Pediatric Syncope: An Examination of Diagnostic Processes, Therapeutic Approaches and the Role of the Tilt Test: Insights from an 18-Year Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
- Definite VVS group: patients who were definitively diagnosed with vasovagal syncope based on a comprehensive evaluation, including medical history; physical examination; electrocardiography; and, when necessary, echocardiography, brain MRI, and electroencephalography.
- Suspected VVS group: patients who lack a confident diagnosis of VVS after the initial assessment and required tilt table testing.
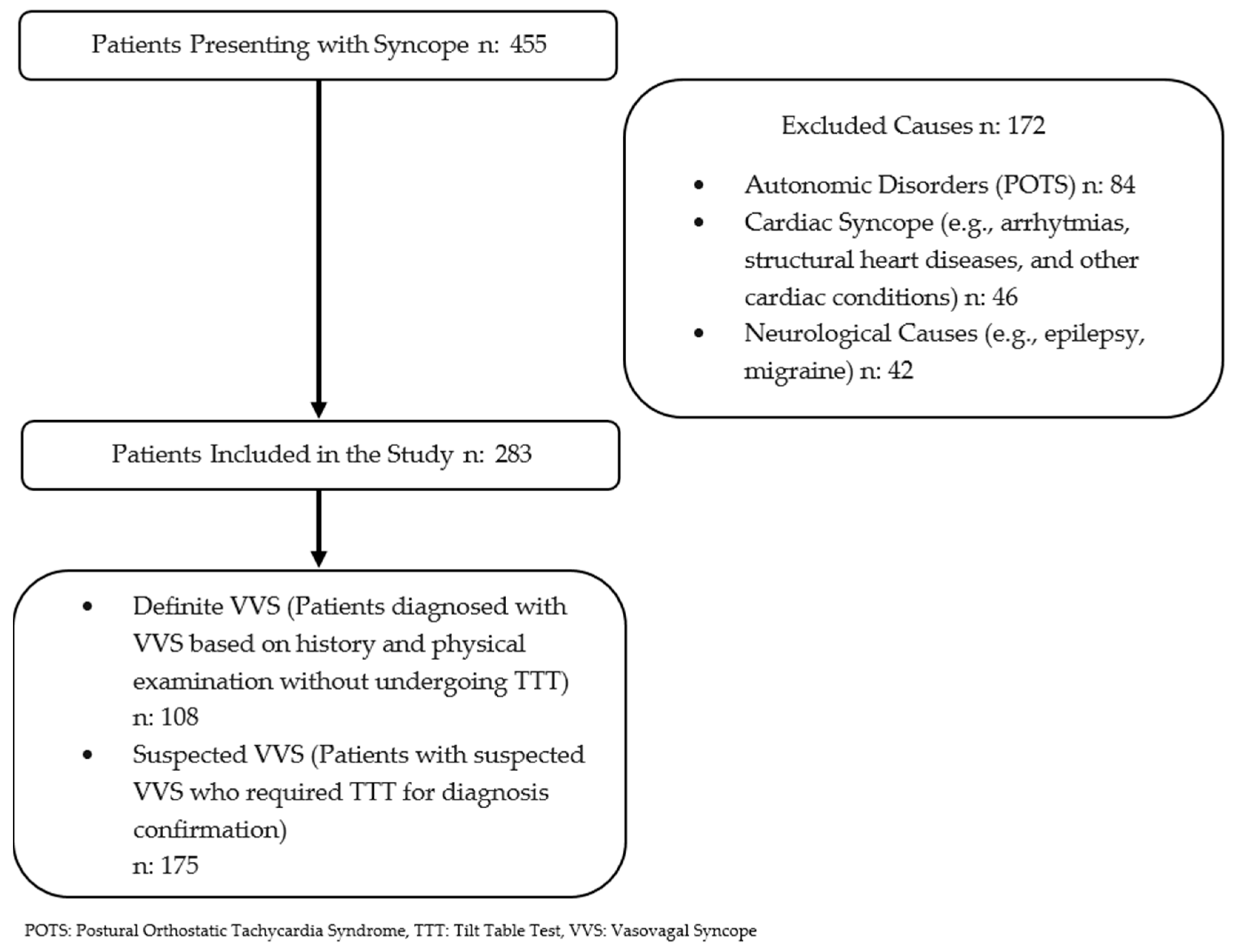
2.1. Diagnostic Methods
2.1.1. Electrocardiography (ECG)
2.1.2. Twenty-Four-Hour Holter Monitoring
2.1.3. Tilt Table Test (TTT)
2.1.4. TTT Protocol
2.1.5. Classification of TTT Responses
- Type 1: Mixed Response
- Type 2: Cardioinhibitory Response
- Type 3: Pure Vasodepressor Response
- Postural Orthostatic Tachycardia Syndrome (POTS)
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NMS | Neurally mediated syncope |
| CS | Cardiac syncope |
| VVS | Vasovagal syncope |
| POTS | Postural orthostatic tachycardia syndrome |
| ECG | Electrocardiography |
| HR | Heart rate |
| AV | Atrioventricular |
| TTT | Tilt table test |
| ESC | European Society of Cardiology |
| ISDN | İsosorbide dinitrate |
| SVT | Supraventricular tachycardia |
| VT | Ventricular tachycardia |
| OR | Odds ratio |
| CI | Confidence interval |
| BP | Blood pressure |
| GTN | Nitroglycerin |
References
- Cheng, W.; Yaqi, L.; Ying, L.; Hong, T.; Min, H.; Xiangyu, D.; Lin, S.; Jinghui, S.; Hongfang, J.; Junbao, D.; et al. Chinese Pediatric Cardiology Society (CPCS) guideline for diagnosis and treatment of syncope in children and adolescents. Sci. Bull. 2018, 63, 1558–1564. [Google Scholar] [CrossRef]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A.; et al. ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef] [PubMed]
- Colman, N.; Nahm, K.; van Dijk, J.G.; Reitsma, J.B.; Wieling, W.; Kaufmann, H. Diagnostic value of history taking in reflex syncope. Clin. Auton. Res. 2004, 14 (Suppl. S1), 37–44. [Google Scholar] [CrossRef] [PubMed]
- Mosqueda-Garcia, R.; Furlan, R.; Tank, J.; Fernandez-Violante, R. The elusive pathophysiology of neurally mediated syncope. Circulation 2000, 102, 2898–2906. [Google Scholar] [CrossRef]
- Colman, N.; Nahm, K.; Ganzeboom, K.S.; Shen, W.K.; Reitsma, J.; Linzer, M.; Wieling, W.; Kaufmann, H. Epidemiology of reflex syncope. Clin. Auton. Res. 2004, 14 (Suppl. 1), 9–17. [Google Scholar] [CrossRef]
- Thijs, R.D.; Wieling, W.; Kaufmann, H.; van Dijk, G. Defining and classifying syncope. Clin. Auton. Res. 2004, 14 (Suppl. 1), 4–8. [Google Scholar] [CrossRef]
- Tony Reybrouck, T.; Ector, H. Syncope and Assessment of Autonomic Function in Children. In Moss and Adam’s Heart Disease in Infants, Children and Adolescent, 7th ed.; Allen, H.D., Driscoll, D.J., Shaddy, R.E., Feltes, T.F., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 269–274. [Google Scholar]
- Johnsrude, C.L. Current approach to pediatric syncope. Pediatr. Cardiol. 2000, 21, 522–531. [Google Scholar] [CrossRef]
- McLeod, K.A. Syncope in childhood. Arch. Dis. Child. 2003, 88, 350–353. [Google Scholar] [CrossRef]
- Levine, M.M. Neurally mediated syncope in children: Results of tilt testing, treatment, and long-term follow-up. Pediatr. Cardiol. 1999, 20, 331–335. [Google Scholar] [CrossRef]
- Kenny, R.A.; Ingram, A.; Bayliss, J.; Sutton, R. Head-up tilt: A useful test for investigating unexplained syncope. Lancet 1986, 1, 1352–1355. [Google Scholar] [CrossRef]
- Brignole, M.; Alboni, P.; Benditt, D.; Bergfeldt, L.; Blanc, J.J.; Bloch Thomsen, P.E.; van Dijk, J.G.; Fitzpatrick, A.; Hohnloser, S.; Janousek, J.; et al. Guidelines on management (diagnosis and treatment) of syncope. Eur. Heart J. 2001, 22, 1256–1306. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Sutton, R.; Ammirati, F.; Blanc, J.J.; Brignole, M.; Dahm, J.B.; Deharo, J.C.; Gajek, J.; Gjesdal, K.; Krahn, A.; et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur. Heart J. 2009, 30, 2631–2671. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liao, Y.; Zhang, Q.; Yan, H.; Liu, P.; Wang, Y.; Sun, Y.; Xu, W.; Liu, X.; Du, J.; et al. Spectrum of underlying diseases in syncope and treatment of neurally-mediated syncope in children and adolescents over the past 30 years: A single center study. Front. Cardiovasc. Med. 2022, 9, 1017505. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagels, W.A.; Padberg, G.W.; Overweg, J.; van der Velde, E.A.; Roos, R.A. Transient loss of consciousness: The value of the history for distinguishing seizure from syncope. J. Neurol. 1991, 238, 39–43. [Google Scholar] [CrossRef]
- Sheldon, R.; Rose, S.; Ritchie, D.; Connolly, S.J.; Koshman, M.L.; Lee, M.A.; Frenneaux, M.; Fisher, M.; Murphy, W. Historical criteria that distinguish syncope from seizures. J. Am. Coll. Cardiol. 2002, 40, 142–148. [Google Scholar] [CrossRef]
- Alboni, P.; Brignole, M.; Menozzi, C.; Raviele, A.; Del Rosso, A.; Dinelli, M.; Solano, A.; Bottoni, N. Diagnostic value of history in patients with syncope with or without heart disease. J. Am. Coll. Cardiol. 2001, 37, 1921–1928. [Google Scholar] [CrossRef]
- Brignole, M.; Alboni, P.; Benditt, D.G.; Bergfeldt, L.; Blanc, J.J.; Bloch Thomsen, P.E.; van Dijk, J.G.; Fitzpatrick, A.; Hohnloser, S.; Janousek, J.; et al. Guidelines on management (diagnosis and treatment) of syncope--update 2004. Europace 2004, 6, 467–537. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Khan, P.; Panting, J.; Nadar, S. Tilt-table test: Its role in modern practice. Clin. Med. 2013, 13, 227–232. [Google Scholar] [CrossRef]
- Ferdowsi, M.; Kwan, B.H.; Tan, M.P.; Saedon, N.I.; Subramaniam, S.; Abu Hashim, N.F.I.; Mohd Nasir, S.S.; Zainal Abidin, I.; Chee, K.H.; Goh, C.H. Classification of vasovagal syncope from physiological signals on tilt table testing. Biomed. Eng. Online 2024, 23, 37. [Google Scholar] [CrossRef]
- Zavala, R.; Metais, B.; Tuckfield, L.; DelVecchio, M.; Aronoff, S. Pediatric Syncope: A Systematic Review. Pediatr. Emerg. Care 2020, 36, 442–445. [Google Scholar] [CrossRef]
- Soteriades, E.S.; Evans, J.C.; Larson, M.G.; Chen, M.H.; Chen, L.; Benjamin, E.J.; Levy, D. Incidence and prognosis of syncope. N. Engl. J. Med. 2002, 347, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Wieling, W.; Jardine, D.L.; de Lange, F.J.; Brignole, M.; Nielsen, H.B.; Stewart, J.; Sutton, R. Cardiac output and vasodilation in the vasovagal response: An analysis of the classic papers. Heart Rhythm. 2016, 13, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Mereu, R.; De Barbieri, G.; Perrone, T.; Mugellini, A.; Di Toro, A.; Bernardi, L. Heart rate/blood pressure ratio as predictor of neuromediated syncope. Int. J. Cardiol. 2013, 167, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A.; Chapleau, M.W. Increased cardiac sympathetic activity: Cause or compensation in vasovagal syncope? Clin. Auton. Res. 2018, 28, 265–266. [Google Scholar] [CrossRef]
- Gregson, H.; Ivkov, A. Sex and age impact prevalence and symptoms of vasovagal syncope: Implications for accurate diagnosis. STEM Fellowsh. J. 2021, 7, 13–15. [Google Scholar] [CrossRef]
- Morillo, C.A.; Klein, G.J.; Zandri, S.; Yee, R. Diagnostic accuracy of a low-dose isoproterenol head-up tilt protocol. Am. Heart J. 1995, 129, 901–906. [Google Scholar] [CrossRef]
- Bartoletti, A.; Alboni, P.; Ammirati, F.; Brignole, M.; Del Rosso, A.; Foglia Manzillo, G.; Menozzi, C.; Raviele, A.; Sutton, R. ‘The Italian Protocol’: A simplified head-up tilt testing potentiated with oral nitroglycerin to assess patients with unexplained syncope. Europace 2000, 2, 339–342. [Google Scholar] [CrossRef]
- Parry, S.W.; Gray, J.C.; Newton, J.L.; Reeve, P.; O’Shea, D.; Kenny, R.A. ‘Front-loaded’ head-up tilt table testing: Validation of a rapid first line nitrate-provoked tilt protocol for the diagnosis of vasovagal syncope. Age Ageing 2008, 37, 411–415. [Google Scholar] [CrossRef]
- Bartoletti, A.; Gaggioli, G.; Menozzi, C.; Bottoni, N.; Del Rosso, A.; Mureddu, R.; Musso, G.; Foglia-Manzillo, G.; Bonfigli, B.; Brignole, M. Head-up tilt testing potentiated with oral nitroglycerin: A randomized trial of the contribution of a drug-free phase and a nitroglycerin phase in the diagnosis of neurally mediated syncope. Europace 1999, 1, 183–186. [Google Scholar] [CrossRef]
- Dindar, A.; Cetin, B.; Ertuğrul, T.; Cantez, T. Sublingual isosorbide dinitrate-stimulated tilt test for diagnosis of vasovagal syncope in children and adolescents. Pediatr. Cardiol. 2003, 24, 270–273. [Google Scholar] [CrossRef]
- Furukawa, T.; Maggi, R.; Solano, A.; Croci, F.; Brignole, M. Effect of clinical triggers on positive responses to tilt-table testing potentiated with nitroglycerin or clomipramine. Am. J. Cardiol. 2011, 107, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.P.; Theodorakis, G.; Vardas, P.; Sutton, R. Methodology of head-up tilt testing in patients with unexplained syncope. J. Am. Coll. Cardiol. 1991, 17, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.M.; Slotwiner, D.J.; Mittal, S.; Scheiner, M.; Markowitz, S.M.; Lerman, B.B. Formal analysis of the optimal duration of tilt testing for the diagnosis of neurally mediated syncope. Am. Heart J. 2001, 141, 282–288. [Google Scholar] [CrossRef]
- Raj, S.R.; Freeman, R. Highlights in clinical autonomic neurosciences: Vasovagal syncope—Insights on diagnosis, pathophysiology and treatment. Auton. Neurosci. 2012, 168, 1–3. [Google Scholar] [CrossRef]
- Ventura, R.; Maas, R.; Zeidler, D.; Schoder, V.; Nienaber, C.A.; Schuchert, A.; Meinertz, T. A randomized and controlled pilot trial of beta-blockers for the treatment of recurrent syncope in patients with a positive or negative response to head-up tilt test. Pacing Clin. Electrophysiol. 2002, 25, 816–821. [Google Scholar] [CrossRef]
- Sheldon, R.; Connolly, S.; Rose, S.; Klingenheben, T.; Krahn, A.; Morillo, C.; Talajic, M.; Ku, T.; Fouad-Tarazi, F.; Ritchie, D.; et al. Prevention of Syncope Trial (POST): A randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation 2006, 113, 1164–1170. [Google Scholar] [CrossRef]
- Flevari, P.; Livanis, E.G.; Theodorakis, G.N.; Zarvalis, E.; Mesiskli, T.; Kremastinos, D.T. Vasovagal syncope: A prospective, randomized, crossover evaluation of the effect of propranolol, nadolol and placebo on syncope recurrence and patients’ well-being. J. Am. Coll. Cardiol. 2002, 40, 499–504. [Google Scholar] [CrossRef]
- Madrid, A.H.; Ortega, J.; Rebollo, J.G.; Manzano, J.G.; Segovia, J.G.; Sánchez, A.; Peña, G.; Moro, C. Lack of efficacy of atenolol for the prevention of neurally mediated syncope in a highly symptomatic population: A prospective, double-blind, randomized and placebo-controlled study. J. Am. Coll. Cardiol. 2001, 37, 554–559. [Google Scholar] [CrossRef]
- Atici, A.; Rasih-Sonsoz, M.; Ali-Barman, H.; Durmaz, E.; Demirkiran, A.; Gulsen, K.; Elitok, A.; Onur, I.; Sahin, I.; Bilge, A.K. The Role of Beta-1 Receptor Gene Polymorphism in Beta-Blocker Therapy for Vasovagal Syncope. Rev. Investig. Clin. 2020, 72, 300–307. [Google Scholar] [CrossRef]
- Kouakam, C.; Vaksmann, G.; Pachy, E.; Lacroix, D.; Rey, C.; Kacet, S. Long-term follow-up of children and adolescents with syncope; predictor of syncope recurrence. Eur. Heart J. 2001, 22, 1618–1625. [Google Scholar] [CrossRef]
- Salim, M.A.; Ware, L.E.; Barnard, M.; Alpert, B.S.; DiSessa, T.G. Syncope recurrence in children: Relation to tilt-test results. Pediatrics 1998, 102, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Xu, B.; Liao, Y.; Li, X.; Jin, H.; Du, J. Predictor of Syncopal Recurrence in Children With Vasovagal Syncope Treated With Metoprolol. Front. Pediatr. 2022, 10, 870939. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R. Should we treat severe vasovagal syncope with a pacemaker? J. Intern. Med. 2017, 281, 554–561. [Google Scholar] [CrossRef] [PubMed]

| Variables (n) | Total (283) | Clinically Diagnosed with Vasodepressor Syncope (108) | Undergoing TTT (175) |
|---|---|---|---|
| Age (years) | 13.5 ± 1.6 | 13.5 ± 1.5 | 13.5 ± 1.6 |
| Sex | |||
| Female | 196 (69%) | 76 (70%) | 120 (69%) |
| Male | 87 (31%) | 32 (30%) | 55 (31%) |
| Number of syncope episodes | 3 (1–6) | 2 (1–5) | 3 (1–6) |
| Monitoring period (years) | 4 (1–8) | 3 (1–6) | 4 (1–8) |
| Variables (n) | Negative TTT (44) | Basal TTT Positive (57) | Positive After ISDN (74) | p-Value |
|---|---|---|---|---|
| Age (years) | 13.3 ± 1.9 (8–18) | 13.6 ± 1.5 (9–18) | 13.6 ± 1.6 (9–17) | 0.91 |
| Male sex | 15 (34%) | 17 (30%) | 23 (31%) | 0.89 |
| Initiation of beta-blockers | 0 ab | 46 (80%) ac | 48 (65%) bc | <0.001 |
| Initiation of Fludrocortisone | 0 | 0 | 2, (3%) | 0.25 |
| Initiation of Midodrine | 0 | 1 (2%) | 0 | 0.35 |
| Relapse | 14 (32%) | 11 (19%) | 12 (16%) | 0.12 |
| Syncope type | 0.017 | |||
| Mixed | 29 (51%) | 39 (53%) | ||
| Cardioinhibitory type 2A | 11 (19%) | 4 (5.4%) | ||
| Cardioinhibitory type 2B | 7 (12%) | 5 (6.6%) | ||
| Vasodepressor | 10 (18%) | 26 (35%) | ||
| Monitoring period (years) | 4 ± 1.3 | 4.1 ± 1.2 | 4.1 ± 1.1 | 0.17 |
| Number of syncope episodes | 2.7 ± 0.8 ab | 3.1 ± 0.8 a | 3.1 ± 0.9 b | 0.008 |
| Duration of medication use (months) | 21.8 ± 5.1 | 21.4 ± 5 | 0.71 | |
| Time to positive basal tilt (minutes) | 13 ± 4 | |||
| Time to positivity after ISDN (minutes) | 6.8 ± 1.8 |
| Variables (n) | Clinically Diagnosed with Vasodepressor Syncope (108) | Undergoing TTT (175) | p-Value |
|---|---|---|---|
| Age | 13.5 ± 1.5 | 13.5 ± 1.6 | 0.84 |
| Male sex | 32 (30%) | 55 (31%) | 0.75 |
| Relapse | 16 (15%) | 94 (54%) | <0.001 |
| Initiation of beta-blockers | 16 (15%) | 94 (54%) | <0.001 |
| Duration of medication use (months) | 23.4 ± 2.7 | 21.6 ± 5 | 0.25 |
| Number of syncope episodes | 2.4 ± 0.8 | 3 ± 0.9 | <0.001 |
| Monitoring period (years) | 2.9 ± 1.1 | 4.1 ± 1.2 | <0.001 |
| (A) | ||||||
|---|---|---|---|---|---|---|
| Syncope Type (n) | Mixed (68) | Cardioinhibitor Type 2A (15) | Cardioinhibitor Type 2B (12) | Vasodepressor (36) | p-Value | |
| Age | 13.5 ± 1.4 | 13.2 ± 1.7 | 13.8 ± 1.6 | 13.9 ± 1.8 | 0.4 | |
| Male sex | 21 (31%) | 4 (27%) | 5 (42%) | 10 (28%) | 0.8 | |
| Initiation of beta-blockers | 67 (99%) | 15 (100%) | 12 (100%) | 0 | <0.001 | |
| Initiation of Fludrocortisone | 0 | 1 (1%) | 1 (1%) | 0 | 0.047 | |
| Initiation of Midodrine | 0 | 0 | 1 (1%) | 0 | 0.02 | |
| Relapse | 3 (4%) | 2 (1%) | 7 (60%) | 11 (31%) | <0.001 | |
| Time to positive basal tilt (minutes) | 12.6 ± 3.9 | 12.3 ± 3.6 | 12.9 ± 3.1 | 15 ± 5 | 0.5 | |
| Time to positivity after ISDN (minutes) | 6.6 ± 7.8 | 7.8 ± 2.6 | 7 ± 1 | 6.9 ± 2.2 | 0.6 | |
| Monitoring period (years) | 4.1 ± 1 | 4.2 ± 1.8 | 4.3 ± 1.4 | 4 ± 1.1 | 0.9 | |
| Number of syncope episodes | 3 ± 0.7 | 3.4 ± 1 | 3.6 ± 1.2 | 3 ± 0.9 | 0.2 | |
| Duration of medication use (months) | 21.2 ± 4.7 | 23.1 ± 5.1 | 24.7 ± 4.2 b | 16.7 ± 5.4 | 0.003 | |
| (B) | ||||||
| Variables | p1 | p2 | p3 | p4 | p5 | p6 |
| Initiation of beta-blockers | 1 | 1 | <0.001 | - | <0.001 | <0.001 |
| Initiation of Fludrocortisone | 0.12 | 0.068 | - | 1 | 0.46 | 0.32 |
| Initiation of Midodrine | - | 0.068 | - | 1 | - | 0.32 |
| Relapse | 0.76 | <0.001 | <0.001 | 0.056 | 0.8 | 0.34 |
| Duration of medication use (months) | 0.44 | 0.032 | 0.12 | 1 | 0.68 | 0.024 |
| Variables | Univariate Regression | Multivariate Regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age | 0.9 | 0.74–1.13 | 0.42 | |||
| Sex | 1.4 | 0.68–3 | 0.35 | |||
| Initiation of beta-blockers | 0.3 | 0.15–0.71 | 0.004 | |||
| Number of syncope episodes | 3.2 | 1.4–7.2 | 0.005 | 1.7 | 1.3–2.5 | 0.03 |
| Duration of medication use | 2.2 | 1.5–3.2 | <0.001 | 2.1 | 1.3–3.2 | 0.001 |
| Syncope Type | ||||||
| Mixed | 0.1 | 0.03–0.35 | <0.001 | |||
| Cardioinhibitory type 2A | 0.7 | 0.15–3.3 | 0.65 | |||
| Cardioinhibitory type 2B | 9 | 2.5–31.8 | <0.001 | 2.3 | 1.1–4 | 0.01 |
| Vasodepressor | 3 | 1.2–7.7 | 0.02 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaca, S.; Özbingöl, D.; Karaca Özer, P.; Yavuz, M.L.; Nişli, K. Pediatric Syncope: An Examination of Diagnostic Processes, Therapeutic Approaches and the Role of the Tilt Test: Insights from an 18-Year Single-Center Experience. Children 2025, 12, 459. https://doi.org/10.3390/children12040459
Karaca S, Özbingöl D, Karaca Özer P, Yavuz ML, Nişli K. Pediatric Syncope: An Examination of Diagnostic Processes, Therapeutic Approaches and the Role of the Tilt Test: Insights from an 18-Year Single-Center Experience. Children. 2025; 12(4):459. https://doi.org/10.3390/children12040459
Chicago/Turabian StyleKaraca, Serra, Doruk Özbingöl, Pelin Karaca Özer, Mustafa Lütfi Yavuz, and Kemal Nişli. 2025. "Pediatric Syncope: An Examination of Diagnostic Processes, Therapeutic Approaches and the Role of the Tilt Test: Insights from an 18-Year Single-Center Experience" Children 12, no. 4: 459. https://doi.org/10.3390/children12040459
APA StyleKaraca, S., Özbingöl, D., Karaca Özer, P., Yavuz, M. L., & Nişli, K. (2025). Pediatric Syncope: An Examination of Diagnostic Processes, Therapeutic Approaches and the Role of the Tilt Test: Insights from an 18-Year Single-Center Experience. Children, 12(4), 459. https://doi.org/10.3390/children12040459





