Plasma Calmodulin as a Biomarker of Subclinical Cardiovascular Disease in Pediatric Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Clinical Assessments and Specimen Collection
2.3. Anthropometric Measurements
2.4. Office BP and ABPM
2.5. Echocardiography and Carotid Artery Ultrasonography
2.6. Plasma Calmodulin Measurement
2.7. Statistical Analysis
3. Results
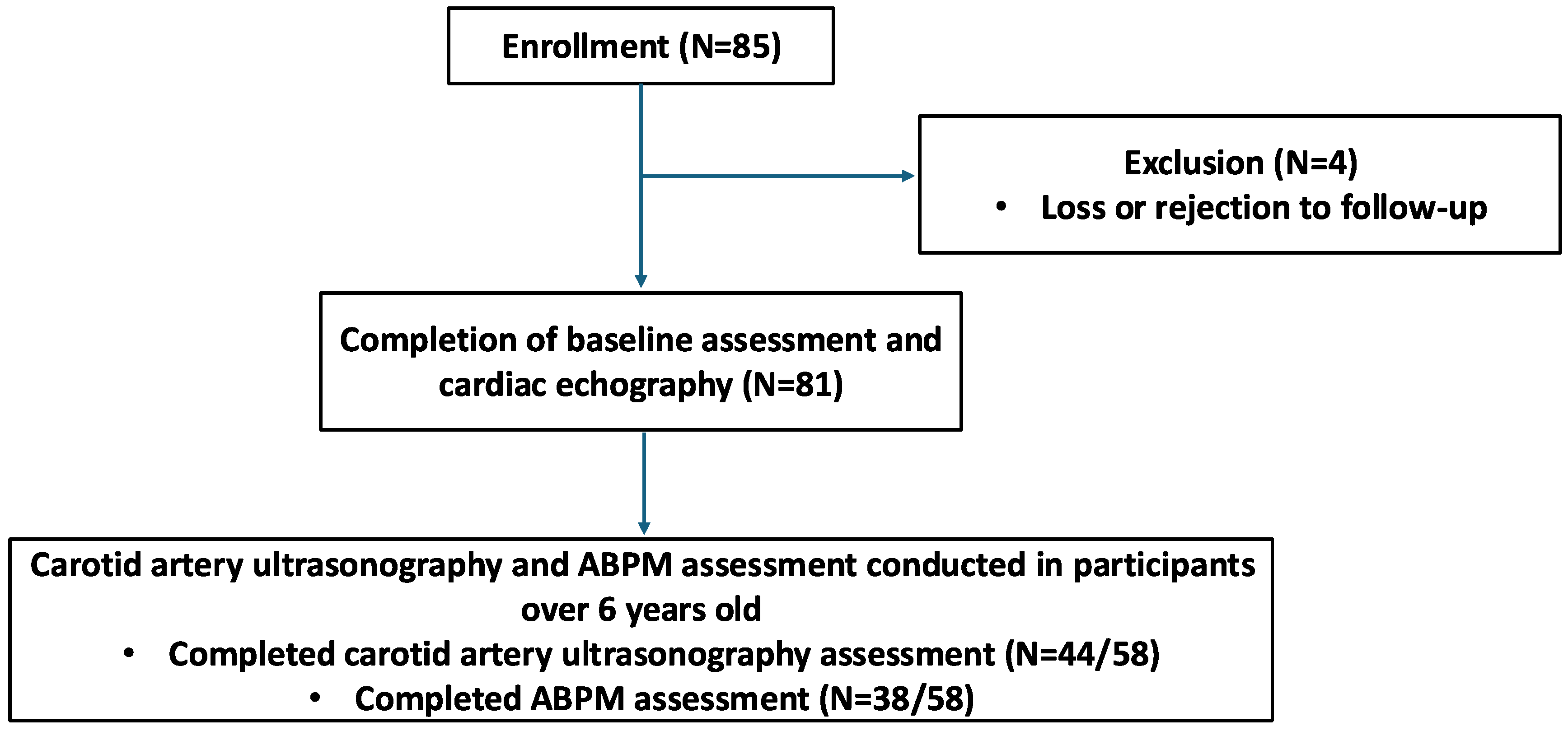
3.1. Participant Enrollment and Data Collection
3.2. Participant Characteristics
3.2.1. Traditional Cardiovascular Risk Factors
3.2.2. CKD-Specific Cardiovascular Risk Factors
3.2.3. Plasma Calmodulin Levels
3.3. Correlation Between Calmodulin and Baseline Characteristics
3.4. Correlation Between Calmodulin and Cardiovascular/Imaging Parameters
3.4.1. Echocardiography
3.4.2. Carotid Artery Ultrasonography
3.4.3. 24 h ABPM
3.5. Association Between Clinical Variables and Abnormal BP
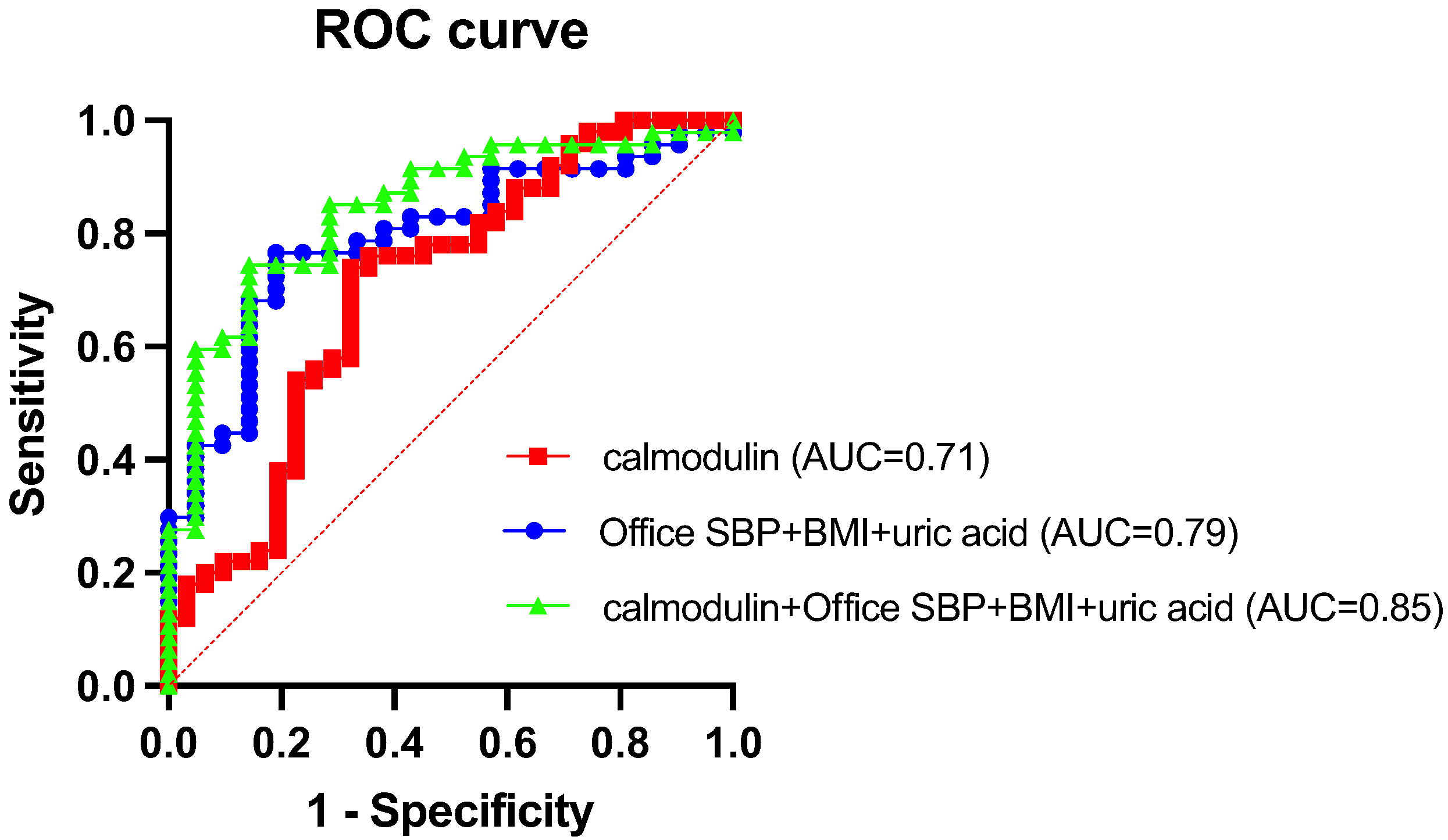
3.6. Predictive Performance of Calmodulin for Abnormal BP
3.7. Diagnostic Value of Plasma Calmodulin
4. Discussion
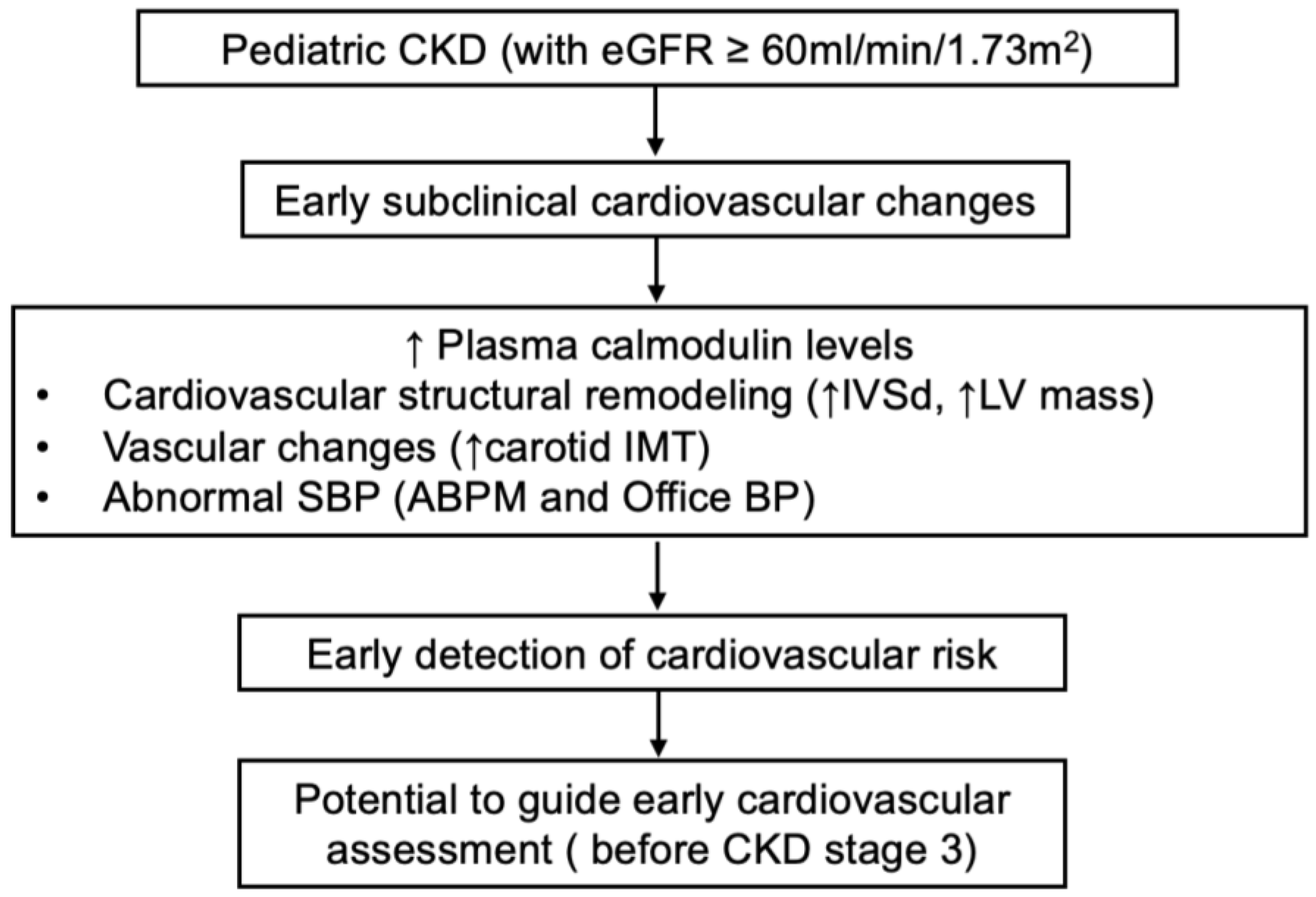
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABPM | ambulatory blood pressure monitoring |
| BMI | body mass index |
| BP | blood pressure |
| BUN | blood urea nitrogen |
| CAKUT | congenital anomalies of the kidney and urinary tract |
| CKD | chronic kidney disease |
| CVD | cardiovascular disease |
| Calmodulin | calcium-binding messenger protein |
| DBP | diastolic blood pressure |
| eGFR | estimated glomerular filtration rate |
| EF | ejection fraction |
| FS | fractional shortening |
| IVSd | interventricular septal thickness at end-diastole |
| LDL-C | low-density lipoprotein cholesterol |
| LV mass | left ventricular mass |
| LVIDd | left ventricular internal diameter at end-diastole |
| LVPWd | left ventricular posterior wall thickness at end-diastole |
| AI | augmentation index |
| IMT | intima–media thickness |
| PWV | pulse wave velocity |
| beta | β-stiffness index |
| NPV | negative predictive value |
| PPV | positive predictive value |
| Pi | inorganic phosphate |
| SBP | systolic blood pressure |
| UPCR | urinary protein-to-creatinine ratio |
| WBC | white blood cell count |
| eGFR | estimated glomerular filtration rate |
References
- Taşdemir, M.; Eroğlu, A.G.; Canpolat, N.; Konukoğlu, D.; Ağbaş, A.; Sevim, M.D.; Çalışkan, S.; Sever, L. Cardiovascular alterations do exist in children with stage-2 chronic kidney disease. Clin. Exp. Nephrol. 2016, 20, 926–933. [Google Scholar] [CrossRef]
- Schaefer, F.; Doyon, A.; Azukaitis, K.; Bayazit, A.; Canpolat, N.; Duzova, A.; Niemirska, A.; Sözeri, B.; Thurn, D.; Anarat, A.; et al. Cardiovascular Phenotypes in Children with CKD: The 4C Study. Clin. J. Am. Soc. Nephrol. 2017, 12, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.R.; Kimball, T.R.; Morrison, J.A.; Khoury, P.; Meyer, R.A. Indexing left ventricular mass to account for differences in body size in children and adolescents without cardiovascular disease. Am. J. Cardiol. 1995, 76, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Mitsnefes, M.M.; Kimball, T.R.; Kartal, J.; Witt, S.A.; Glascock, B.J.; Khoury, P.R.; Daniels, S.R. Progression of left ventricular hypertrophy in children with early chronic kidney disease: 2-year follow-up study. J. Pediatr. 2006, 149, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Samuels, J.; Ng, D.; Flynn, J.T.; Mitsnefes, M.; Poffenbarger, T.; Warady, B.A.; Furth, S. Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension 2012, 60, 43–50. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Hsu, C.-N. Cardiovascular Risks of Hypertension: Lessons from Children with Chronic Kidney Disease. Children 2022, 9, 1650. [Google Scholar] [CrossRef]
- Chien, S.-J.; Lin, I.C.; Hsu, C.-N.; Lo, M.-H.; Tain, Y.-L. Homocysteine and Arginine-to-Asymmetric Dimethylarginine Ratio Associated with Blood Pressure Abnormalities in Children With Early Chronic Kidney Disease. Circ. J. 2015, 79, 2031–2037. [Google Scholar] [CrossRef]
- Wang, S.; Vicente, F.B.; Miller, A.; Brooks, E.R.; Price, H.E.; Smith, F.A. Measurement of arginine derivatives in pediatric patients with chronic kidney disease using high-performance liquid chromatography-tandem mass spectrometry. Clin. Chem. Lab. Med. 2007, 45, 1305–1312. [Google Scholar] [CrossRef]
- Mitsnefes, M.M.; Betoko, A.; Schneider, M.F.; Salusky, I.B.; Wolf, M.S.; Jüppner, H.; Warady, B.A.; Furth, S.L.; Portale, A.A. FGF23 and Left Ventricular Hypertrophy in Children with CKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 45–52. [Google Scholar] [CrossRef]
- Grund, A.; Sinha, M.D.; Haffner, D.; Leifheit-Nestler, M. Fibroblast Growth Factor 23 and Left Ventricular Hypertrophy in Chronic Kidney Disease-A Pediatric Perspective. Front. Pediatr. 2021, 9, 702719. [Google Scholar] [CrossRef]
- Sinha, M.D.; Turner, C.; Booth, C.J.; Waller, S.; Rasmussen, P.; Goldsmith, D.J.A.; Simpson, J.M. Relationship of FGF23 to indexed left ventricular mass in children with non-dialysis stages of chronic kidney disease. Pediatr. Nephrol. 2015, 30, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Tain, Y.L.; Chen, H.E.; Hsu, C.N. Cardiovascular Disease Risk in Children with Chronic Kidney Disease: Impact of Apolipoprotein C-II and Apolipoprotein C-III. Front. Pediatr. 2021, 9, 706323. [Google Scholar] [CrossRef] [PubMed]
- Holle, J.; Querfeld, U.; Kirchner, M.; Anninos, A.; Okun, J.; Thurn-Valsassina, D.; Bayazit, A.; Niemirska, A.; Canpolat, N.; Bulut, I.K.; et al. Indoxyl sulfate associates with cardiovascular phenotype in children with chronic kidney disease. Pediatr. Nephrol. 2019, 34, 2571–2582. [Google Scholar] [CrossRef]
- Means, A.R. Calmodulin: Properties, Intracellular Localization, and Multiple Roles in Cell Regulation. Recent Prog. Horm. Res. 1981, 37, 333–367. [Google Scholar] [CrossRef]
- Sperelakis, N. Properties of calcium channels in cardiac muscle and vascular smooth muscle. Mol. Cell. Biochem. 1990, 99, 97–109. [Google Scholar] [CrossRef]
- Francis, A.; Shroff, R.; Earley, A.; Foster, B.J. KDIGO 2024 Guidelines—Key Points for Pediatricians. JAMA Pediatr. 2025, 179, 114–116. [Google Scholar] [CrossRef]
- Höcht, C. Blood Pressure Variability: Prognostic Value and Therapeutic Implications. Int. Sch. Res. Not. 2013, 2013, 398485. [Google Scholar] [CrossRef]
- Schutte, A.E.; Kollias, A.; Stergiou, G.S. Blood pressure and its variability: Classic and novel measurement techniques. Nat. Rev. Cardiol. 2022, 19, 643–654. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Bolton, K.; Culleton, B.; Harvey, K.S.; Ikizler, T.A.; Johnson, C.A.; Kausz, A.; Kimmel, P.L.; Kusek, J.; et al. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef]
- Health Promotion Administration, Ministry of Health and Welfare. Recommended BMI Reference Values for Growth in Children and Adolescents. Available online: https://www.hpa.gov.tw/542/9547/n (accessed on 2 April 2025).
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef]
- Mac Neil, S.; Walker, S.W.; Seid, J.; Tomlinson, S. Calmodulin in human serum and the specific release of calmodulin from calmodulin-rich platelets. Biosci. Rep. 1984, 4, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.S.; Carson, C.W.; Esmon, C.T. Endothelial cells and extracellular calmodulin inhibit monocyte tumor necrosis factor release and augment neutrophil elastase release. J. Biol. Chem. 1997, 272, 11778–11785. [Google Scholar] [CrossRef]
- Ono, K.; Niwa, M.; Suzuki, H.; Kobayashi, N.B.; Yoshida, T.; Sawada, M. Calmodulin as a Key Regulator of Exosomal Signal Peptides. Cells 2022, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Esteras, N.; Alquézar, C.; de la Encarnación, A.; Villarejo, A.; Bermejo-Pareja, F.; Martín-Requero, A. Calmodulin levels in blood cells as a potential biomarker of Alzheimer’s disease. Alzheimers Res. Ther. 2013, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.M.; Morgan, D.A.; Nuno, D.W.; Ketsawatsomkron, P.; Bair, T.B.; Venema, A.N.; Dibbern, M.E.; Kutschke, W.J.; Weiss, R.M.; Lamping, K.G.; et al. Calcium/calmodulin-dependent kinase II inhibition in smooth muscle reduces angiotensin II-induced hypertension by controlling aortic remodeling and baroreceptor function. J. Am. Heart Assoc. 2015, 4, e001949. [Google Scholar] [CrossRef]
- Muthalif, M.M.; Karzoun, N.A.; Benter, I.F.; Gaber, L.; Ljuca, F.; Uddin, M.R.; Khandekar, Z.; Estes, A.; Malik, K.U. Functional significance of activation of calcium/calmodulin-dependent protein kinase II in angiotensin II--induced vascular hyperplasia and hypertension. Hypertension 2002, 39, 704–709. [Google Scholar] [CrossRef]
- Usui, T.; Okada, M.; Hara, Y.; Yamawaki, H. Exploring calmodulin-related proteins, which mediate development of hypertension, in vascular tissues of spontaneous hypertensive rats. Biochem. Biophys. Res. Commun. 2011, 405, 47–51. [Google Scholar] [CrossRef]
- Huang, S.L.; Wen, Y.I.; Kupranycz, D.B.; Pang, S.C.; Schlager, G.; Hamet, P.; Tremblay, J. Abnormality of calmodulin activity in hypertension. Evidence of the presence of an activator. J. Clin. Investig. 1988, 82, 276–281. [Google Scholar] [CrossRef]
- Zuidscherwoude, M.; Grigore, T.; van de Langenberg, B.; Witte, G.; van der Wijst, J.; Hoenderop, J.G. Calmodulin regulates TRPV5 intracellular trafficking and plasma membrane abundance. J. Physiol. 2024, 602, 6871–6888. [Google Scholar] [CrossRef]
- Basu, U.; Case, A.J.; Liu, J.; Tian, J.; Li, Y.L.; Zimmerman, M.C. Redox-sensitive calcium/calmodulin-dependent protein kinase IIα in angiotensin II intra-neuronal signaling and hypertension. Redox Biol. 2019, 27, 101230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tandan, S.; Cheng, J.; Yang, C.; Nguyen, L.; Sugianto, J.; Johnstone, J.L.; Sun, Y.; Hill, J.A. Ca2+/calmodulin-dependent protein kinase II-dependent remodeling of Ca2+ current in pressure overload heart failure. J. Biol. Chem. 2008, 283, 25524–25532. [Google Scholar] [CrossRef] [PubMed]
- Schulman, H.; Anderson, M.E. Ca/Calmodulin-dependent Protein Kinase II in Heart Failure. Drug Discov. Today Dis. Mech. 2010, 7, e117–e122. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, P.D.; Purohit, A.; Hund, T.J.; Anderson, M.E. Calmodulin-dependent protein kinase II: Linking heart failure and arrhythmias. Circ. Res. 2012, 110, 1661–1677. [Google Scholar] [CrossRef]
| Total Participants | n = 81 | |
|---|---|---|
| Baseline Characteristics | Median [IQR] | Unit |
| Age | 9.48 [6.70, 12.86] | Years |
| Male | 47 (58.0%) | n (%) |
| CAKUT | 60 (74.1%) | n (%) |
| eGFR | 104.4 [90.31, 117.2] | mL/min/1.73 m2 |
| Height | 138.7 [117.9, 155.9] | cm |
| Weight | 32.7 [21.85, 44.55] | kg |
| BMI | 17.35 [15.34, 21.15] | kg/m2 |
| Underweight | 9 (11.1%) | n (%) |
| Overweight/obesity | 26 (32.1%) | n (%) |
| Office SBP | 110.0 [101.0, 118.8] | mmHg |
| Office DBP | 71.5 [67.0, 77.0] | mmHg |
| Elevated office BP/office HTN | 50 (61.7%) | n (%) |
| LDL-C | 93.0 [75.25, 118.5] | mg/dL |
| Triglycerides | 67.0 [45.0, 97.25] | mg/dL |
| Fasting Plasma Glucose | 85.5 [82.0, 89.75] | mg/dL |
| Uric Acid | 4.8 [4.2, 6.1] | mg/dL |
| BUN | 13.0 [10.0, 15.0] | mg/dL |
| Creatinine | 0.54 [0.45, 0.67] | mg/dL |
| UPCR | 54.9 [38.75, 93.35] | mg/g |
| Hemoglobin | 13.1 [12.65, 14.3] | g/dL |
| WBC | 6.6 [5.65, 8.1] | ×103/μL |
| Platelets | 297.0 [245.5, 357.0] | ×103/μL |
| Sodium | 140.0 [139.0, 141.0] | mEq/L |
| Potassium | 4.4 [4.1, 4.5] | mEq/L |
| Calcium | 10.1 [9.8, 10.4] | mg/dL |
| Inorganic Phosphate | 4.8 [4.4, 5.1] | mg/dL |
| Calmodulin | 137.9 [83.35, 201] | ×102 pg/mL |
| Baseline Characteristics | n = 81 | |
|---|---|---|
| Parameter | r | p Value |
| Age | 0.1594 | 0.1552 |
| eGFR | −0.09152 | 0.4194 |
| Height | 0.1016 | 0.3701 |
| Weight | 0.2717 | 0.0141 (*) |
| BMI | 0.3265 | 0.0031 (**) |
| Office SBP | −0.1003 | 0.4156 |
| Office DBP | 0.07021 | 0.5694 |
| LDL-C | 0.1113 | 0.3255 |
| Triglycerides | −0.0147 | 0.897 |
| Fasting Plasma Glucose | 0.04954 | 0.6625 |
| Uric acid | 0.1273 | 0.2576 |
| BUN | −0.06777 | 0.5478 |
| Creatinine | 0.00657 | 0.9536 |
| UPCR | −0.04278 | 0.7045 |
| Hemoglobin | 0.2011 | 0.0718 |
| WBC | 0.01874 | 0.0939 |
| Platelets | 0.05743 | 0.6106 |
| Sodium | 0.01947 | 0.863 |
| Potassium | 0.03792 | 0.7368 |
| Calcium | 0.3268 | 0.0029 (**) |
| Inorganic Phosphate | −0.136 | 0.226 |
| Echocardiography | n = 81 | |
|---|---|---|
| Parameter | r | p Value |
| LVPWd | 0.1254 | 0.2678 |
| LVIDd | 0.1164 | 0.3038 |
| IVSd | 0.2492 | 0.0258 (*) |
| LV mass | 0.2472 | 0.0271 (*) |
| FS | 0.03405 | 0.7643 |
| EF | 0.03019 | 0.7904 |
| Carotid Artery Ultrasound | n = 44 | |
| Parameter | r | p Value |
| Left catotid IMT | 0.2748 | 0.071 |
| Left catotid beta | 0.1433 | 0.3533 |
| Left catotid AI | 0.001298 | 0.9933 |
| Left catotid PWV | 0.1766 | 0.2514 |
| Left catotid artery diameter maximum | 0.08736 | 0.5728 |
| Left catotid artery diameter mininum | 0.06935 | 0.6547 |
| Right catotid IMT | 0.3836 | 0.0104 (**) |
| Right catotid beta | 0.1562 | 0.3111 |
| Right catotid AI | −0.01228 | 0.9369 |
| Right catotid PWV | 0.1721 | 0.2623 |
| Right catotid artery diameter maximum | 0.1086 | 0.4831 |
| Right catotid artery diameter mininum | 0.09321 | 0.5473 |
| ABPM | n = 38 | |
| Parameter | r | p Value |
| Overall SBP | 0.4934 | 0.0016 (**) |
| Overall DBP | 0.294 | 0.0732 |
| Overall Mean BP | 0.008937 | 0.9575 |
| Overall SBP load | 0.4746 | 0.0026 (**) |
| Overall DBP load | 0.233 | 0.1592 |
| Awake SBP | 0.5094 | 0.0011 (**) |
| Awake DBP | 0.3118 | 0.0567 |
| Awake Mean BP | −0.01725 | 0.9181 |
| Awake SBP Load | 0.4955 | 0.0016 (**) |
| Awake DBP load | 0.2329 | 0.1549 |
| Asleep SBP | 0.3798 | 0.0187 (*) |
| Asleep DBP | 0.2017 | 0.2247 |
| Asleep Mean BP | −0.0304 | 0.8562 |
| Asleep SBP load | 0.3681 | 0.023 (*) |
| Asleep DBP load | 0.1892 | 0.2552 |
| SBP Asleep Dipping percentage | 0.138 | 0.4088 |
| DBP Asleep Dipping percentage | 0.06934 | 0.06791 |
| AASI | 0.1226 | 0.4634 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p Value | Adjusted OR | 95% CI | p Value |
| Age (years) | 0.9588 | 0.8500 to 1.079 | 0.4855 | |||
| Male (vs. Female) | 1.53 | 0.6170 to 3.818 | 0.3583 | |||
| CAKUT (vs. non-CAKUT) | 1.329 | 0.4256 to 4.029 | 0.6154 | |||
| eGFR (mL/min/1.73 m2) | 0.993 | 0.9730 to 1.012 | 0.471 | |||
| BMI (kg/m2) | 1.158 | 1.021 to 1.342 | 0.0339 (*) | 0.9376 | 0.7654 to 1.141 | 0.5188 |
| Office SBP (mmHg) | 1.1 | 1.044 to 1.174 | 0.0013 (**) | 1.14 | 1.063 to 1.245 | 0.001 (**) |
| Office DBP (mmHg) | 1.078 | 1.013 to 1.168 | 0.0361 (*) | |||
| Fasting Plasma Glucose (mg/dL) | 1.014 | 0.9581 to 1.077 | 0.6374 | |||
| LDL-C (mg/dL) | 1.005 | 0.9914 to 1.020 | 0.5066 | |||
| Triglycerides (mg/dL) | 0.9998 | 0.9931 to 1.007 | 0.9502 | |||
| Uric Acid (mg/dL) | 1.339 | 1.013 to 1.916 | 0.0705 | 1.039 | 0.7154 to 1.611 | 0.8505 |
| UPCR (mg/g) | 1 | 0.9999 to 1.001 | 0.5979 | |||
| Hemoglobin (g/dL) | 0.9926 | 0.6955 to 1.402 | 0.9661 | |||
| Sodium (mEq/L) | 0.9575 | 0.7064 to 1.282 | 0.7721 | |||
| Potassium (mEq/L) | 2.227 | 0.5677 to 9.800 | 0.2651 | |||
| Calcium (mg/dL) | 1.143 | 0.4238 to 3.040 | 0.7856 | |||
| Inorganic Phosphorus (mg/dL) | 1.618 | 0.7031 to 3.906 | 0.2652 | |||
| Calmodulin (×102 pg/mL) | 1.008 | 1.002 to 1.016 | 0.0167 (*) | 1.012 | 1.003 to 1.024 | 0.029 (*) |
| Calmodulin (×102 pg/mL) | Abnormal BP Profile | Normal BP Profile | Total | |
|---|---|---|---|---|
| ≥131.04 | 37 | 10 | 47 | PPV: 78.72% |
| <131.04 | 13 | 21 | 34 | NPV: 61.76% |
| Total | 50 | 31 | ||
| Sensitivity: 74% | Specificity: 67.74% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Liao, W.-T.; Hsu, C.-N.; Tain, Y.-L.; Lu, P.-C. Plasma Calmodulin as a Biomarker of Subclinical Cardiovascular Disease in Pediatric Chronic Kidney Disease. Children 2025, 12, 599. https://doi.org/10.3390/children12050599
Lee H-J, Liao W-T, Hsu C-N, Tain Y-L, Lu P-C. Plasma Calmodulin as a Biomarker of Subclinical Cardiovascular Disease in Pediatric Chronic Kidney Disease. Children. 2025; 12(5):599. https://doi.org/10.3390/children12050599
Chicago/Turabian StyleLee, Hsin-Jung, Wei-Ting Liao, Chien-Ning Hsu, You-Lin Tain, and Pei-Chen Lu. 2025. "Plasma Calmodulin as a Biomarker of Subclinical Cardiovascular Disease in Pediatric Chronic Kidney Disease" Children 12, no. 5: 599. https://doi.org/10.3390/children12050599
APA StyleLee, H.-J., Liao, W.-T., Hsu, C.-N., Tain, Y.-L., & Lu, P.-C. (2025). Plasma Calmodulin as a Biomarker of Subclinical Cardiovascular Disease in Pediatric Chronic Kidney Disease. Children, 12(5), 599. https://doi.org/10.3390/children12050599






