Z. Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. AJNR Am. J. Neuroradiol. 2010, 31, 288–294. [Google Scholar] [CrossRef]
- Law, N.; Greenberg, M.; Bouffet, E.; Taylor, M.D.; Laughlin, S.; Strother, D.; Fryer, C.; McConnell, D.; Hukin, J.; Kaise, C.; et al. Clinical and neuroanatomical predictors of cerebellar mutism syndrome. Neuro-Oncology 2012, 14, 1294–1303. [Google Scholar] [CrossRef]
- Albazron, F.M.; Bruss, J.; Jones, R.M.; Yock, T.I.; Pulsifer, M.B.; Cohen, A.L.; Nopoulos, P.C.; Abrams, A.N.; Sato, M.; Boes, A.D. Pediatric postoperative cerebellar cognitive affective syndrome follows outflow pathway lesions. Neurology 2019, 93, e1561–e1571. [Google Scholar] [CrossRef] [PubMed]
- Krienen, F.M.; Buckner, R.L. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb. Cortex 2009, 19, 2485–2497. [Google Scholar] [CrossRef] [PubMed]
- Marek, S.; Siegel, J.S.; Gordon, E.M.; Raut, R.V.; Gratton, C.; Newbold, D.J.; Ortega, M.; Laumann, T.O.; Adeyemo, B.; Miller, D.B.; et al. Spatial and Temporal Organization of the Individual Human Cerebellum. Neuron 2018, 100, 977–993.e7. [Google Scholar] [CrossRef] [PubMed]
- Mariën, P.; De Smet, H.J.; Wijgerde, E.; Verhoeven, J.; Crols, R.; De Deyn, P.P. Posterior fossa syndrome in adults: A new case and comprehensive survey of the literature. Cortex J. Devoted Study Nerv. Syst. Behav. 2013, 49, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Suresh, H.; Morgan, B.R.; Mithani, K.; Warsi, N.M.; Yan, H.; Germann, J.; Boutet, A.; Loh, A.; Gouveia, F.V.; Young, J.; et al. Postoperative cerebellar mutism syndrome is an acquired autism-like network disturbance. Neuro-Oncology 2024, 26, 950–964. [Google Scholar] [CrossRef]
- Sanduleanu, S.; Woodruff, H.C.; De Jong, E.E.; Van Timmeren, J.E.; Jochems, A.; Dubois, L.; Lambin, P. Tracking tumor biology with radiomics: A systematic review utilizing a radiomics quality score. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 127, 349–360. [Google Scholar] [CrossRef]
- Traverso, A.; Wee, L.; Dekker, A.; Gillies, R. Repeatability and Reproducibility of Radiomic Features: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1143–1158. [Google Scholar] [CrossRef]
- Wang, S.; Wang, G.; Zhang, W.; He, J.; Sun, W.; Yang, M.; Sun, Y.; Peet, A. MRI-based whole-tumor radiomics to classify the types of pediatric posterior fossa brain tumor. Neurochirurgie 2022, 68, 601–607. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, Y.; Liang, C.; Zhao, Y.; Lv, X.; Zou, Y.; Yan, K.; Zheng, H.; Liang, D.; Li, Z.C. Biologic Pathways Underlying Prognostic Radiomics Phenotypes from Paired MRI and RNA Sequencing in Glioblastoma. Radiology 2021, 301, 654–663. [Google Scholar] [CrossRef]
- Fan, X.; Li, J.; Huang, B.; Lu, H.; Lu, C.; Pan, M.; Wang, X.; Zhang, H.; You, Y.; Wang, X.; et al. Noninvasive radiomics model reveals macrophage infiltration in glioma. Cancer Lett. 2023, 573, 216380. [Google Scholar] [CrossRef] [PubMed]
- Pacchiano, F.; Tortora, M.; Doneda, C.; Izzo, G.; Arrigoni, F.; Ugga, L.; Cuocolo, R.; Parazzini, C.; Righini, A.; Brunetti, A. Radiomics and artificial intelligence applications in pediatric brain tumors. World J. Pediatr. WJP 2024, 20, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J.; Valera, E.M.; Schmahmann, J.D. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. NeuroImage 2012, 59, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Hernandez-Castillo, C.R.; Poldrack, R.A.; Ivry, R.B.; Diedrichsen, J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat. Neurosci. 2019, 22, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Geva, S.; Schneider, L.M.; Roberts, S.; Green, D.W.; Price, C.J. The Effect of Focal Damage to the Right Medial Posterior Cerebellum on Word and Sentence Comprehension and Production. Front. Hum. Neurosci. 2021, 15, 664650. [Google Scholar] [CrossRef]
- Ardakani, A.A.; Bureau, N.J.; Ciaccio, E.J.; Acharya, U.R. Interpretation of radiomics features—A pictorial review. Comput. Methods Programs Biomed. 2022, 215, 106609. [Google Scholar] [CrossRef]
- Laukamp, K.R.; Shakirin, G.; Baeßler, B.; Thiele, F.; Zopfs, D.; Hokamp, N.G.; Timmer, M.; Kabbasch, C.; Perkuhn, M.; Borggrefe, J. Accuracy of Radiomics-Based Feature Analysis on Multiparametric Magnetic Resonance Images for Noninvasive Meningioma Grading. World Neurosurg. 2019, 132, e366–e390. [Google Scholar] [CrossRef]
- Committeri, U.; Fusco, R.; Di Bernardo, E.; Abbate, V.; Salzano, G.; Maglitto, F.; Dell’Aversana Orabona, G.; Piombino, P.; Bonavolontà, P.; Arena, A.; et al. Radiomics Metrics Combined with Clinical Data in the Surgical Management of Early-Stage (cT1-T2 N0) Tongue Squamous Cell Carcinomas: A Preliminary Study. Biology 2022, 11, 468. [Google Scholar] [CrossRef]
- Li, N.-Y.; Shi, B.; Chen, Y.L.; Wang, P.P.; Wang, C.B.; Chen, Y.; Ge, Y.Q.; Dong, J.N.; Wei, C. The Value of MRI Findings Combined With Texture Analysis in the Differential Diagnosis of Primary Ovarian Granulosa Cell Tumors and Ovarian Thecoma-Fibrothecoma. Front. Oncol. 2021, 11, 758036. [Google Scholar] [CrossRef]
- Shim, K.Y.; Chung, S.W.; Jeong, J.H.; Hwang, I.; Park, C.K.; Kim, T.M.; Park, S.H.; Won, J.K.; Lee, J.H.; Lee, S.T.; et al. Radiomics-based neural network predicts recurrence patterns in glioblastoma using dynamic susceptibility contrast-enhanced MRI. Sci. Rep. 2021, 11, 9974. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, R.; Tang, O.; Hu, C.; Tang, L.; Chang, K.; Shen, Q.; Wu, J.; Zou, B.; Xiao, B.; et al. Automatic Machine Learning to Differentiate Pediatric Posterior Fossa Tumors on Routine MR Imaging. AJNR Am. J. Neuroradiol. 2020, 41, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
| Overall (N = 247) | Training (N = 174) | Validation (N = 73) | p-Value | ||
|---|---|---|---|---|---|
| 247 | 174 | 73 | |||
| CMS (%) | Non-CMS | 174 (70.4) | 128 (73.6) | 46 (63.0) | 0.132 |
| CMS | 73 (29.6) | 46 (26.4) | 27 (37.0) | ||
| Location weight [Q1, Q3] | 1.72 [0.14, 4.62] | 1.72 [0.17, 4.14] | 1.43 [0.14, 5.34] | 0.593 | |
| Age at surgery [Q1, Q3] | 4.88 [2.89, 7.78] | 4.85 [2.61, 7.60] | 5.06 [3.05, 8.37] | 0.658 | |
| Gender (%) | Female | 104 (42.1) | 67 (38.5) | 37 (50.7) | 0.104 |
| Male | 143 (57.9) | 107 (61.5) | 36 (49.3) | ||
| Size [Q1, Q3] | 48.25 [40.00, 55.53] | 48.19 [38.50, 54.91] | 48.72 [40.00, 57.80] | 0.192 | |
| Consistency (%) | Non-solid | 35 (14.2) | 27 (15.5) | 8 (11.0) | 0.461 |
| Solid | 212 (85.8) | 147 (84.5) | 65 (89.0) | ||
| Hydrocephalus, n (%) | No | 131 (53.0) | 98 (56.3) | 33 (45.2) | 0.145 |
| Yes | 116 (47.0) | 76 (43.7) | 40 (54.8) | ||
| Paraventricular edema (%) | No | 95 (38.5) | 72 (41.4) | 23 (31.5) | 0.19 |
| Yes | 152 (61.5) | 102 (58.6) | 50 (68.5) | ||
| Presurgical VP shunt (%) | No | 222 (89.9) | 152 (87.4) | 70 (95.9) | 0.072 |
| Yes | 25 (10.1) | 22 (12.6) | 3 (4.1) | ||
| Surgical Route (%) | R1 | 63 (25.5) | 48 (27.6) | 15 (20.5) | 0.411 |
| R2 | 58 (23.5) | 43 (24.7) | 15 (20.5) | ||
| R3 | 55 (22.3) | 35 (20.1) | 20 (27.4) | ||
| R4 | 71 (28.7) | 48 (27.6) | 23 (31.5) | ||
| Pathology (%) | Other | 156 (63.2) | 114 (65.5) | 42 (57.5) | 0.297 |
| MB | 91 (36.8) | 60 (34.5) | 31 (42.5) | ||
| Midline location (%) | No | 68 (27.5) | 48 (27.6) | 20 (27.4) | 1 |
| Yes | 179 (72.5) | 126 (72.4) | 53 (72.6) |
| Overall(N = 247) | Non-CMS (N = 174) | CMS(N = 73) | p-Value | ||
|---|---|---|---|---|---|
| Location weight [Q1, Q3] | 1.72 [0.14, 4.62] | 0.85 [0.00, 3.36] | 3.68 [1.73, 7.18] | <0.001 | |
| Age at surgery (years), median [Q1, Q3] | 4.88 [2.89, 7.78] | 4.59 [2.64, 7.62] | 5.41 [3.67, 7.81] | 0.146 | |
| Gender, n (%) | Female | 104 (42.1) | 81 (46.6) | 23 (31.5) | 0.041 |
| Male | 143 (57.9) | 93 (53.4) | 50 (68.5) | ||
| Size [Q1, Q3] | 48.25 [40.00, 55.53] | 47.94 [39.50, 55.70] | 48.32 [40.00, 55.20] | 0.825 | |
| Consistency, n (%) | Non-solid | 35 (14.2) | 28 (16.1) | 7 (9.6) | 0.255 |
| Solid | 212 (85.8) | 146 (83.9) | 66 (90.4) | ||
| Hydrocephalus, n (%) | No | 131 (53.0) | 96 (55.2) | 35 (47.9) | 0.369 |
| Yes | 116 (47.0) | 78 (44.8) | 38 (52.1) | ||
| Paraventricular edema, n (%) | No | 95 (38.5) | 73 (42.0) | 22 (30.1) | 0.11 |
| Yes | 152 (61.5) | 101 (58.0) | 51 (69.9) | ||
| Presurgical VP shunt, n (%) | No | 222 (89.9) | 158 (90.8) | 64 (87.7) | 0.607 |
| Yes | 25 (10.1) | 16 (9.2) | 9 (12.3) | ||
| Surgical Route, n (%) | R1 | 63 (25.5) | 41 (23.6) | 22 (30.1) | 0.001 |
| R2 | 58 (23.5) | 32 (18.4) | 26 (35.6) | ||
| R3 | 55 (22.3) | 48 (27.6) | 7 (9.6) | ||
| R4 | 71 (28.7) | 53 (30.5) | 18 (24.7) | ||
| Pathology, n (%) | Other | 156 (63.2) | 120 (69.0) | 36 (49.3) | 0.005 |
| MB | 91 (36.8) | 54 (31.0) | 37 (50.7) | ||
| Midline location, n (%) | No | 68 (27.5) | 58 (33.3) | 10 (13.7) | 0.003 |
| Yes | 179 (72.5) | 116 (66.7) | 63 (86.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, X.; Yang, W.; Cai, Y.; Peng, X.; Qiu, X.; Ling, M.; Yang, P.; Chen, J.; Zhang, H.; Ma, W.; et al. Integrating Radiomics and Lesion Mapping for Cerebellar Mutism Syndrome Prediction. Children 2025, 12, 667. https://doi.org/10.3390/children12060667
Chai X, Yang W, Cai Y, Peng X, Qiu X, Ling M, Yang P, Chen J, Zhang H, Ma W, et al. Integrating Radiomics and Lesion Mapping for Cerebellar Mutism Syndrome Prediction. Children. 2025; 12(6):667. https://doi.org/10.3390/children12060667
Chicago/Turabian StyleChai, Xinyi, Wei Yang, Yingjie Cai, Xiaojiao Peng, Xuemeng Qiu, Miao Ling, Ping Yang, Jiashu Chen, Hong Zhang, Wenping Ma, and et al. 2025. "Integrating Radiomics and Lesion Mapping for Cerebellar Mutism Syndrome Prediction" Children 12, no. 6: 667. https://doi.org/10.3390/children12060667
APA StyleChai, X., Yang, W., Cai, Y., Peng, X., Qiu, X., Ling, M., Yang, P., Chen, J., Zhang, H., Ma, W., Ni, X., & Ge, M. (2025). Integrating Radiomics and Lesion Mapping for Cerebellar Mutism Syndrome Prediction. Children, 12(6), 667. https://doi.org/10.3390/children12060667






