The Evaluation of Anemia Among Stunted Children Aged 6–24 Months in Bandung District, West Java, Indonesia
Abstract
1. Introduction
2. Materials and Methods
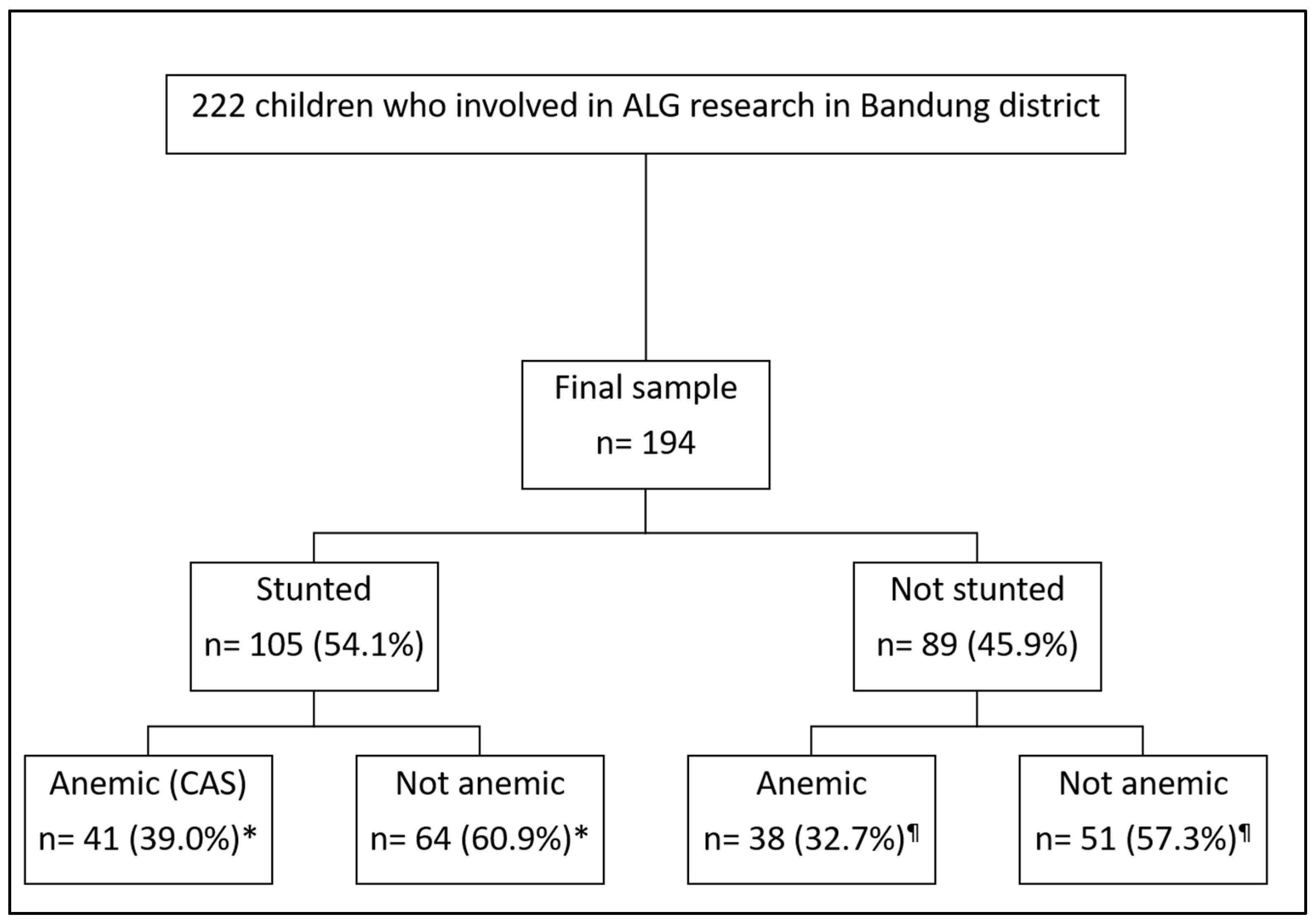
2.1. Study Design, Setting, and Participants
2.2. Data Collection
2.3. Diagnostic Criteria
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Sociodemographic Characteristics of the Study
3.2. Prevalence of Anemia and CAS
3.3. Factors Associated with IDA
3.4. Factors Associated with the Co-Occurrence of Anemia and Stunting (CAS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAP | American Academy of Pediatrics |
| AOR | Adjusted odds ratio |
| CAS | Co-occurrence of anemia and stunting |
| CI | Confidence interval |
| Hb | Hemoglobin |
| ID | Iron deficiency |
| IDA | Iron-deficiency anemia |
| LBW | Low birth weight |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| MCV | Mean corpuscular volume |
| MI | Mentzer index |
| RBC | Red blood cells |
| Ret-He | Reticulocyte-hemoglobin |
| SD | Standard deviation |
| SLI | Shine and Lal Index |
| UNICEF | United Nations Children’s Fund |
| WHO | World Health Organization |
References
- World Health Organization. Accelerating Anaemia Reduction: A Comprehensive Framework for Action; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Chaparro, C.M.; Suchdev, P.S. Anemia Epidemiology, Pathophysiology, and Etiology in Low-and Middle-income Countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef]
- Indonesia, K.K.R. Hasil Riset Kesehatan Dasar Nasional Tahun 2018; Kementerian Kesehatan Republik Indonesia: South Jakarta, Indonesia, 2018. [Google Scholar]
- Tran, T.D.; Biggs, B.-A.; Holton, S.; Nguyen, H.T.M.; Hanieh, S.; Fisher, J. Co-Morbid Anaemia and Stunting among Children of Pre-School Age in Low-and Middle-Income Countries: A Syndemic. Public Health Nutr. 2019, 22, 35–43. [Google Scholar] [CrossRef]
- Gaston, R.T.; Habyarimana, F.; Ramroop, S. Joint Modelling of Anaemia and Stunting in Children Less than Five Years of Age in Lesotho: A Cross-Sectional Case Study. BMC Public Health 2022, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Mustakim, M.R.D.; Irawan, R.; Irmawati, M.; Setyoboedi, B. Impact of Stunting on Development of Children Between 1–3 Years of Age. Ethiop. J. Health Sci. 2022, 32, 569–578. [Google Scholar]
- Laksono, A.D.; Wulandari, R.D.; Amaliah, N.; Wisnuwardani, R.W. Stunting among Children under Two Years in Indonesia: Does Maternal Education Matter? PLoS ONE 2022, 17, e0271509. [Google Scholar] [CrossRef]
- Mohammed, S.H.; Larijani, B.; Esmaillzadeh, A. Concurrent Anemia and Stunting in Young Children: Prevalence, Dietary and Non-Dietary Associated Factors. Nutr. J. 2019, 18, 10. [Google Scholar] [CrossRef]
- Orsango, A.Z.; Loha, E.; Lindtjørn, B.; Engebretsen, I.M.S. Co-Morbid Anaemia and Stunting among Children 2–5 Years Old in Southern Ethiopia: A Community-Based Cross-Sectional Study. BMJ Paediatr Open 2021, 5, e001039. [Google Scholar] [CrossRef]
- Malako, B.G.; Asamoah, B.O.; Tadesse, M.; Hussen, R.; Gebre, M.T. Stunting and Anemia among Children 6–23 Months Old in Damot Sore District, Southern Ethiopia. BMC Nutr 2019, 5, 3. [Google Scholar] [CrossRef]
- Rahman, M.S.; Mushfiquee, M.; Masud, M.S.; Howlader, T. Association between Malnutrition and Anemia in Under-Five Children and Women of Reproductive Age: Evidence from Bangladesh Demographic and Health Survey 2011. PLoS ONE 2019, 14, e0219170. [Google Scholar] [CrossRef]
- Htay, Z.W.; Swe, T.; Hninn, T.S.S.; Myar, M.T.; Wai, K.M. Factors Associated with Syndemic Anemia and Stunting among Children in Myanmar: A Cross-Sectional Study from a Positive Deviance Approach. Arch. Pédiatrie 2023, 30, 372–377. [Google Scholar] [CrossRef]
- Rivera, A.; Marín, V.; Romaní, F. Concurrence of Anemia and Stunting and Associated Factors among Children Aged 6 to 59 Months in Peru. PLoS Glob. Public Health 2024, 4, e0002914. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaoud, N.M.; Al-Shami, E.; Prakash, P. Anemia and Associated Factors among Kuwaiti Preschool Children and Their Mothers. Alex. J. Med. 2015, 51, 161–166. [Google Scholar] [CrossRef]
- Gosdin, L.; Martorell, R.; Bartolini, R.M.; Mehta, R.; Srikantiah, S.; Young, M.F. The Co-Occurrence of Anaemia and Stunting in Young Children. Matern. Child Nutr. 2018, 14, e12597. [Google Scholar] [CrossRef] [PubMed]
- Castejon, H.V.; Ortega, P.; Amaya, D.; Gomez, D.; Leal, J.; Castejon, O.J. Co-Existence of Anemia, Vitamins A Deficiency and Growth Retardation Among Children 24–84 Months Old in Maracaibo, Venezuela. Nutr. Neurosci. 2004, 7, 113–119. [Google Scholar] [CrossRef]
- Albalak, R.; Ramakrishnan, U.; Stein, A.D.; Van Der Haar, F.; Haber, M.J.; Schroeder, D.; Martorell, R. Community and International Nutrition Research Communication Co-Occurrence of Nutrition Problems in Honduran Children. J. Nutr. 2000, 130, 2271–2273. [Google Scholar] [CrossRef]
- World Health Organization. Stunting Prevalence Among Children Under 5 Years of Age; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- World Health Organization. Levels and Trends in Child Malnutrition Child Malnutrition: UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates: Key Findings of the 2023 Edition; World Health Organization: Geneva, Switzerland, 2023; ISBN 9240073795. [Google Scholar]
- Kementerian Kesehatan Republik Indonesia. Hasil Survei Status Gizi Indonesia (SSGI) 2022; Kementerian Kesehatan Republik Indonesia: South Jakarta, Indonesia, 2023. [Google Scholar]
- Sanità, D. WHO Child Growth Standards: Length/Height-For-Age, Weight-For-Age, Weight-For-Length, Weight-For-Height And Body Mass Index-For-Age: Methods And Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- United Nations Children’s Fund (UNICEF). UNICEF Conceptual Framework on Maternal and Child Nutrition; UNICEF Indonesia: Jakarta, Indonesia, 2021. [Google Scholar]
- Karlsson, O.; Kim, R.; Moloney, G.M.; Hasman, A.; Subramanian, S.V. Patterns in Child Stunting by Age: A Cross-sectional Study of 94 Low-and Middle-income Countries. Matern. Child Nutr. 2023, 19, e13537. [Google Scholar] [CrossRef]
- Lukman, T.N.E.; Anwar, F.; Riyadi, H.; Harjomidjojo, H.; Martianto, D. Birth Weight and Length Associated with Stunting among Children Under-Five in Indonesia. J. Gizi Pangan 2021, 16, 99–108. [Google Scholar]
- Sartika, A.N.; Khoirunnisa, M.; Meiyetriani, E.; Ermayani, E.; Pramesthi, I.L.; Nur Ananda, A.J. Prenatal and Postnatal Determinants of Stunting at Age 0–11 Months: A Cross-Sectional Study in Indonesia. PLoS ONE 2021, 16, e0254662. [Google Scholar] [CrossRef]
- United Nations Children’s Fund (UNICEF). Towards a Future in Indonesia Without Child Undernutrition; UNICEF Indonesia: Jakarta, Indonesia, 2021. [Google Scholar]
- Siramaneerat, I.; Astutik, E.; Agushybana, F.; Bhumkittipich, P.; Lamprom, W. Examining Determinants of Stunting in Urban and Rural Indonesian: A Multilevel Analysis Using the Population-Based Indonesian Family Life Survey (IFLS). BMC Public Health 2024, 24, 1371. [Google Scholar] [CrossRef]
- Asmare, A.A.; Agmas, Y.A. Determinants of Coexistence of Stunting, Wasting, and Underweight Among Children Under Five Years in the Gambia; Evidence from 2019/20 Gambian Demographic Health Survey: Application of Multivariate Binary Logistic Regression Model. BMC Public Health 2022, 22, 1621. [Google Scholar] [CrossRef]
- Beal, T.; Tumilowicz, A.; Sutrisna, A.; Izwardy, D.; Neufeld, L.M. A Review of Child Stunting Determinants in Indonesia. Matern. Child Nutr. 2018, 14, e12617. [Google Scholar] [CrossRef]
- Soliman, A.; De Sanctis, V.; Alaaraj, N.; Ahmed, S.; Alyafei, F.; Hamed, N.; Soliman, N. Early and Long-Term Consequences of Nutritional Stunting: From Childhood to Adulthood. Acta Biomed. 2021, 92, e2021168. [Google Scholar] [CrossRef]
- McCarthy, E.K.; Murray, D.M.; Kiely, M.E. Iron Deficiency during the First 1000 Days of Life: Are We Doing Enough to Protect the Developing Brain? Proc. Nutr. Soc. 2022, 81, 108–118. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Populations; World Health Organization: Geneva, Switzerland, 2020; ISBN 9240000127. [Google Scholar]
- Baker, R.D.; Greer, F.R.; Nutrition, T.C. Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0–3 Years of Age). Pediatrics 2010, 126, 1040–1050. [Google Scholar] [CrossRef]
- Ullrich, C.; Wu, A.; Armsby, C.; Rieber, S.; Wingerter, S.; Brugnara, C.; Shapiro, D.; Bernstein, H. Screening Healthy Infants for Iron Deficiency Using Reticulocyte Hemoglobin Content. JAMA 2005, 294, 924–930. [Google Scholar] [CrossRef]
- Pramantik, D.N.; Ratnaningsih, T.; Mulyono, B. Iron Deficiency Screening with Content Hemoglobin Reticulocyte (Chr) in Children Aged 6 Months to 5 Years. J. Med. Sci. (Berk. Ilmu Kedokt.) 2016, 47, 3. [Google Scholar] [CrossRef]
- Mahagna, L.; Tanous, O.; Dujovny, T.; Eitam, H.; Masalha, R.; Colodner, R.; Koren, A.; Levin, C. Leptin Is Associated with the Degree of Anemia and the Erythropoietin Levels in β Thalassemia Patients. Blood 2017, 130, 950. [Google Scholar] [CrossRef]
- Oktarina, C.; Dilantika, C.; Sitorus, N.L.; Basrowi, R.W. Relationship between Iron Deficiency Anemia and Stunting in Pediatric Populations in Developing Countries: A Systematic Review and Meta-Analysis. Children 2024, 11, 1268. [Google Scholar] [CrossRef]
- Mutumba, R.; Mbabazi, J.; Pesu, H.; Greibe, E.; Olsen, M.F.; Briend, A.; Mølgaard, C.; Ritz, C.; Mupere, E.; Filteau, S. Micronutrient Status and Other Correlates of Hemoglobin Among Children with Stunting: A Cross-Sectional Study in Uganda. Nutrients 2023, 15, 3785. [Google Scholar] [CrossRef] [PubMed]
- Dessie, G.; Li, J.; Nghiem, S.; Doan, T. Prevalence and Determinants of Stunting-Anemia and Wasting-Anemia Comorbidities and Micronutrient Deficiencies in Children Under 5 in the Least-Developed Countries: A Systematic Review and Meta-Analysis. Nutr. Rev. 2024, 83, nuae063. [Google Scholar] [CrossRef] [PubMed]
- El-Shafie, A.M.; Kasemy, Z.A.; Omar, Z.A.; Alkalash, S.H.; Salama, A.A.; Mahrous, K.S.; Hewedy, S.M.; Kotb, N.M.; Abd El-Hady, H.S.; Eladawy, E.S.; et al. Prevalence of Short Stature and Malnutrition among Egyptian Primary School Children and Their Coexistence with Anemia. Ital. J. Pediatr. 2020, 46, 91. [Google Scholar] [CrossRef]
- Bustan, W.N.; Hadju, V.; Indriasari, R.; Hasan, N.; Salmah, A.U.; Daud, N.A.; Nirmalasari, R. Coexistence of Anemia and Stunting among Adolescent Girls Aged 13–15 Years in A Coastal Area of Indonesia: Prevalence and Related Causal Factors. Azerbaijan Med. J. 2023, 63, 7567–7575. [Google Scholar]
- Sahiledengle, B.; Mwanri, L.; Petrucka, P.; Agho, K.E. Coexistence of Anaemia and Stunting among Children Aged 6–59 Months in Ethiopia: Findings from the Nationally Representative Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 6251. [Google Scholar] [CrossRef]
- Amare, H.H.; Lindtjorn, B. Concurrent Anemia and Stunting Among Schoolchildren in Wonago District in Southern Ethiopia: A Cross-Sectional Multilevel Analysis. PeerJ 2021, 9, e11158. [Google Scholar] [CrossRef]
- Omer, A.; Hailu, D.; Whiting, S.J. Child-Owned Poultry Intervention Effects on Hemoglobin, Anemia, Concurrent Anemia and Stunting, and Morbidity Status of Young Children in Southern Ethiopia: A Cluster Randomized Controlled Community Trial. Int. J. Environ. Res. Public Health 2023, 20, 5406. [Google Scholar] [CrossRef]
- Satriawan, E. Strategi Nasional Percepatan Pencegahan Stunting 2018–2024; Tim Nasional Percepatan Penanggulangan Kemiskinan (TNP2K): Jakata, Indonesia, 2018. [Google Scholar]
- Sardjoko, S. Strategi Nasional Percepatan Penurunan Stunting, Capaian Dan Pelaksanaannya. In Disampaikan pada Rapat Koordinasi Teknis Membangun Dan Memperkuat Komitmen Dalam Percepatan Pencegahan Anak Kerdil (Stunting); Tim Nasional Percepatan Penanggulangan Kemiskinan (TNP2K): Jakarta, Indonesia, 2020. [Google Scholar]
| Characteristics | Stunting (n = 105) | No Stunting (n = 89) | p |
|---|---|---|---|
| Subjects (n = 194) | |||
| Age (months): mean ± SD | 18 ± 7 | 15.7 ± 4.7 | 0.004 * |
| Sex | 0.788 | ||
| Male | 61 (58.1) | 50 (56.2) | |
| Female | 44 (41.9) | 39 (43.8) | |
| Birth weight | 0.027 * | ||
| Normal | 82 (78.1) | 80 (89.9) | |
| Low birth weight | 23 (21.9) | 9 (10.1) | |
| Hemoglobin | 11.2 ± 1.9 | 11.3 ± 1.6 | 0.606 |
| <11 g/dL | 41 (39.0) | 38 (42.7) | |
| ≥11 g/dL | 64 (61.0) | 51 (57.3) | |
| Iron-deficiency anemia | 0.314 | ||
| Yes | 34 (32.4) | 35 (39.3) | |
| No | 71 (67.6) | 54 (60.7) | |
| Meal frequency per day | 0.050 | ||
| 3 times or more | 61 (58.1) | 51 (57.3) | |
| 2 times | 40 (38.1) | 35 (39.3) | |
| Once | 4 (3.8) | 3 (3.4) | |
| Exclusive breastfeeding | 0.430 | ||
| Yes | 74 (70.5) | 58 (65.2) | |
| No | 31 (29.5) | 31 (34.8) | |
| Current formula milk consumption | 0.826 | ||
| Yes | 31 (34.8) | 35 (33.3) | |
| No | 70 (66.7) | 58 (65.2) | |
| Weekly meat consumption | 2 ± 2 | 2.2 ± 2.1 | 0.452 |
| Weekly consumption of legumes and nuts | 2 ± 6 | 2.8 ± 2.8 | 0.607 |
| Weekly egg consumption | 4 ± 5 | 4.2 ± 2.6 | 0.326 |
| Weekly milk consumption | 0 ± 7 | 2.7 ± 3.7 | 0.855 |
| Mother’s age (years): mean ± SD | 29 ± 12 | 29.9 ± 6.4 | 0.372 |
| Gestational age | 0.489 | ||
| Term | 91 (86.7) | 80 (89.9) | |
| Preterm | 14 (13.3) | 9 (10.1) | |
| Father’s age (years): mean ± SD | 33 ± 12 | 33.4 ± 7.6 | 0.372 |
| Mother’s education | 0.179 | ||
| No education | 1 (1) | 1 (1.1) | |
| Primary school | 40 (38.1) | 21 (23.6) | |
| Junior high school | 44 (41.9) | 39 (43.8) | |
| Senior high school | 18 (17.1) | 25 (28.1) | |
| University | 2 (1.9) | 3 (3.4) | |
| Father’s education | 0.060 | ||
| No education | 0 (0.0) | 1 (1.1) | |
| Primary school | 45 (42.9) | 23 (25.8) | |
| Junior high school | 35 (33.3) | 30 (33.7) | |
| Senior high school | 23 (21.9) | 33 (37.1) | |
| University | 2 (1.9) | 2 (2.3) | |
| Family income (per month) | 0.351 | ||
| ≥regional minimum wage standard | 18 (17.1) | 20 (22.5) | |
| <regional minimum wage standard | 87 (82.9) | 69 (77.5) | |
| Weight-for-age | <0.001 * | ||
| Normal | 53 (50.5) | 79 (88.8) | |
| Underweight | 22 (21.0) | 9 (10.1) | |
| Severely underweight | 30 (28.6) | 1 (1.1) | |
| Weight-for-length | 0.351 | ||
| Normal | 73 (69.5) | 75 (84.3) | |
| Overweight | 1 (1.0) | 0 (0.0) | |
| Wasted | 14 (13.3) | 7 (7.9) | |
| Severely wasted | 17 (16.2) | 7 (7.9) |
| Variables | OR (95%CI) | p |
|---|---|---|
| Stunting | 0.42 (0.19 to 0.94) | 0.04 * |
| Age | 1.02 (0.94 to 1.09) | 0.69 |
| Normal birth weight | 0.55 (0.19 to 1.57) | 0.26 |
| Weight-for-age | ||
| Normal | 0.83 (0.22 to 3.15) | 0.79 |
| Weight-for-length | ||
| Normal | 3.40 (0.85 to 13.61) | 0.08 |
| Wasted | 7.12 (1.60 to 31.71) | 0.01 * |
| Meal frequency per day | ||
| 3 times or more | 1.36 (0.68 to 2.72) | 0.38 |
| No exclusive breastfeeding | 0.45 (0.19 to 1.02) | 0.06 |
| Formula milk consumption | 0.75 (0.33 to 1.70) | 0.49 |
| Preterm gestational age | 1.14 (0.34 to 3.76) | 0.83 |
| Mother’s education | ||
| Primary school | 0.79 (0.33 to 1.86) | 0.59 |
| Senior high school | 0.83 (0.33 to 2.08) | 0.69 |
| Father’s education | ||
| Primary school | 3.32 (1.35 to 8.20) | 0.01 * |
| Senior high school | 1.06 (0.42 to 2.67) | 0.89 |
| Family income (per month) | ||
| ≥regional minimum wage | 2.09 (0.88 to 4.94) | 0.09 |
| Characteristics | CAS (n = 41) | No CAS (n = 153) | p |
|---|---|---|---|
| Subjects (n = 194) | |||
| Age (months): mean ± SD | 17.0 ± 5.0 | 16.6 ± 4.7 | 0.50 |
| Sex | 0.05 | ||
| Male | 29 (70.7) | 82 (53.6) | |
| Female | 12 (29.3) | 71 (46.4) | |
| Birth weight | 0.56 | ||
| Normal | 33 (80.5) | 129 (84.3) | |
| Low birth weight | 8 (19.5) | 24 (15.7) | |
| Hemoglobin | 9.8 ± 1.0 | 11.6 ± 1.3 | 0.00 * |
| <11 g/dL | (39.0) | 115 (75.2) | |
| ≥11 g/dL | 64 (61.0) | (24.8) | |
| Iron-deficiency anemia | 0.00 * | ||
| Yes | 34 (82.9) | 35 (22.9) | |
| No | 7 (17.1) | 118 (77.1) | |
| Meal frequency per day | 0.68 | ||
| 3 times or more | 26 (63.4) | 86 (56.2) | |
| 2 times | 14 (34.2) | 61 (39.9) | |
| Once | 1 (2.4) | 6 (3.9) | |
| Exclusive breastfeeding | 0.12 | ||
| Yes | 32 (78.0) | 100 (65.4) | |
| No | 9 (22.0) | 53 (34.6) | |
| Current formula milk consumption | 0.14 | ||
| Yes | 10 (24.4) | 56 (36.6) | |
| No | 31 (75.6) | 97 (63.4) | |
| Weekly meat consumption | 1.4 ± 1.3 | 2.2 ± 2.0 | 0.06 |
| Weekly consumption of legumes and nuts | 3.0 ± 2.7 | 2.6 ± 2.7 | 0.36 |
| Weekly egg consumption | 3.6 ± 2.8 | 4.1 ± 2.6 | 0.344 |
| Weekly milk consumption | 2.5 ± 3.2 | 2.6 ± 3.4 | 0.94 |
| Mother’s age (years): mean ± SD | 30.6 ± 7.4 | 29.2 ± 6.7 | 0.27 |
| Gestational age | 0.94 | ||
| Term | 36 (87.8) | 135 (88.2) | |
| Preterm | 5 (12.2) | 18 (11.8) | |
| Father’s age (years): mean ± SD | 34.8 ± 7.9 | 32.3 ± 7.7 | 0.17 |
| Mother’s education | 0.22 | ||
| No education | 1 (2.4) | 1 (0.7) | |
| Primary school | 16 (39.0) | 45 (29.4) | |
| Junior high school | 19 (46.3) | 64 (41.8) | |
| Senior high school | 4 (9.8) | 39 (25.5) | |
| University | 2 (1.9) | 3 (3.4) | |
| Father’s education | 0.05 | ||
| No education | 0 (0.0) | 1 (0.7) | |
| Primary school | 21 (51.2) | 47 (30.7) | |
| Junior high school | 14 (33.5) | 51 (33.3) | |
| Senior high school | 5 (12.2) | 51 (33.3) | |
| University | 2 (1.9) | 2 (2.3) | |
| Family income (per month) | 0.66 | ||
| ≥regional minimum wage | 9 (22.0) | 29 (19.0) | |
| <regional minimum wage | 32 (78.0) | 124 (81.0) | |
| Weight-for-age | 0.00 * | ||
| Normal | 17 (41.5) | 115 (75.2) | |
| Underweight | 11 (26.8) | 20 (13.0) | |
| Severely underweight | 13 (31.7) | 18 (11.8) | |
| Weight-for-length | 0.07 | ||
| Normal | 27 (65.8) | 121 (79.1) | |
| Overweight | 0 (0.0) | 1 (0.6) | |
| Wasted | 9 (22.0) | 12 (7.8) | |
| Severely wasted | 5 (12.2) | 19 (12.4) |
| Variables | OR (95 %CI) | p |
|---|---|---|
| Normal birth weight | 1.46 (0.42 to 5.12) | 0.55 |
| Weight-for-age | ||
| Underweight | 0.93 (0.22 to 3.96) | 0.92 |
| Normal | 0.48 (0.11 to 2.07) | 0.32 |
| Weight-for-length | ||
| Normal | 2.59 (0.49 to 13.65) | 0.26 |
| Wasted | 5.89 (0.95 to 36.45) | 0.06 |
| Meal frequency per day | ||
| 3 times or more | 1.38 (0.52 to 3.63) | 0.51 |
| No exclusive breastfeeding | 0.69 (0.21 to 2.25) | 0.54 |
| Formula milk consumption | 0.43 (0.13 to 1.38) | 0.16 |
| Preterm gestational age | 0.92 (0.19 to 4.39) | 0.92 |
| Mother’s education | ||
| Primary school | 1.14 (0.14 to 90.26) | 0.95 |
| Junior high school | 1.38 (0.02 to 106.53) | 0.88 |
| Senior high school | 0.45 (0.01 to 26.25) | 0.70 |
| Father’s education | ||
| Primary school | 0.69 (0.01 to 40.27) | 0.86 |
| Junior high school | 0.37 (0.01 to 20.70) | 0.63 |
| Senior high school | 0.19 (0.00 to 8.75) | 0.40 |
| Family income (per month) | ||
| ≥regional minimum wage | 4.23 (1.02 to 17.60) | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susanah, S.; Marcelius, D.; Rakhmilla, L.E.; Rossanti, R.; Febrianti, S.A.; Sakinah, S.; Winyarti, W.; Lutfia, S.S.; Judistiani, R.T.D.; Gurnida, D.A.; et al. The Evaluation of Anemia Among Stunted Children Aged 6–24 Months in Bandung District, West Java, Indonesia. Children 2025, 12, 638. https://doi.org/10.3390/children12050638
Susanah S, Marcelius D, Rakhmilla LE, Rossanti R, Febrianti SA, Sakinah S, Winyarti W, Lutfia SS, Judistiani RTD, Gurnida DA, et al. The Evaluation of Anemia Among Stunted Children Aged 6–24 Months in Bandung District, West Java, Indonesia. Children. 2025; 12(5):638. https://doi.org/10.3390/children12050638
Chicago/Turabian StyleSusanah, Susi, David Marcelius, Lulu Eva Rakhmilla, Rini Rossanti, Sindy Amalia Febrianti, Siti Sakinah, Winyarti Winyarti, Safira Satyani Lutfia, Raden Tina Dewi Judistiani, Dida Akhmad Gurnida, and et al. 2025. "The Evaluation of Anemia Among Stunted Children Aged 6–24 Months in Bandung District, West Java, Indonesia" Children 12, no. 5: 638. https://doi.org/10.3390/children12050638
APA StyleSusanah, S., Marcelius, D., Rakhmilla, L. E., Rossanti, R., Febrianti, S. A., Sakinah, S., Winyarti, W., Lutfia, S. S., Judistiani, R. T. D., Gurnida, D. A., & Setiabudiawan, B. (2025). The Evaluation of Anemia Among Stunted Children Aged 6–24 Months in Bandung District, West Java, Indonesia. Children, 12(5), 638. https://doi.org/10.3390/children12050638






