Early Mobilization Protocols in Critically Ill Pediatric Patients: A Scoping Review of Strategies, Tools and Perceived Barriers
Abstract
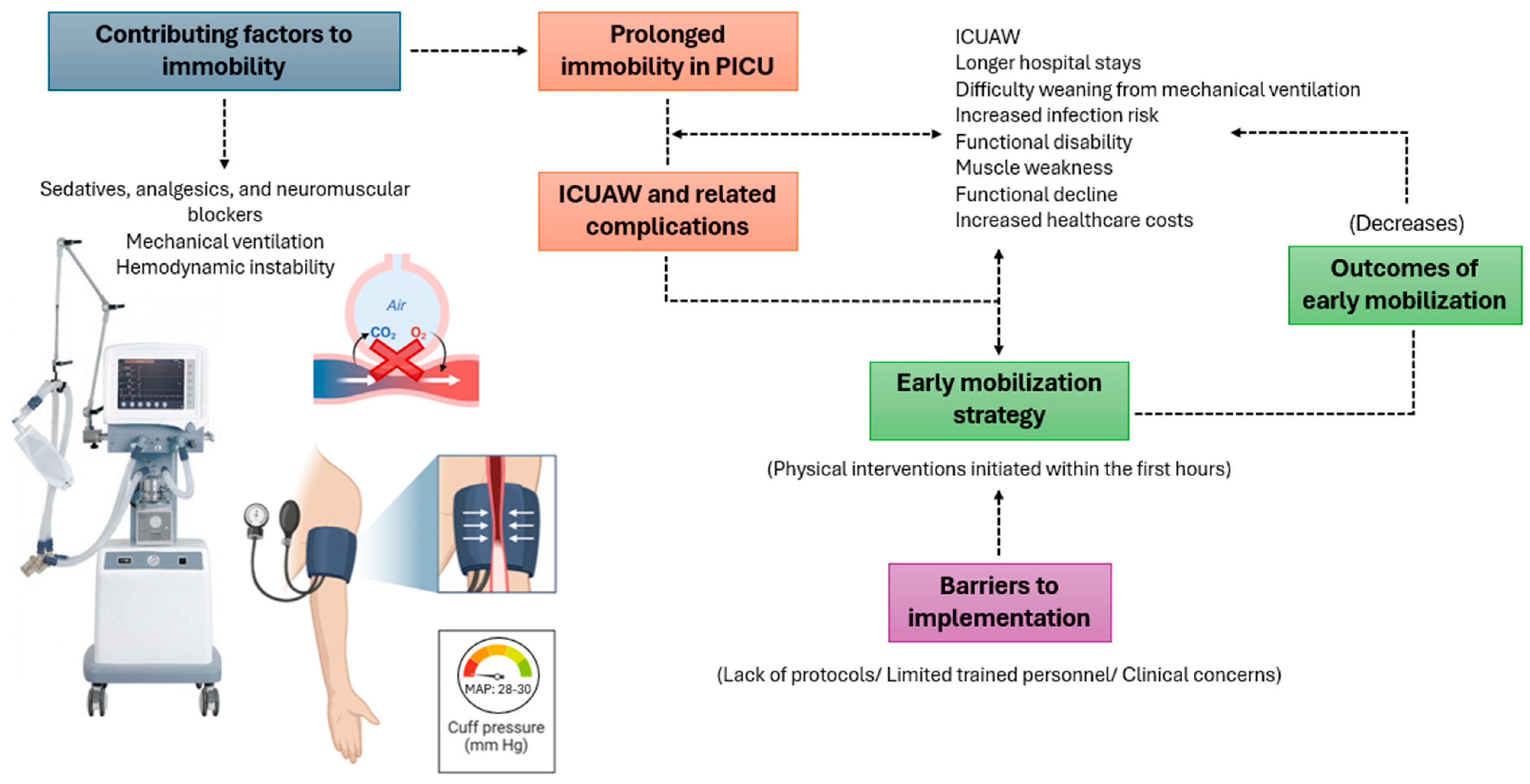
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.2.1. Search Sources
2.2.2. Review Question
- ▪
- Population: Critically ill pediatric patients in intensive care units;
- ▪
- Intervention: Early mobilization;
- ▪
- Comparison: Not applicable;
- ▪
- Outcomes: Identification of early mobilization protocols used, strategies implemented, and barriers perceived by healthcare teams in their implementation for this population.
2.3. Search Terms
2.4. Eligibility Criteria
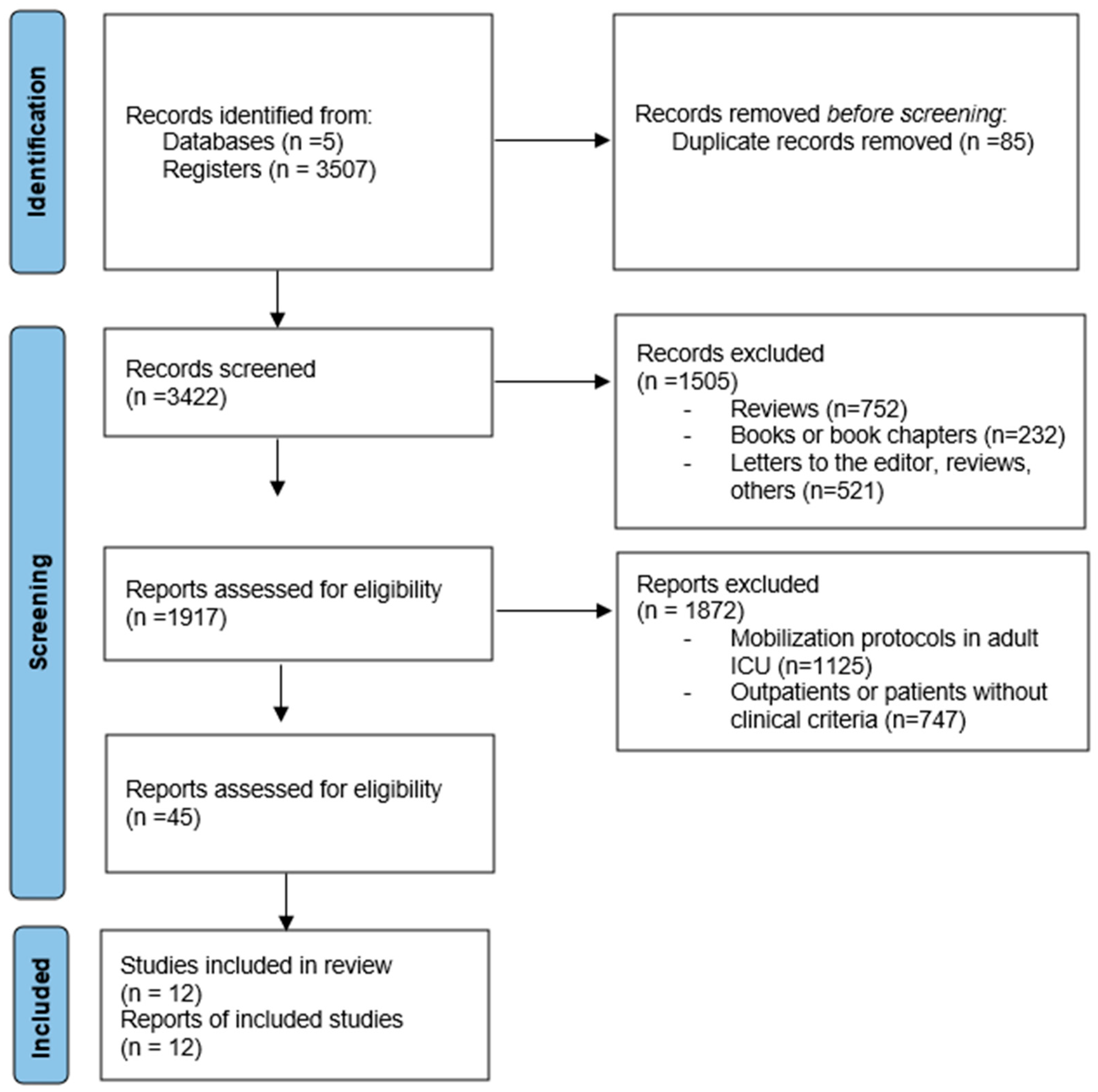
2.5. Study Selection
2.6. Quality Assessment of Studies
2.7. Data Extraction and Synthesis
2.8. Transparency and Reproducibility
3. Results
3.1. Methodological Quality of Studies
3.2. Analysis of Study Design, PICU Stay, Measurements and Interventions
3.3. Description and Analysis of Early Mobilization in PICUs
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PICUs | Pediatric Intensive Care Units |
| ICUAW | Intensive Care Unit-acquired Weakness |
| NIMV | Non-Invasive Mechanical Ventilation |
| IMV | Invasive Mechanical Ventilation |
| MAP | Mean Arterial Pressure |
| PCPC | Pediatric Cerebral Performance Category |
| ECMO | Extracorporeal Membrane Oxygenation |
| POPC | Pediatric Overall Performance Category |
| CAP-D | Pediatric Delirium Assessment |
| FSS | Functional Status Scale |
| PEDI-CAT | Pediatric Evaluation of Disability Inventory-CAT |
| WiiMT | Wii Motion Therapy for Upper Extremities |
Appendix A. Database Search Queries
| Database | PubMed | Scopus | Web of Science | Dimensions AI | ScienceDirect |
| Search Date | 22 December 2024 | 22 December 2024 | 08 December 2024 | 14 December 2024 | 10 December 2024 |
| Search Fields | All fields | All fields | All fields | Title and abstract | All fields |
| Search Equation | (((((Early Ambulation) OR (Mobilization)) AND (Pediatrics)) OR (Child)) AND (Respiration Artificial)) AND (Hypotension) | (((((Early Ambulation) OR (Mobilization)) AND (Pediatrics)) OR (Child)) AND (Respiration Artificial)) AND (Hypotension) | (((((Early Ambulation) OR (Mobilization)) AND (Pediatrics)) OR (Child)) AND (Respiration Artificial)) AND (Hypotension) | (((((Early Ambulation) OR (Mobilization)) AND (Pediatrics)) OR (Child)) AND (Respiration Artificial)) AND (Hypotension) | (((((Early Ambulation) OR (Mobilization)) AND (Pediatrics)) OR (Child)) AND (Respiration Artificial)) AND (Hypotension) |
| Records Identified | 258 | 1.497 | 3 | 129 | 1620 |
Appendix B
Appendix B.1. The Newcastle–Ottawa Scale (NOS) for Assessing Study Quality—Cohorts
| Selection | Comparability | Exposure | Score | |||||
| First Author & Reference | Representativeness of the Cohort Exposed | Selection of the Unexposed Cohort | Demonstration that the Outcome of Interest Was Not Present at the Start of the Study | Comparability of Cohorts Based on Design or Analysis | Evaluation of the Result | The Follow-Up Was Enough Prolonged for Them to Occur the Results? | Adequacy of Cohort Monitoring | Total |
| Choong, Karen MB, et al., 2014 [1] | * | * | * | * | * | * | * | 7–9 |
| Simpson C et al., 2022 [31] | * | * | * | ** | * | * | * | 8–9 |
| Newcastle-Ottawa Scale (NOS) Criteria: Each star represents the achievement of a specific criterion on the scale. *: one criterion, **: two criteria. | ||||||||
Appendix B.2. The Newcastle–Ottawa Scale (NOS) for Assessing Study Quality—Cross-Sectional and Longitudinal Studies
| First Author & Reference | Kudchadkar, S, et al., 2020 [28] | Wieczorek, B, et al., 2016 [29] | Cui, LR, et al., 2017 [32] | Colwell B, et al., 2018 [35] | Herbsman, J, et al., 2020 [36] | Ista E, et al., 2020 [37] | Simonassi JI, et al., 2022 [38] |
| Are eligibility criteria specified? | * | * | * | * | * | * | * |
| Representativeness of the sample | * | * | * | - | * | * | * |
| Sample selection/sample size | * | * | - | - | * | * | * |
| Definition of subjects not included | - | * | - | * | * | * | * |
| Comparability between participants | ** | ** | ** | ** | ** | ** | ** |
| Outcome assessment | * | * | * | * | * | * | * |
| Same method of outcome assessment for the entire sample | * | * | * | * | * | * | * |
| Statistical test | * | * | * | * | * | * | * |
| Quantitative | 9-8 | 9-9 | 9-7 | 9-7 | 9-9 | 9-9 | 9-9 |
| Newcastle-Ottawa Scale (NOS) Criteria: Each star represents the achievement of a specific criterion on the scale. *: one criterion, **: two criteria | |||||||
Appendix B.3. The Jadad Scale for Assessing the Quality of Randomized Clinical Trials
| First Author and Reference | Fink, E, et al., 2019 [30] | Choong, K, et al., 2017 [33] |
| Is the study described as randomized? | 1 | 1 |
| Is the method used for random sequence generation described? | 1 | 1 |
| Is the method for generating the random sequence adequate? | 1 | 1 |
| Is the study described as double-blind? | 1 | 0 |
| Is the blinding method described? | 1 | 0 |
| Is the blinding method adequate? | 1 | 0 |
| Are the losses and withdrawals from the study described? | 1 | 1 |
| Quantitative score | 7 | 4 |
| Qualitative score | Good quality | Good quality |
Appendix B.4. The MINORS Scale for Assessing the Quality of Non-Randomized Studies
| First Author and Reference | Abdulsatara, F, et al., 2013 [34] |
| Established objective | |
| Inclusion of patients consecutively | |
| Prospective data collection | |
| Appropriate data collection according to study objectives | |
| Unbiased outcome assessment | |
| Appropriate follow-up period according to study objectives | |
| Follow-up loss of less than 5% | |
| Sample size calculation (95% CI) | |
| Adequate control group | |
| Control and study groups managed simultaneously | |
| Baseline equivalence of groups | |
| Adequate statistical analysis | |
| Quantitative score | 20 |
| Qualitative score | Good quality |
| Green: 2 points; Yellow: 1 point; Red: 0 points. | |
References
- Choong, K.; Foster, G.; Fraser, D.D.; Hutchison, J.S.; Joffe, A.R.; Jouvet, P.A.; Menon, K.; Pullenayegum, E.; Ward, R.E. Acute rehabilitation practices in critically ill children: A multicenter study. Pediatr. Crit. Care Med. 2014, 15, e270–e279. [Google Scholar] [CrossRef] [PubMed]
- Choong, K.M.; Koo, K.K.Y.M.; Clark, H.B.; Chu, R.; Thabane, L.; Burns, K.E.A.M.; Cook, D.J.M.; Herridge, M.S.M.; Meade, M.O.M. Early mobilization in critically ill children: A survey of Canadian practice. Crit. Care Med. 2013, 41, 1745–1753. [Google Scholar] [CrossRef]
- Cameron, S.; Ball, I.; Cepinskas, G.; Choong, K.; Doherty, T.J.; Ellis, C.G.; Martin, C.M.; Mele, T.S.; Sharpe, M.; Shoemaker, J.K.; et al. Early mobilization in the critical care unit: A review of adult and pediatric literature. J. Crit. Care 2015, 30, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.C.; Kudchadkar, S.R. Early mobilization in the pediatric intensive care unit. Transl. Pediatr. 2018, 7, 308–313. [Google Scholar] [CrossRef]
- Morris, H.; Nilan, K.; Burkhardt, M.; Wood, A.; Passarella, M.; Gibbs, K.; DeMauro, S.B. Early progressive mobility to improve neurodevelopment of infants with severe bronchopulmonary dysplasia at a level IV neonatal intensive care unit: A prospective cohort study. J. Perinatol. 2024, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Piva, T.C.; Ferrari, R.S.; Schaan, C.W. Early mobilization protocols for critically ill pediatric patients: Systematic review. Rev. Bras. Ter. Intensiv. 2019, 31, 248–257. [Google Scholar] [CrossRef]
- Hermans, G.; van den Berghe, G. Clinical review: Intensive care unit acquired weakness. Crit. Care 2015, 19, 274. [Google Scholar] [CrossRef]
- Kasinathan, A.; Sharawat, I.K.; Singhi, P.; Jayashree, M.; Sahu, J.K.; Sankhyan, N. Intensive Care Unit—Acquired Weakness in Children: A Prospective Observational Study Using Simplified Serial Electrophysiological Testing (PEDCIMP Study). Neurocritical Care 2021, 34, 927–934. [Google Scholar] [CrossRef]
- Bone, M.F.; Feinglass, J.M.; Goodman, D.M. Risk factors for acquiring functional and cognitive disabilities during admission to a PICU*. Pediatr. Crit. Care Med. 2014, 15, 640–648. [Google Scholar] [CrossRef]
- Pereira, G.A.; Schaan, C.W.; Ferrari, R.S. Functional evaluation of pediatric patients after discharge from the intensive care unit using the Functional Status Scale. Rev. Bras. Ter. Intensiv. 2017, 29, 460–465. [Google Scholar] [CrossRef]
- Al Harbi, S. Early Mobilization in Pediatric Critical Care: Exploring the Gap Between Theory and Practice in Saudi Arabia. Med. Sci. Monit. 2024, 30, e942467. [Google Scholar] [CrossRef] [PubMed]
- Appleton, R.T.; Kinsella, J.; Quasim, T. The incidence of intensive care unit-acquired weakness syndromes: A systematic review. J. Intensiv. Care Soc. 2014, 16, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Khilnani, P.; Shamim, M.; Kukreti, V. Intensive care unit acquired weakness in children: Critical illness polyneuropathy and myopathy. Indian J. Crit. Care Med. 2014, 18, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhang, W.; Peng, W.; Fan, Y.; He, X.; Xing, R.; Yan, X.; Zhou, S.; Peng, Y.; Luo, W. Knowledge, attitudes and practices regarding children with ICU-acquired weakness in pediatric intensive care unit among chinese medical staff: A cross-sectional survey. BMC Nurs. 2023, 22, 162. [Google Scholar] [CrossRef]
- Joyce, C.L.; Taipe, C.; Sobin, B.; Spadaro, M.; Gutwirth, B.; Elgin, L.; Silver, G.; Greenwald, B.M.; Traube, C. Provider Beliefs Regarding Early Mobilization in the Pediatric Intensive Care Unit. J. Pediatr. Nurs. 2018, 38, 15–19. [Google Scholar] [CrossRef]
- Prevalence of Acute Rehabilitation for Kids in the PICU (PARK-PICU) Investigators; Redivo, J.; Kannan, H.; Souza, A.A.F.; Junior, J.C.; Kudchadkar, S.R. Physical rehabilitation in Brazilian pediatric intensive care units: A multicenter point prevalence study. Crit. Care Sci. 2023, 35, 290–301. [Google Scholar] [CrossRef]
- Namachivayam, P.; Shann, F.; Shekerdemian, L.; Taylor, A.; van Sloten, I.; Delzoppo, C.; Daffey, C.; Butt, W. Three decades of pediatric intensive care: Who was admitted, what happened in intensive care, and what happened afterward*. Pediatr. Crit. Care Med. 2010, 11, 549–555. [Google Scholar] [CrossRef]
- Rennick, J.E.; Childerhose, J.E. Redefining success in the PICU: New patient populations shift targets of care. Pediatrics 2015, 135, e289–e291. [Google Scholar] [CrossRef]
- Scholefield, B.R.; Menzies, J.C.; McAnuff, J.; Thompson, J.Y.; Manning, J.C.; Feltbower, R.G.; Geary, M.; Lockley, S.; Morris, K.P.; Moore, D.; et al. Implementing early rehabilitation and mobilisation for children in UK paediatric intensive care units: The PERMIT feasibility study. Health Technol. Assess. 2023, 27, 1–155. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, H.; Liu, Y.; Tian, Y.; Nie, M.; Jing, W.; Du, A. Development of an early mobilization practice for critically ill patients in intensive care units: A Delphi method study. Am. J. Transl. Res. 2024, 16, 3875–3885. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, W.; Cai, Z.; Liu, J.; Wu, J.; Deng, Y.; Yu, K.; Chen, X.; Zhu, L.; Ma, J.; et al. Early mobilization of critically ill patients in the intensive care unit: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0223185. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, D.; Liu, Y.; Wang, Y.; Zhang, B.; Xiao, Q. Effects of early mobilization on the prognosis of critically ill patients: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2020, 110, 103708. [Google Scholar] [CrossRef]
- Canci, F.; Clark, H.; Hopkins, R.O.; Kudchadkar, S.R.; Lati, J.; Morrow, B.; Neu, C.; Wieczorek, B.; Zebuhr, C.; Choong, K. Practice Recommendations for Early Mobilization in Critically Ill Children. J. Pediatr. Intensiv. Care 2018, 7, 014–026. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Kudchadkar, S.R.; Nelliot, A.; Awojoodu, R.; Vaidya, D.; Traube, C.; Walker, T.; Needham, D.M.; for the Prevalence of Acute Rehabilitation for Kids in the PICU (PARK-PICU) Investigators and the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Physical Rehabilitation in Critically Ill Children: A Multicenter Point Prevalence Study in the United States. Crit. Care Med. 2020, 48, 634–644. [Google Scholar] [CrossRef]
- Wieczorek, B.D.; Ascenzi, J.D.; Kim, Y.M.; Lenker, H.P.; Potter, C.M.; Shata, N.J.M.; Mitchell, L.M.; Haut, C.D.; Berkowitz, I.M.; Pidcock, F.; et al. PICU Up!: Impact of a Quality Improvement Intervention to Promote Early Mobilization in Critically Ill Children*. Pediatr. Crit. Care Med. 2016, 17, e559–e566. [Google Scholar] [CrossRef]
- Fink, E.L.; Beers, S.R.; Houtrow, A.J.; Richichi, R.; Burns, C.; Doughty, L.; Ortiz-Aguayo, R.; Madurski, C.A.; Valenta, C.; Chrisman, M.; et al. Early Protocolized Versus Usual Care Rehabilitation for Pediatric Neurocritical Care Patients: A Randomized Controlled Trial. Pediatr. Crit. Care Med. 2019, 20, 540–550. [Google Scholar] [CrossRef]
- Simpson, C.E.; Esterman, A.J.; Ganu, S.S.; Maki, K.; Keeley, S.R.; Ward, E.J.; Tsiros, M.D. Early mobilisation in critically ill children: Does routine patient screening reduce time to commencing mobilisation? Aust. Crit. Care 2023, 36, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.R.; LaPorte, M.; Civitello, M.; Stanger, M.; Orringer, M.; Casey, F.; Kuch, B.A.; Beers, S.R.; Valenta, C.A.; Kochanek, P.M.; et al. Physical and occupational therapy utilization in a pediatric intensive care unit. J. Crit. Care 2017, 40, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Choong, K.; Awladthani, S.; Khawaji, A.; Clark, H.; Borhan, A.; Cheng, J.; Laskey, S.; Neu, C.; Sarti, A.; Thabane, L.; et al. Early Exercise in Critically Ill Youth and Children, a Preliminary Evaluation: The wEECYCLE Pilot Trial*. Pediatr. Crit. Care Med. 2017, 18, e546–e554. [Google Scholar] [CrossRef]
- Abdulsatar, F.; Walker, R.G.; Timmons, B.W.; Choong, K. “Wii-Hab” in critically ill children: A pilot trial. J. Pediatr. Rehabil. Med. 2013, 6, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Colwell, B.R.L.; Williams, C.N.; Kelly, S.P.; Ibsen, L.M. Mobilization Therapy in the Pediatric Intensive Care Unit: A Multidisciplinary Quality Improvement Initiative. Am. J. Crit. Care 2018, 27, 194–203. [Google Scholar] [CrossRef]
- Herbsman, J.M.; D’agati, M.; Klein, D.; O’donnell, S.; Corcoran, J.R.; Folks, T.D.R.; Al-Qaqaa, Y.M. Early Mobilization in the Pediatric Intensive Care Unit: A Quality Improvement Initiative. Pediatr. Qual. Saf. 2020, 5, e256. [Google Scholar] [CrossRef]
- Ista, E.; Scholefield, B.R.; Manning, J.C.; Harth, I.; Gawronski, O.; Bartkowska-Śniatkowska, A.; Ramelet, A.-S.; Kudchadkar, S.R. Mobilization practices in critically ill children: A European point prevalence study (EU PARK-PICU). Crit. Care 2020, 24, 368. [Google Scholar] [CrossRef]
- Simonassi, J.I.; Canzobre, M.T. Movilización temprana en el paciente pediátrico crítico con soporte ventilatorio. experiencia de un centro de alta complejidad. Rev. Fac. Cien Med. Univ. Nac. Cordoba 2022, 79, 334–340. [Google Scholar] [CrossRef]
- Azamfirei, R.; Mennie, C.; Dinglas, V.D.; Fatima, A.; Colantuoni, E.; Gurses, A.P.; Balas, M.C.; Needham, D.M.; Kudchadkar, S.R.; Alqahtani, M.; et al. Impact of a multifaceted early mobility intervention for critically ill children—The PICU Up! trial: Study protocol for a multicenter stepped-wedge cluster randomized controlled trial. Trials 2023, 24, 191. [Google Scholar] [CrossRef]
- Morrow, B.M. Building a culture of early mobilization in the pediatric intensive care unit—A nuts and bolts approach. Transl. Pediatr. 2021, 10, 2845–2857. [Google Scholar] [CrossRef]
- Dubb, R.; Nydahl, P.; Hermes, C.; Schwabbauer, N.; Toonstra, A.; Parker, A.M.; Kaltwasser, A.; Needham, D.M. Barriers and Strategies for Early Mobilization of Patients in Intensive Care Units. Ann. Am. Thorac. Soc. 2016, 13, 724–730. [Google Scholar] [CrossRef]
- Geven, B.M.; Maaskant, J.M.; van Woensel, J.B.M.; Verbruggen, S.C.A.T.; Ista, E. Barriers and perceived benefits of early mobilisation programmes in Dutch paediatric intensive care units. Nurs. Crit. Care 2023, 28, 519–525. [Google Scholar] [CrossRef]
- Schweickert, W.D.; Pohlman, M.C.; Pohlman, A.S.; Nigos, C.; Pawlik, A.J.; Esbrook, C.L.; Spears, L.; Miller, M.; Franczyk, M.; Deprizio, D.; et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet 2009, 373, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Zanni, J.M.; Korupolu, R.; Fan, E.; Pradhan, P.; Janjua, K.; Palmer, J.B.; Brower, R.G.; Needham, D.M. Rehabilitation therapy and outcomes in acute respiratory failure: An observational pilot project. J. Crit. Care 2010, 25, 254–262. [Google Scholar] [CrossRef]
- Thompson, J.Y.; Menzies, J.C.; Manning, J.C.; McAnuff, J.; Brush, E.C.; Ryde, F.; Rapley, T.; Pathan, N.; Brett, S.; Moore, D.J.; et al. Early mobilisation and rehabilitation in the PICU: A UK survey. BMJ Paediatr. Open 2022, 6, e001300. [Google Scholar] [CrossRef] [PubMed]
- Adel, T.Z.D.; van Dijk, M.; de Heer, M.; Hoekstra, S.; Steenhorst, J.; van Rosmalen, J.; Verbruggen, S.; Toussaint-Duyster, L.; Ista, E. Quality improvement intervention to stimulate early mobilisation of critically ill children. Nurs. Crit. Care 2023, 28, 545–553. [Google Scholar] [CrossRef]
- Pollack, M.M.; Holubkov, R.; Funai, T.; Clark, A.; Berger, J.T.; Meert, K.; Newth, C.J.L.; Shanley, T.; Moler, F.; Carcillo, J.; et al. Pediatric Intensive Care Outcomes: Development of new morbidities during pediatric critical care. Pediatr. Crit. Care Med. 2014, 15, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Hanna, E.S.; Zhao, S.; Shannon, C.N.; Betters, K.A. Changes in Provider Perceptions Regarding Early Mobility in the PICU*. Pediatr. Crit. Care Med. 2020, 21, E30–E38. [Google Scholar] [CrossRef]
- Cho, J.; Park, H.; Kang, D.; Park, E.; Chung, C.R.; Cho, J.; Kudchadkar, S.R. Rehabilitation in critically ill children: Findings from the Korean National Health Insurance database. PLoS ONE 2022, 17, e0266360. [Google Scholar] [CrossRef]
- Zheng, K.; Sarti, A.; Boles, S.; Cameron, S.; Carlisi, R.; Clark, H.; Khawaji, A.; Awladthani, S.; Al-Harbi, S.; Choong, K. Impressions of Early Mobilization of Critically Ill Children—Clinician, Patient, and Family Perspectives*. Pediatr. Crit. Care Med. 2018, 19, e350–e357. [Google Scholar] [CrossRef]
- Dirkes, S.M.; Kozlowski, C. Early Mobility in the Intensive Care Unit: Evidence, Barriers, and Future Directions. Crit. Care Nurse 2019, 39, 33–42. [Google Scholar] [CrossRef]
- Salcedo, H.A.P.; Heredia, L.G.T.; Cardoza, V.S.; Zape, J.L.E. Evaluación de la fuerza muscular por dinamometría de prensión manual en las unidades de cuidado intensivo: Revisión de literatura. Med. Crit. 2024, 38, 108–113. [Google Scholar] [CrossRef]
- Sood, S.; Ganatra, H.A.; Marques, F.P.; Langner, T.R. Complications during mechanical ventilation—A pediatric intensive care perspective. Front. Med. 2023, 10, 1016316. [Google Scholar] [CrossRef] [PubMed]
- Alaparthi, G.K.; Gatty, A.; Samuel, S.R.; Amaravadi, S.K. Effectiveness, Safety, and Barriers to Early Mobilization in the Intensive Care Unit. Crit. Care Res. Pract. 2020, 2020, 7840743. [Google Scholar] [CrossRef] [PubMed]
- Cuello-Garcia, C.A.; Mai, S.H.C.; Simpson, R.; Al-Harbi, S.; Choong, K. Early Mobilization in Critically Ill Children: A Systematic Review. J. Pediatr. 2018, 203, 25–33.e6. [Google Scholar] [CrossRef]
| Author/Year | Kudchadkar, S, et al., 2020 [28] | Wieczorek, B, et al., 2016 [29] | Fink, E, et al., 2019 [30] | Simpson, C, et al., 2022 [31] | Cui, LR, et al., 2017 [32] | Choong, Karen MB, et al., 2017 [33] | Abdulsatar et al., 2013 [34] | Colwell BRL, et al., 2018 [35] | Herbsman, J, et al., 2020 [36] | Ista, E, et al., 2020 [37] | Simonassi, JI, et al., 2022 [38] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Objective | Analyze prevalence and barriers in pediatric rehabilitation | Evaluate safety and feasibility of early mobilization | Evaluate protocolized rehabilitation in neurocritically ill patients | Evaluate the impact of a checklist for early mobilization | Characterize the use of physical and occupational therapy in the PICU | Feasibility of bed cycling in the PICU | Evaluate the safety of virtual reality exercise in the PICU | Implement early mobilization protocol in the PICU | Increase early mobilization in orthopedic and neurosurgical patients | Prevalence and factors of physical rehabilitation in PICUs in Europe | Mobilization in the PICU with ventilatory support in a Latin American hospital |
| Sample | 3098 | 200 | 110 | 71 | 138 | 30 | 8 | 567 | 403 | 456 | 196 |
| Country | USA | USA | USA | Australia | USA | Canada | Canada | USA | USA | Europe | Argentina |
| Study design | Observational, cross-sectional | Observational, longitudinal | Randomized controlled trial | Cohort study | Observational, cross-sectional | Pilot randomized controlled trial | Pilot clinical trial | Observational cross-sectional | Observational longitudinal | Multicenter cross-Sectional | Observational, cross-sectional retrospective |
| Cause of PICU admission | Cardiac diseases | Critical illnesses | Traumatic brain injury, cardiac arrest, stroke | Critical illnesses | Pulmonary, gastrointestinal, neurological, transplant, cancer | Pulmonary, neurological, cardiac | Critical illnesses | Chronic diseases | Trauma, respiratory difficulty | Cardiorespiratory and post-surgical diseases | Acute respiratory infection |
| Age (years) | 0–18 | 0–17 | 17–3 | ≥0.7–18 | 1 week–18 weeks | 17–3 | 18–3 | 0–<4 | ≥18 months | 0–<18 | <18 |
| PICU length of stay | ≥72 h | ≥72 h | ≥48 h | >48 h | ≥72 h | ≥48 h | >48 h | Not available | ≥48 h | ≥72 h | >24 h |
| Interventions and duration | Mobilization in and out of bed, within the first 3 days after admission | PICU Up program: passive and active mobilizations, sleep hygiene, delirium control, within the first 3 days | PT, OT, SLT within the first 3 days after admission | Mobilization in and out of bed, within the first 2 days after admission | PT and OT Mobilization in and out of bed, within the first 3 days after admission | Bed cycling with ergometer + habitual physiotherapy, 210 min per week for 5 days | Virtual reality exercise (WiiMT), 10 min twice a day for 2 days | Mobilization in and out of bed, 2–3 times per day | Mobilization in and out of bed between 18 and 48 h after admission | Mobilization in and out of bed for patients admitted >72 h | Mobilization in and out of bed from 72 h after admission |
| Author/ Year | Choong, Karen MB, et al., 2014 [1] | Kudchadkar, S, et al., 2020 [28] | Wieczorek, B, et al., 2016 [29] | Fink, E, et al., 2020 [30] | Simpson, C, et al., 2022 [31] | Cui, LR, et al., 2017 [32] | Choong, K, et al., 2017 [33] | Abdulsatara, F, et al., 2013 [34] | Colwell, B, et al., 2018 [35] | Herbsman, J, et al., 2020 [36] | Ista E, et al., 2020 [37] | Simonassi JI, et al., 2022 [38] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurement Scales | PCPC, POPC | PCPC | PICU Up! Questionnaire | FSS, POPC, PCPC | Early Mobilization Checklist | POPC | PEDI-CAT | Accelerometers, Grip-A.MT, PCPC, POPC | POPC | Algorithm: “ready for mobilization” | PCPC | POPC, CAP-D |
| Description of mobilization activities | Strengthening exercises, ambulation, transfers, chest physiotherapy | Passive mobility, sitting/standing, transfers, walking | Passive and active mobilization, positioning, ambulation, bed transfers | Positioning, passive/active range of motion, transfers, ambulation | In-bed mobility, edge of bed mobility, out-of-bed mobility, ambulation | Passive/active range of motion, transfers, resistance exercises, ambulation | In-bed cycling (30 min/day, 5 days/week), regular physiotherapy | WiiMT boxing exercises (10 min, twice a day for 2 days) | Passive and active movement, sitting, standing, transfers, ambulation | Active mobilization in bed, sitting on edge of bed, standing, ambulation | Passive range of motion, bed exercises, transfers, walking | Passive/active mobilization, transfers, ambulation, coordination exercises, strengthening |
| Contraindications for early mobilization | Excessive sedation, vasoactive infusions | Cardiovascular instability, excessive sedation | ECMO, open chest/abdomen, unstable fracture | Imminent death, PCPC 4-5 | Not specified | Tachycardia, desaturation | Hemodynamic instability | Cardiopulmonary instability | Desaturation, tachypnea, emesis | Severe illness, incomplete data | Cardiac instability, sedation | Clinical severity, seizure disorders |
| % Ventilated | Not specified | 59% | Not specified | 74% | 44% | 65% | Not specified | 50% | Not specified | 13.11% | IMV 39.0%, NIMV 11.8%, MVTQT 12.9% | 63.3% VMI, 37.7% VMNI |
| Perceived and declared barriers | Sedation, neuromuscular blockade, vasoactive infusions | Medical contraindications, hemodynamic instability, excessive sedation, lack of medical order | Medical procedures, hemodynamic instability, bed rest orders, equipment availability | Hemodynamic instability, abnormal intracranial pressure, parental/nursing refusal | Sedation, mechanical ventilation, | Hemodynamic instability, nursing request, patient absence | Availability of physiotherapist | Excessive sedation, ward transfers | Hemodynamic instability, lack of personnel | Lack of resources, equipment, lines/drains, patient agitation, confusion | Hemodynamic instability, excessive sedation | Not specified |
| Response to early mobilization | Improved peripheral and respiratory muscle strength and physical function and increased ventilator-free days | Safe mobilization; improved ambulation in children ≥ 3 years | Increased mobilization post-implementation | Functional improvement | Not applicable | Post-PICU functional improvement (28% ambulated) | Safe and feasible; enhanced intensity and duration of mobilization | Significant upper extremity activity increase | Increased mobilization; some ventilated patients able to walk | Early mobilization reduced hospital stay by 35% | Increased mobilization in children ≥ 3 years with family present | Early mobilization feasible in critically ill children on ventilatory support |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noreña-Buitrón, L.D.; Sanclemente-Cardoza, V.; Espinosa-Cifuentes, M.A.; Payán-Salcedo, H.A.; Estela-Zape, J.L. Early Mobilization Protocols in Critically Ill Pediatric Patients: A Scoping Review of Strategies, Tools and Perceived Barriers. Children 2025, 12, 633. https://doi.org/10.3390/children12050633
Noreña-Buitrón LD, Sanclemente-Cardoza V, Espinosa-Cifuentes MA, Payán-Salcedo HA, Estela-Zape JL. Early Mobilization Protocols in Critically Ill Pediatric Patients: A Scoping Review of Strategies, Tools and Perceived Barriers. Children. 2025; 12(5):633. https://doi.org/10.3390/children12050633
Chicago/Turabian StyleNoreña-Buitrón, Lizeth Dayana, Valeria Sanclemente-Cardoza, Maria Alejandra Espinosa-Cifuentes, Harold Andrés Payán-Salcedo, and Jose Luis Estela-Zape. 2025. "Early Mobilization Protocols in Critically Ill Pediatric Patients: A Scoping Review of Strategies, Tools and Perceived Barriers" Children 12, no. 5: 633. https://doi.org/10.3390/children12050633
APA StyleNoreña-Buitrón, L. D., Sanclemente-Cardoza, V., Espinosa-Cifuentes, M. A., Payán-Salcedo, H. A., & Estela-Zape, J. L. (2025). Early Mobilization Protocols in Critically Ill Pediatric Patients: A Scoping Review of Strategies, Tools and Perceived Barriers. Children, 12(5), 633. https://doi.org/10.3390/children12050633






