Parental Attitude Toward the Engagement in Physical Activity of Their Children with Type 1 Diabetes Mellitus in Hungary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Study Variables
2.4. Family History
2.5. Data Analysis
3. Results
3.1. Socio-Economic Factors Influencing Sports Behavior
3.2. Physical and Health Status of Children
3.3. Sporting Habits of Children
3.4. Parents’ Opinion on the Physical Activity of Their Children
3.5. Impact of Diabetes on Children’s Quality of Life
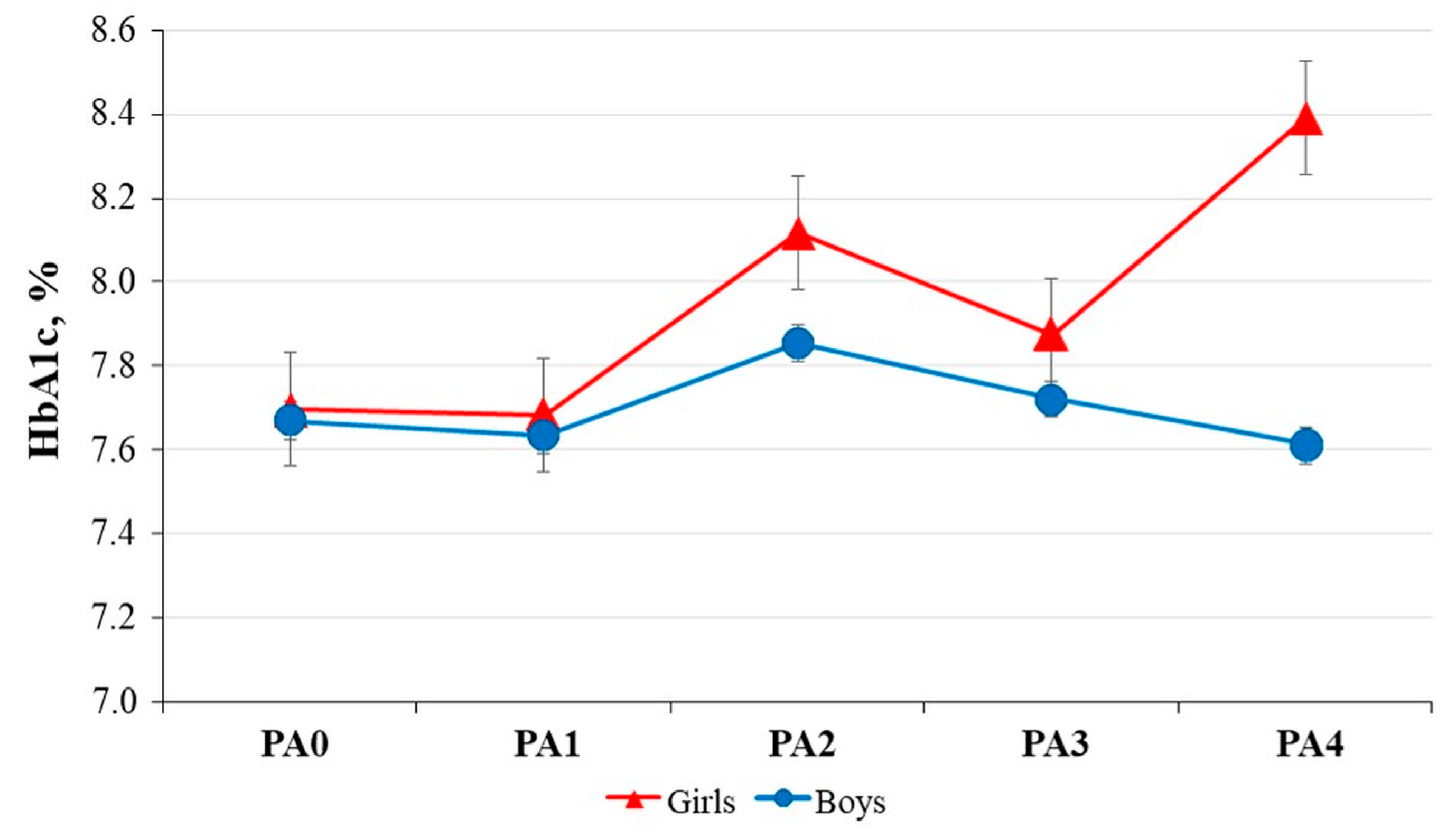
3.6. Gender-Based Analysis of Physical Activity of Children with Diabetes and Healthy Children
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batista, M.B.; Romanzini, C.L.P.; Barbosa, C.C.L.; Blasquez Shigaki, G.; Romanzini, M.; Ronque, E.R.V. Participation in sports in childhood and adolescence and physical activity in adulthood: A systematic review. J. Sports Sci. 2019, 37, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Quirk, H.; Blake, H.; Dee, B.; Glazebrook, C. “You can’t just jump on a bike and go”: A qualitative study exploring parents’ perceptions of physical activity in children with type 1 diabetes. BMC Pediatr. 2014, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.J.; Boyko, E.J. IDF DIABETES ATLAS, 10th ed.; International Diabetes Federation, Brussels, Belgium. 2021. Available online: https://www.ncbi.nlm.nih.gov/pubmed/35914061 (accessed on 24 April 2024).
- Writing Group for the SEARCH for Diabetes in Youth Study Group; Dabelea, D.; Bell, R.A.; D’Agostino, R.B., Jr.; Imperatore, G.; Johansen, J.M.; Linder, B.; Liu, L.L.; Loots, B.; Marcovina, S.; et al. Incidence of diabetes in youth in the United States. JAMA 2007, 297, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.C.; Dahlquist, G.G.; Gyürüs, E.; Green, A.; Soltész, G.; EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: A multicentre prospective registration study. Lancet 2009, 373, 2027–2033. [Google Scholar] [CrossRef]
- Pettitt, D.J.; Talton, J.; Dabelea, D.; Divers, J.; Imperatore, G.; Lawrence, J.M.; Liese, A.D.; Linder, B.; Mayer-Davis, E.J.; Pihoker, C.; et al. Prevalence of diabetes in U.S. youth in 2009: The SEARCH for diabetes in youth study. Diabetes Care 2014, 37, 402–408. [Google Scholar] [CrossRef]
- International Diabetes Federation Europe Annual Report. 2023. Available online: https://idf.org/europe/resources/?resource-type=annual-reports (accessed on 8 June 2024).
- Dahlquist, G.G.; Nyström, L.; Patterson, C.C.; Swedish Childhood Diabetes Study Group; Diabetes Incidence in Sweden Study Group. Incidence of type 1 diabetes in Sweden among individuals aged 0–34 years, 1983–2007: An analysis of time trends. Diabetes Care 2011, 34, 1754–1759. [Google Scholar] [CrossRef]
- Gyurus, E.; Patterson, C.; Soltesz, G. “Constantly rising or peaks and plateaus?” Incidence of childhood type 1 diabetes in Hungary (1989–2009). Orv. Hetil. 2011, 152, 1692–1697. [Google Scholar] [CrossRef]
- Zhang, K.; Kan, C.; Han, F.; Zhang, J.; Ding, C.; Guo, Z.; Huang, N.; Zhang, Y.; Hou, N.; Sun, X. Global, Regional, and National Epidemiology of Diabetes in Children From 1990 to 2019. JAMA Pediatr. 2023, 177, 837–846. [Google Scholar] [CrossRef]
- Barkai, L.; Kiss, Z.; Rokszin, G.; Abonyi-Tóth, Z.; Jermendy, G.; Wittmann, I.; Kempler, P. Changes in the incidence and prevalence of type 1 and type 2 diabetes among 2 million children and adolescents in Hungary between 2001 and 2016—A nationwide population-based study. Arch. Med. Sci. 2020, 16, 34–41. [Google Scholar] [CrossRef]
- Berg, C.A.; King, P.S.; Butler, J.M.; Pham, P.; Palmer, D.; Wiebe, D.J. Parental involvement and adolescents’ diabetes management: The mediating role of self-efficacy and externalizing and internalizing behaviors. J. Pediatr. Psychol. 2011, 36, 329–339. [Google Scholar] [CrossRef]
- Young, M.T.; Lord, J.H.; Patel, N.J.; Gruhn, M.A.; Jaser, S.S. Good cop, bad cop: Quality of parental involvement in type 1 diabetes management in youth. Curr. Diab Rep. 2014, 14, 546. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.S.; Kozikowski, C.; Lee, J.M.; Wysocki, T. Type 1 diabetes in very young children: A model of parent and child influences on management and outcomes. Pediatr. Diabetes 2017, 18, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Turin, A.; Radobuljac, M.D. Psychosocial factors affecting the etiology and management of type 1 diabetes mellitus: A narrative review. World J. Diabetes 2021, 12, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Stanek, K.R.; Noser, A.E.; Patton, S.R.; Clements, M.A.; Youngkin, E.M.; Majidi, S. Stressful life events, parental psychosocial factors, and glycemic management in school-aged children during the 1 year follow-up of new-onset type 1 diabetes. Pediatr. Diabetes 2020, 21, 673–680. [Google Scholar] [CrossRef]
- Mohammed, J.; Deda, L.; Clarson, C.L.; Stein, R.I.; Cuerden, M.S.; Mahmud, F.H. Assessment of habitual physical activity in adolescents with type 1 diabetes. Can. J. Diabetes 2014, 38, 250–255. [Google Scholar] [CrossRef]
- Willeboordse, M.; van de Kant, K.D.; van der Velden, C.A.; van Schayck, C.P.; Dompeling, E. Associations between asthma, overweight and physical activity in children: A cross-sectional study. BMC Public Health 2016, 16, 919. [Google Scholar] [CrossRef]
- Muth, N.D.; Bolling, C.; Hannon, T.; Sharifi, M.; Section on Obesity; Committee on Nutrition. The Role of the Pediatrician in the Promotion of Healthy, Active Living. Pediatrics 2024, 153, e2023065480. [Google Scholar] [CrossRef]
- Baran, J.; Weres, A.; Wyszyńska, J.; Pitucha, G.; Czenczek-Lewandowska, E.; Rusek, W.; Leszczak, J.; Mazur, A. 60 Minutes Per Day in Moderate to Vigorous Physical Activity as a Natural Health Protector in Young Population. Int. J. Environ. Res. Public Health 2020, 17, 8918. [Google Scholar] [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- American Diabetes Association, 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. 1), S13–S28. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; American Public Health Association; National Resource Center for Health and Safety in Child Care and Early Education. Caring for Our Children: National Health and Safety Performance Standards; Guidelines for Early Care and Education Programs. 2019. Available online: https://nrckids.org/files/CFOC4%20pdf-%20FINAL.pdf (accessed on 8 June 2024).
- Ogle, G.; Middlehurst, A.; Silink, M.; Hanas, R. Pocketbook for Management of Diabetes in Childhood and Adolescence in Under-Resourced Countries, 2nd ed.; International Diabetes Federation: Brussels, Belgium, 2017; Available online: https://idf.org/media/uploads/2023/05/attachments-56.pdf (accessed on 8 June 2024).
- Strong, W.B.; Malina, R.M.; Blimkie, C.J.; Daniels, S.R.; Dishman, R.K.; Gutin, B.; Hergenroeder, A.C.; Must, A.; Nixon, P.A.; Pivarnik, J.M.; et al. Evidence based physical activity for school-age youth. J. Pediatr. 2005, 146, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef]
- Jabbour, G.; Henderson, M.; Mathieu, M.E. Barriers to Active Lifestyles in Children with Type 1 Diabetes. Can. J. Diabetes 2016, 40, 170–172. [Google Scholar] [CrossRef]
- Lascar, N.; Kennedy, A.; Hancock, B.; Jenkins, D.; Andrews, R.C.; Greenfield, S.; Narendran, P. Attitudes and barriers to exercise in adults with type 1 diabetes (T1DM) and how best to address them: A qualitative study. PLoS ONE 2014, 9, e108019. [Google Scholar] [CrossRef]
- Miculis, C.P.; De Campos, W.; da Silva Boguszweski, M.C. Correlation between glycemic control and physical activity level in adolescents and children with type 1 diabetes. J. Phys. Act. Health 2015, 12, 232–237. [Google Scholar] [CrossRef]
- Niranjan, V.; McBrayer, D.G.; Ramirez, L.C.; Raskin, P.; Hsia, C.C. Glycemic control and cardiopulmonary function in patients with insulin-dependent diabetes mellitus. Am. J. Med. 1997, 103, 504–513. [Google Scholar] [CrossRef]
- Reddy, R.; Wittenberg, A.; Castle, J.R.; El Youssef, J.; Winters-Stone, K.; Gillingham, M.; Jacobs, P.G. Effect of Aerobic and Resistance Exercise on Glycemic Control in Adults With Type 1 Diabetes. Can. J. Diabetes 2019, 43, 406–414.e1. [Google Scholar] [CrossRef]
- Codella, R.; Terruzzi, I.; Luzi, L. Why should people with type 1 diabetes exercise regularly? Acta Diabetol. 2017, 54, 615–630. [Google Scholar] [CrossRef]
- De Ferranti, S.D.; de Boer, I.H.; Fonseca, V.; Fox, C.S.; Golden, S.H.; Lavie, C.J.; Magge, S.N.; Marx, N.; McGuire, D.K.; Orchard, T.J.; et al. Type 1 diabetes mellitus and cardiovascular disease: A scientific statement from the American Heart Association and American Diabetes Association. Circulation 2014, 130, 1110–1130. [Google Scholar] [CrossRef] [PubMed]
- Quirk, H.; Blake, H.; Tennyson, R.; Randell, T.L.; Glazebrook, C. Physical activity interventions in children and young people with Type 1 diabetes mellitus: A systematic review with meta-analysis. Diabet. Med. 2014, 31, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Tully, C.; Aronow, L.; Mackey, E.; Streisand, R. Physical Activity in Youth With Type 1 Diabetes: A Review. Curr. Diab Rep. 2016, 16, 85. [Google Scholar] [CrossRef]
- Plamper, M.; Gohlke, B.; Woelfle, J.; Konrad, K.; Rohrer, T.; Hofer, S.; Bonfig, W.; Fink, K.; Holl, R.W. Interaction of Pubertal Development and Metabolic Control in Adolescents with Type 1 Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 8615769. [Google Scholar] [CrossRef] [PubMed]
- Rawshani, A.; Sattar, N.; Franzén, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.M.; Eliasson, B.; Gudbjörnsdottir, S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: A nationwide, register-based cohort study. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef]
- Czenczek-Lewandowska, E.; Grzegorczyk, J.; Mazur, A. Physical activity in children and adolescents with type 1 diabetes and contem-porary methods of its assessment. Pediatr. Endocrinol. Diabetes Metab. 2018, 24, 179–184. [Google Scholar] [CrossRef]
- Wu, N.; Bredin, S.S.D.; Guan, Y.; Dickinson, K.; Kim, D.D.; Chua, Z.; Kaufman, K.; Warburton, D.E.R. Cardiovascular Health Benefits of Exercise Training in Persons Living with Type 1 Diabetes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 253. [Google Scholar] [CrossRef]
- Leclair, E.; Kerdanet, M.; Riddell, M.; Heyman, E. Type 1 Diabetes and Physical Acrivity in Children and Adolescents. J. Diabetes Metab. 2013, S10. [Google Scholar] [CrossRef]
- Beraki, A.; Magnuson, A.; Särnblad, S.; Åman, J.; Samuelsson, U. Increase in physical activity is associated with lower HbA1c levels in children and adolescents with type 1 diabetes: Results from a cross-sectional study based on the Swedish pediatric diabetes quality registry (SWEDIABKIDS). Diabetes Res. Clin. Pract. 2014, 105, 119–125. [Google Scholar] [CrossRef]
- Kaza, M.; Tsentidis, C.; Vlachopapadopoulou, E.; Karanasios, S.; Sakou, I.I.; Mastorakos, G.; Karavanaki, K. The Role of Exercise on Cardiometabolic Profile and Body Composition in Youth with Type 1 Diabetes. Children 2022, 9, 1840. [Google Scholar] [CrossRef]
- Nguyen, T.; Obeid, J.; Walker, R.G.; Krause, M.P.; Hawke, T.J.; McAssey, K.; Vandermeulen, J.; Timmons, B.W. Fitness and physical activity in youth with type 1 diabetes mellitus in good or poor glycemic control. Pediatr. Diabetes 2015, 16, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, W.R.; Gabbay, M.A.; Castro, M.L.; Saraiva, G.L.; Chacra, A.R.; de Barros Neto, T.L.; Dib, S.A. Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatr. Diabetes 2005, 6, 145–149. [Google Scholar] [CrossRef]
- Williams, B.K.; Guelfi, K.J.; Jones, T.W.; Davis, E.A. Lower cardiorespiratory fitness in children with Type 1 diabetes. Diabet. Med. 2011, 28, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.S.; Espindola, C.F.; do Prado, C.; Amarins, M.B.; Potenza, A.L.; Pacheco, L.; Santos, E.; Vieira, T.C.A. Type 1 diabetes does not impair the physical capacity of non-sedentary adolescents. Diabetol. Metab. Syndr. 2017, 9, 100. [Google Scholar] [CrossRef]
- Ilkowitz, J.R.; Wu, F.; Chen, Y.; Gallagher, M.P. Perspectives on the role of exercise in the treatment of pediatric type 1 diabetes. Pediatr. Diabetes 2020, 21, 466–472. [Google Scholar] [CrossRef]
- Absil, H.; Baudet, L.; Robert, A.; Lysy, P.A. Benefits of physical activity in children and adolescents with type 1 diabetes: A systematic review. Diabetes Res. Clin. Pract. 2019, 156, 107810. [Google Scholar] [CrossRef]
- Michaud, I.; Henderson, M.; Legault, L.; Mathieu, M.-E. Physical activity and sedentary behavior levels in children and adolescents with type 1 diabetes using insulin pump or injection therapy—The importance of parental activity profile. J. Diabetes Complicat. 2017, 31, 381–386. [Google Scholar] [CrossRef]
- Ryninks, K.; Sutton, E.; Thomas, E.; Jago, R.; Shield, J.P.; Burren, C.P. Attitudes to Exercise and Diabetes in Young People with Type 1 Diabetes Mellitus: A Qualitative Analysis. PLoS ONE 2015, 10, e0137562. [Google Scholar] [CrossRef]
- Giblin, S.; Scully, P.; Dalton, N.; Connolly, M.; McCaffrey, A.; Sheikhi, A.; Neylon, O.; O’Gorman, C. Parent and child perceptions of physical activity with type 1 diabetes. BMJ Open Diabetes Res. Care 2022, 10, e002977. [Google Scholar] [CrossRef]
- Valerio, G.; Spagnuolo, M.I.; Lombardi, F.; Spadaro, R.; Siano, M.; Franzese, A. Physical activity and sports participation in children and adolescents with type 1 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 376–382. [Google Scholar] [CrossRef]
- Kennedy, A.; Narendran, P.; Andrews, R.C.; Daley, A.; Greenfield, S.M. Attitudes and barriers to exercise in adults with a recent diagnosis of type 1 diabetes: A qualitative study of participants in the Exercise for Type 1 Diabetes (EXTOD) study. BMJ Open 2018, 8, e017813. [Google Scholar] [CrossRef] [PubMed]
- Matson, R.I.B.; Leary, S.D.; Cooper, A.R.; Thompson, C.; Narendran, P.; Andrews, R.C. Objective Measurement of Physical Activity in Adults With Newly Diagnosed Type 1 Diabetes and Healthy Individuals. Front. Public Health 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- KSH, Hungarian Central Statistical Office, Fókuszban a Vármegyék. 2023. Available online: https://ksh.hu/docs/hun/xftp/megy/233/index.html (accessed on 8 June 2024).
- Acs, P.; Betlehem, J.; Oláh, A.; Bergier, J.; Melczer, C.; Prémusz, V.; Makai, A. Measurement of public health benefits of physical activity: Validity and reliability study of the international physical activity questionnaire in Hungary. BMC Public Health 2020, 20 (Suppl. 1), 1198. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Balatoni, I.; Szépné, V.H.; Müller, A.; Kovács, S.; Kosztin, N.; Csernoch, L. Sporting habits of university students in Hungary. Balt. J. Health Phys. Act. 2019, 11, 27–37. [Google Scholar] [CrossRef]
- Szépné, V.H.; Csernoch, L.; Balatoni, I. E-sports versus physical activity among adolescents. Balt. J. Health Phys. Act. 2019, 11, 38–47. [Google Scholar] [CrossRef]
- Varni, J.W.; Burwinkle, T.M.; Jacobs, J.R.; Gottschalk, M.; Kaufman, F.; Jones, K.L. The PedsQL in type 1 and type 2 diabetes: Reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care 2003, 26, 631–637. [Google Scholar] [CrossRef]
- Lukacs, A.; Simon, N.; Varga, B.; Kiss-Tóth, E.; Barkai, L. [Hungarian adaptation of the Pediatric Quality of Life Inventory 3.0 Diabetes Module]. Orv. Hetil. 2011, 152, 1837–1842. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile And Cut Off Points. In StatPearls; Ineligible Companies: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31082114 (accessed on 24 June 2024).
- Hales, C.M.; Fryar, C.D.; Carroll, M.D.; Freedman, D.S.; Ogden, C.L. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. JAMA 2018, 319, 1723–1725. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Kith, N.; Csernoch, L.; Balatoni, I. Sport habits in North-Eastern Hungary. J. Health Sci. 2014, 4, 46–59. [Google Scholar] [CrossRef]
- Dalal, M.; Cazorla-Lancaster, Y.; Chu, C.G.; Agarwal, N. Healthy From the Start-Lifestyle Interventions in Early Childhood. Am. J. Lifestyle Med. 2022, 16, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Kahn, E.B.; Ramsey, L.T.; Brownson, R.C.; Heath, G.W.; Howze, E.H.; Powell, K.E.; Stone, E.J.; Rajab, M.W.; Corso, P. The effectiveness of interventions to increase physical activity. A systematic review. Am. J. Prev. Med. 2002, 22 (Suppl. 4), 73–107. [Google Scholar] [CrossRef] [PubMed]
- Kavookjian, J.; Elswick, B.M.; Whetsel, T. Interventions for being active among individuals with diabetes: A systematic review of the literature. Diabetes Educ. 2007, 33, 962–988, discussion 989–990. [Google Scholar] [CrossRef]
- Balatoni, I.; Szépné, H.V.; Kiss, T.; Adamu, U.G.; Szulc, A.M.; Csernoch, L. The Importance of Physical Activity in Preventing Fatigue and Burnout in Healthcare Workers. Healthcare 2023, 11, 1915. [Google Scholar] [CrossRef]
- Smith, L.B.; Terry, A.; Bollepalli, S.; Rechenberg, K. School-Based Management of Pediatric Type 1 Diabetes: Recommendations, Advances, and Gaps in Knowledge. Curr. Diab Rep. 2019, 19, 37. [Google Scholar] [CrossRef]


| Variables | Definition | ||
|---|---|---|---|
| Demographic | Gender (boy, girl) Age (4–10 years, 11–14 years, 15–18 years) Place of residence (village, town, Debrecen, other county, Budapest) Education (elementary school, high school, vocational high school, technical college) | ||
| Metabolic | BMI (<5th percentile for underweight, 5th–85th percentile for normal, 85th–95th percentile for overweight, ≥95th percentile for obese) HbA1c (<7% for good glycemic control, ≥7 for poor glycemic control) | ||
| Clinical | Years of disease (<6 months, 6–12 months, >12 months) Treatment (MDIs, pump) | ||
| Physical activity | Measured by IPAQ | ||
| Other daily activities, how often, what and where the child likes to play sports | |||
| Parents’ opinions on the relationship between physical activity and diabetes; the items used are as follows: | Likert scale from 1 to 5: 1 meaning “strongly disagree” and 5 “strongly agree” | ||
| PA is important | |||
| The child likes vigorous PA | |||
| Certain sports are better | |||
| Same amount of PA as healthy peers | |||
| Diabetes is improved by PA | |||
| PA makes diabetes worse | |||
| PA is dangerous | |||
| Can cope with the requirements of PE lessons | |||
| Can participate fully in PE lessons | |||
| Daily PE lessons help | |||
| Daily PE has positively influenced their attitude | |||
| The transition from MDIs to insulin pumps has positively influenced their attitude | |||
| Quality of life | Measured by PedsQL 3.0; the diabetes symptom items used are the following: | Scale from 0 to 4: 0 meaning “never” and 7 “almost always” | |
| I felt hungry | |||
| I felt thirsty | |||
| I have to go to the bathroom too often | |||
| I have stomach aches | |||
| I have headaches I go “low” I felt tired or fatigued I become shaky I become sweaty I have trouble sleeping I get irritable | |||
| Children with T1DM (n = 140) | Healthy Children (n = 178) | |
|---|---|---|
| Gender (%) | ||
| Girls | 48.2 | 55.1 |
| Boys | 51.8 | 44.9 |
| Age groups (%) | ||
| 4–10 | 22.5 | 26.1 |
| 11–14 | 38.4 | 42.6 |
| 15–18 | 39.1 | 31.3 |
| Mean ± SD | 13.1 ± 3.2 | 12.6 ± 2.8 |
| Education (%) | ||
| Elementary school | 54.6 | 63.6 |
| High school | 29.2 | 17.9 |
| Vocational high school | 10 | 11 |
| Technical college | 6.2 | 7.5 |
| Place of residence (%) | ||
| Village | 26.1 | 22.6 |
| Town | 49.3 | 51.8 |
| Debrecen | 21.6 | 19 |
| Other county | 2.2 | 6.5 |
| Budapest | 0.7 | 0 |
| BMI * (%) | ||
| Underweight (<5th percentile) | 6.7 | 7.9 |
| Normal (5th–85th percentile) | 64.2 | 55.8 |
| Overweight (85th–95th percentile) | 17.9 | 15.8 |
| Obese (≥95th percentile) | 11.2 | 20.6 |
| Mean (kg/m2) ± SD | 20.9 ± 6.5 | 21.4 ± 5.9 |
| HbA1c (%) | ||
| Mean ± SD | 7.8 ± 1 | – |
| Diabetes present (%) | ||
| <6 months | 2.9 | – |
| 6–12 months | 5 | – |
| >12 months | 92.1 | – |
| Mean (years) ± SD | 6.2 ± 3.7 | – |
| Insulin method (%) | ||
| MDIs | 53.6 | – |
| Pump | 46.4 | – |
| Relatives have T1DM (%) | 27.1 | 23.7 |
| Physical activity (%) | ||
| Yes | 57.5 | 67.6 |
| No | 42.5 | 32.4 |
| Frequency of PA (%) | ||
| Over five times per week | 22.6 | 14.3 |
| Three to four times per week | 36.9 | 36.1 |
| One to two times per week | 35.7 | 46.2 |
| Three times per month or less | 4.8 | 3.4 |
| Statements | Children with T1DM, n = 140 | Healthy Children, n = 178 | ||||
|---|---|---|---|---|---|---|
| Physical Activity | Physical Inactivity | p-Value a | Physical Activity | Physical Inactivity | p-Value a | |
| Physical activity is important for children with diabetes. | 4.68 (0.68) | 4.23 (0.95) | 0.001 | – | – | – |
| The child likes vigorous physical activity. | 3.44 (1.34) | 2.29 (1.11) | <0.001 | 3.59 (1.17) | 2.50 (1.11) | <0.001 |
| Certain sports are better for children with diabetes. | 3.68 (1.22) | 3.39 (1.14) | 0.124 | – | – | – |
| Children with diabetes can do the same amount of physical activity as their healthy peers. | 4.30 (0.94) | 3.61 (1.33) | 0.002 | – | – | – |
| Diabetes is improved by physical activity. | 4.39 (0.96) | 3.82 (1.29) | 0.007 | – | – | – |
| Physical activity makes diabetes worse. | 1.38 (0.89) | 1.41 (0.81) | 0.429 | – | – | – |
| Physical activity is dangerous for children with diabetes. | 1.76 (1.05) | 1.81 (1.06) | 0.786 | – | – | – |
| My child feels that they can cope with the requirements of PE lessons. | 4.09 (1.43) | 3.91 (1.31) | 0.127 | 4.31 (1.17) | 3.76 (1.24) | <0.001 |
| My child feels that they can participate fully in group sports activities in PE lessons. | 4.58 (0.92) | 4.11 (1.22) | 0.009 | 4.67 (0.69) | 3.86 (1.20) | <0.001 |
| My child’s health has improved following the introduction of daily physical education. | 3.75 (1.32) | 3.02 (1.34) | 0.002 | 3.85 (1.24) | 3.22 (1.33) | 0.003 |
| My child’s attitude toward physical activity has been positively influenced by the introduction of daily PE. | 3.82 (1.27) | 2.83 (1.38) | <0.001 | 3.88 (1.27) | 3.34 (1.26) | 0.008 |
| When my child switched from MDIs to an insulin pump, their attitude toward physical activity changed in a positive direction. | 3.35 (1.53) | 2.76 (1.52) | 0.076 | – | – | – |
| Symptoms | Mean (SD) | p-Value a | |
|---|---|---|---|
| Physical Activity | Physical Inactivity | ||
| Hunger | 2.61 (1.24) | 2.80 (1.17) | 0.332 |
| Thirst | 2.69 (1.22) | 2.65 (1.26) | 0.876 |
| Frequent urination | 2.18 (1.14) | 2.14 (1.10) | 0.930 |
| Stomach pain | 2.03 (1.04) | 1.96 (1.10) | 0.576 |
| Headache | 2.21 (1.03) | 2.16 (0.98) | 0.751 |
| Blood sugar levels decrease | 2.97 (0.82) | 2.91 (0.79) | 0.865 |
| Fatigue | 2.70 (1.16) | 3.20 (1.16) | 0.015 |
| Tremor | 1.93 (0.87) | 1.89 (0.94) | 0.695 |
| Sweating | 1.89 (1.08) | 1.86 (0.94) | 0.896 |
| Bad sleep | 1.83 (0.99) | 1.91 (1.32) | 0.627 |
| Irritability | 2.47 (1.11) | 2.43 (1.33) | 0.623 |
| Statement | Children with T1DM, n = 140 | Healthy Children, n = 178 | ||||
|---|---|---|---|---|---|---|
| Girls | Boys | p-Value a | Girls | Boys | p-Value a | |
| Physical activity is important for children with diabetes. | 4.51 (0.73) | 4.46 (0.93) | 0.755 | – | – | – |
| The child likes vigorous physical activity. | 2.69 (1.33) | 3.23 (1.35) | 0.024 | 3.05 (1.32) | 3.47 (1.14) | 0.072 |
| Certain sports are better for children with diabetes. | 3.68 (1.18) | 3.40 (1.21) | 0.210 | – | – | – |
| Children with diabetes can do the same amount of physical activity as their healthy peers. | 4.08 (1.06) | 3.94 (1.24) | 0.719 | – | – | – |
| Diabetes is improved by physical activity. | 4.06 (1.14) | 4.24 (1.14) | 0.255 | – | – | – |
| Physical activity makes diabetes worse. | 1.45 (0.86) | 1.23 (0.67) | 0.086 | – | – | – |
| Physical activity is dangerous for children with diabetes. | 1.72 (0.97) | 1.76 (1.06) | 0.947 | – | – | – |
| My child feels that they can cope with the requirements of PE lessons. | 3.87 (1.48) | 4.15 (1.27) | 0.347 | 4.15 (1.21) | 4.15 (1.23) | 0.994 |
| My child feels that they can participate fully in group sports activities in PE lessons. | 4.34 (1.12) | 4.48 (0.99) | 0.450 | 4.36 (1.00) | 4.63 (0.87) | 0.382 |
| My child’s health has improved following the introduction of daily physical education. | 3.43 (1.42) | 3.48 (1.31) | 0.935 | 3.41 (1.34) | 3.93 (1.17) | 0.012 |
| My child’s attitude toward physical activity has been positively influenced by the introduction of daily PE. | 3.43 (1.38) | 3.41 (1.44) | 0.988 | 3.49 (1.32) | 3.99 (1.18) | 0.016 |
| When my child switched from MDIs to an insulin pump, their attitude toward physical activity changed in a positive direction. | 3.34 (1.44) | 3.02 (1.62) | 0.365 | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balatoni, I. Parental Attitude Toward the Engagement in Physical Activity of Their Children with Type 1 Diabetes Mellitus in Hungary. Children 2025, 12, 612. https://doi.org/10.3390/children12050612
Balatoni I. Parental Attitude Toward the Engagement in Physical Activity of Their Children with Type 1 Diabetes Mellitus in Hungary. Children. 2025; 12(5):612. https://doi.org/10.3390/children12050612
Chicago/Turabian StyleBalatoni, Ildikó. 2025. "Parental Attitude Toward the Engagement in Physical Activity of Their Children with Type 1 Diabetes Mellitus in Hungary" Children 12, no. 5: 612. https://doi.org/10.3390/children12050612
APA StyleBalatoni, I. (2025). Parental Attitude Toward the Engagement in Physical Activity of Their Children with Type 1 Diabetes Mellitus in Hungary. Children, 12(5), 612. https://doi.org/10.3390/children12050612






