Evaluation of the Effect of Body Mass Index and Waist Circumference on Ocular Health Parameters in Children and Adolescents
Abstract
1. Introduction
2. Materials and Methods
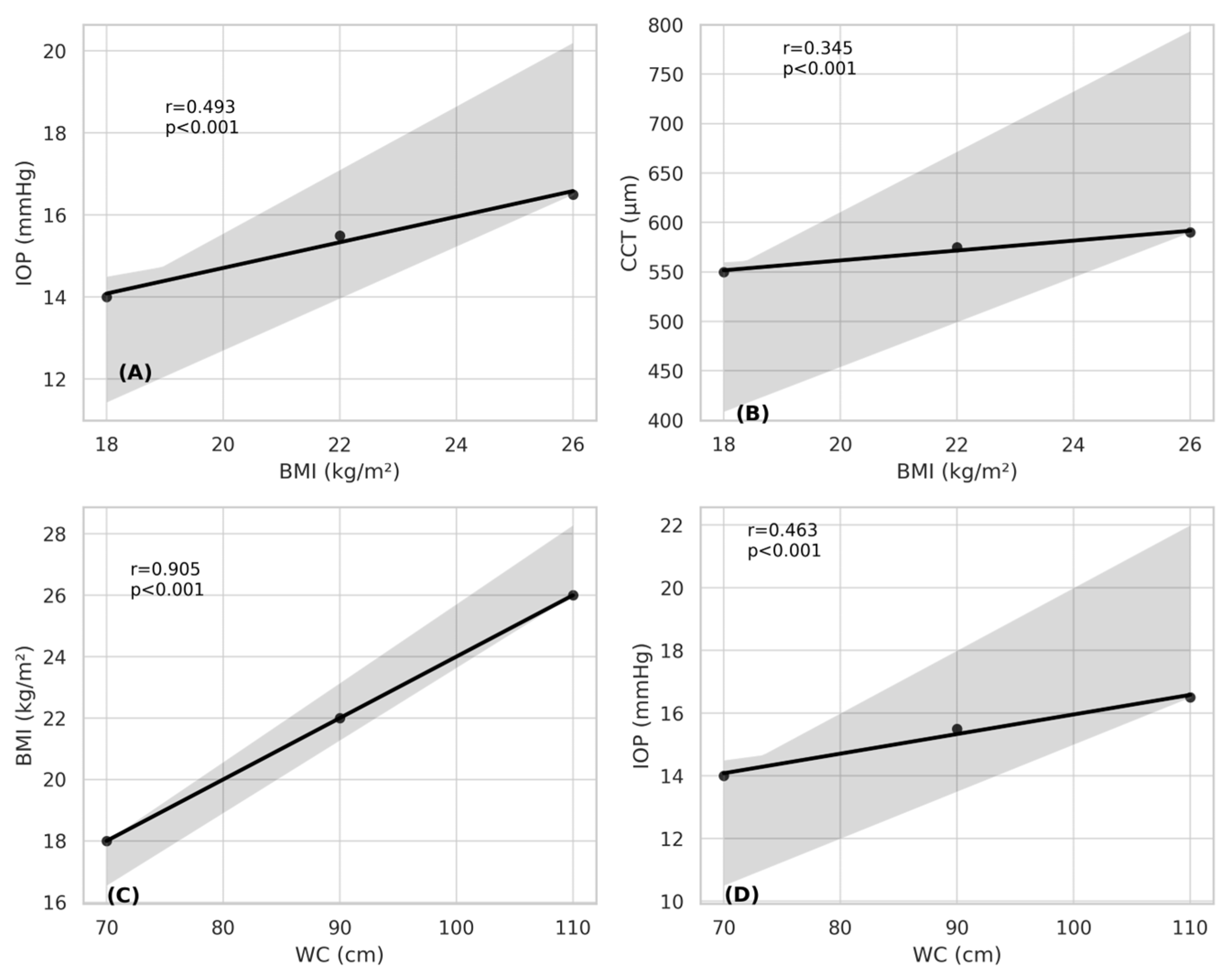
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Onís, M.; Blössner, M.; Borghi, E. Global prevalence and trends of overweight and obesity among preschool children. Am. J. Clin. Nutr. 2010, 92, 1257–1264. [Google Scholar] [PubMed]
- Raghuveer, G. Lifetime cardiovascular risk of childhood obesity. Am. J. Clin. Nutr. 2010, 91, 1514S–1519S. [Google Scholar] [CrossRef] [PubMed]
- Can, M.E.; Kızıltoprak, H.; Buluş, A.D.; Özkoyuncu, D.; Koç, M.; Yıldız, Z.Ö. Corneal biomechanical properties in childhood obesity. J. Pediatr. Ophthalmol. Strabismus 2020, 57, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Wong, T.Y. Obesity and Eye Diseases. Surv. Ophthalmol. 2007, 52, 180–195. [Google Scholar] [CrossRef]
- Kurtul, B.E.; Cąkmak, A.I.; Elbeyli, A.; Karaaslan, A.; El, Ç. Association of childhood obesity with retinal microvasculature and corneal endothelial cell morphology. J. Pediatr. Endocrinol. Metab. 2021, 34, 171–176. [Google Scholar] [CrossRef]
- Panon, N.; Luangsawang, K.; Rugaber, C.; Tongchit, T.; Thongsepee, N.; Cheaha, D.; Kongjaidee, P.; Changtong, A.; Daradas, A.; Chotimol, P. Correlation between body mass index and ocular parameters. Clin. Ophthalmol. 2019, 13, 755–762. [Google Scholar] [CrossRef]
- Hargrave, A.; Courson, J.A.; Pham, V.; Landry, P.; Magadi, S.; Shankar, P.; Hanlon, S.; Das, A.; Rumbaut, R.E.; Wayne Smith, C.; et al. Corneal dysfunction precedes the onset of hyperglycemia in a mouse model of diet-induced obesity. PLoS ONE 2020, 15, e0238750. [Google Scholar] [CrossRef]
- Iqbal, Z.; Kalteniece, A.; Ferdousi, M.; Adam, S.; D’Onofrio, L.; Ho, J.H.; Rao, A.P.; Dhage, S.; Azmi, S.; Liu, Y.; et al. Corneal keratocyte density and corneal nerves are reduced in patients with severe obesity and improve after bariatric surgery. Investig. Ophthalmol. Vis. Sci. 2021, 62. [Google Scholar] [CrossRef]
- Ojaimi, E.; Morgan, I.G.; Robaei, D.; Rose, K.A.; Smith, W.; Rochtchina, E.; Mitchell, P. Effect of stature and other anthropometric parameters on eye size and refraction in a population-based study of Australian children. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4424–4429. [Google Scholar] [CrossRef]
- Sanchis-Gimeno, J.A.; Nalla, S.; Rodriguez-Dieguez, E.; Hasrod, N. Correlation between body mass index and corneal thickness in emmetropic subjects. Afr. Vis. Eye Health 2023, 82, 20. [Google Scholar] [CrossRef]
- Doughty, M.J.; Zaman, M.L. Human corneal thickness and its impact on intraocular pressure measures: A review and meta-analysis approach. Surv. Ophthalmol. 2000, 44, 367–408. [Google Scholar] [CrossRef]
- Zakrzewska, A.; Wiącek, M.P.; Machalińska, A. Impact of corneal parameters on intraocular pressure measurements in different tonometry methods. Int. J. Ophthalmol. 2019, 12, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Altinok, A.; Sen, E.; Yazici, A.; Aksakal, F.N.; Oncul, H.; Koklu, G. Factors influencing central corneal thickness in a Turkish population. Curr. Eye Res. 2007, 32, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Bonnemaijer, P.W.M.; Lo Faro, V.; Sanyiwa, A.J.; Hassan, H.G.; Cook, C.; Van de Laar, S.; Lemij, H.G.; Klaver, C.C.W.; Jansonius, N.M.; Thiadens, A. Differences in clinical presentation of primary open-angle glaucoma between African and European populations. Acta Ophthalmol. 2021, 99, e1118–e1126. [Google Scholar] [CrossRef] [PubMed]
- Fern, K.D.; Manny, R.E.; Gwiazda, J.; Hyman, L.; Weise, K.; Marsh-Tootle, W. Intraocular pressure and central corneal thickness in the COMET cohort. Optom. Vis. Sci. 2012, 89, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Lee, J.W.; Lee, J.S. Intraocular pressure and influencing systemic health parameters in a Korean population. Indian. J. Ophthalmol. 2014, 62, 305–310. [Google Scholar] [CrossRef]
- Shimmyo, M.; Ross, A.J.; Moy, A.; Mostafavi, R. Intraocular pressure, Goldmann applanation tension, corneal thickness, and corneal curvature in Caucasians, Asians, Hispanics, and African Americans. Am. J. Ophthalmol. 2003, 136, 603–613. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Verde, L.; Colao, A. Body Mass Index (BMI): Still be used? Eur. J. Intern. Med. 2023, 117, 50–51. [Google Scholar] [CrossRef]
- Xue, Y.; Yang, X.; Liu, G. Association of combined body mass index and central obesity with cardiovascular disease in middle-aged and older adults: A population-based prospective cohort study. BMC Cardiovasc. Disord. 2024, 24. [Google Scholar] [CrossRef]
- Dogan, B.; Dogan, U.; Erol, M.K.; Habibi, M.; Oruc, M.T. Comparison of anterior segment parameter values obtained with Scheimpflug-Placido topographer, optical low coherence reflectometry and noncontact specular microscopy in morbid obesity. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 438–445. [Google Scholar]
- Nishitsuka, K.; Kawasaki, R.; Kanno, M.; Tanabe, Y.; Saito, K.; Honma, K.; Oizumi, T.; Daimon, M.; Kato, T.; Kayama, T.; et al. Determinants and Risk Factors for Central Corneal Thickness in Japanese Persons: The Funagata Study. Ophthalmic Epidemiol. 2011, 18, 244–249. [Google Scholar] [PubMed]
- Albuquerque, L.L.d.; Gaete, M.I.L.; Figueiroa, J.N.; Alves, J.G.B. The correlation between body mass index and intraocular pressure in children. Arq. Bras. Oftalmol. 2013, 76, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Pihlblad, M.S.; Schaefer, D.P. Eyelid laxity, obesity, and obstructive sleep apnea in keratoconus. Cornea 2013, 32, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Tu, R.; Xu, L.; Gu, Y.; Fan, Q.; Wang, Q.; Zhu, M.; Yin, S.; Pang, C.; Zhao, D.; et al. A high body mass index strengthens the association between the time of eye rubbing and keratoconus in a Chinese population: A case control study. BMC Public. Health 2023, 23, 2032. [Google Scholar] [CrossRef]
- Gencer, B.; Ozgurhan, E.B.; Kara, S.; Tufan, H.A.; Arikan, S.; Bozkurt, E.; Demirok, A. Obesity and obstructive sleep apnea in patients with keratoconus in a Turkish population. Cornea 2014, 33, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Londregan, A.; Rich, C.; Trinkaus-Randall, V. Changes in epithelial and stromal corneal stiffness occur with age and obesity. Bioengineering 2020, 7, 14. [Google Scholar] [CrossRef]
- Neyzi, O.; Bundak, R.; Gökçay, G.; Günöz, H.; Furman, A.; Darendeliler, F.; Baş, F. Reference values for weight, height, head circumference, and body mass index in Turkish children. JCRPE J. Clin. Res. Pediatr. Endocrinol. 2015, 7, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Dezor-Garus, J.; Niechciał, E.; Kędzia, A.; Gotz-Więckowska, A. Obesity-induced ocular changes in children and adolescents: A review. Front. Pediatr. 2023, 11, 1133965. [Google Scholar] [CrossRef]
- Akinci, A.; Cetinkaya, E.; Aycan, Z.; Oner, O. Relationship between intraocular pressure and obesity in children. J. Glaucoma 2007, 16, 627–630. [Google Scholar] [CrossRef]
- Aydemir, G.A.; Aydemir, E.; Asik, A.; Bolu, S. Changes in ocular pulse amplitude and choroidal thickness in childhood obesity patients with and without insulin resistance. Eur. J. Ophthalmol. 2022, 32, 2018–2025. [Google Scholar] [CrossRef]
- Baran, R.T.; Baran, S.O.; Toraman, N.F.; Filiz, S.; Demirbilek, H. Evaluation of intraocular pressure and retinal nerve fiber layer, retinal ganglion cell, central macular thickness, and choroidal thickness using optical coherence tomography in obese children and healthy controls. Niger. J. Clin. Pract. 2019, 22, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, S.A.; Unsal, A.I.A.; Verdi, F.; Omurlu, İ.K.; Unuvar, T.; Anık, A. The Effect of Childhood Obesity on Intraocular Pressure, Corneal Biomechanics, Retinal Nerve Fiber Layer, and Central Macular Thickness. J. Glaucoma 2024, 33, 417–421. [Google Scholar]
- Jiang, W.J.; Wu, J.F.; Hu, Y.Y.; Wu, H.; Sun, W.; Lu, T.L.; Wang, X.R.; Bi, H.S.; Jonas, J.B. Intraocular pressure and associated factors in children: The Shandong children eye study. Invest. Ophthalmol. Vis. Sci. 2014, 55, 4128–4134. [Google Scholar] [CrossRef] [PubMed]
- Verdi, F.; Akyüz Ünsal, A.İ.; Aydın Eroğlu, S.; Dündar, S.; Ünüvar, T.; Anık, A.; Kurt Ömürlü, İ. The Association Between Body Mass Index, Intraocular Pressure and Central Corneal Thickness in Children. Meandros Med. Dent. J. 2022, 23, 515–519. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, D.H.; Han, K.; Ha, S.G.; Kim, Y.H.; Kim, J.W.; Park, J.Y.; Yoon, S.J.; Jung, D.W.; Park, S.W.; et al. Relationship between Intraocular Pressure and Parameters of Obesity in Korean Adults: The 2008-2010 Korea National Health and Nutrition Examination Survey. Curr. Eye Res. 2015, 40, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Lee, E.H.; Jargal, G.; Paek, D.; Cho, S.I. The distribution of intraocular pressure and its association with metabolic syndrome in a community. J. Prev. Med. Public. Health 2010, 43, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Kim, M.H.; Pastor-Barriuso, R.; Chang, Y.; Ryu, S.; Zhang, Y.; Rampal, S.; Shin, H.; Kim, J.M.; Friedman, D.S.; et al. A Longitudinal Study of Association between Adiposity Markers and Intraocular Pressure: The Kangbuk Samsung Health Study. PLoS ONE 2016, 11, e0146057. [Google Scholar]
- Elflein, H.M.; Pfeiffer, N.; Hoffmann, E.M.; Hoehn, R.; Kottler, U.B.; Lorenz, K.; Zwiener, I.; Wild, P.S.; Mirshahi, A. Correlations Between Central Corneal Thickness and General Anthropometric Characteristics and Cardiovascular Parameters in a Large European Cohort From the Gutenberg Health Study. Cornea 2014, 33, 359–365. [Google Scholar]
- Zhang, Y.; Bian, A.; Hang, Q.; Li, L.; Zhang, S.; Cheng, G.; Zhou, Q. Corneal Biomechanical Properties of Various Types of Glaucoma and Their Impact on Measurement of Intraocular Pressure. Ophthalmic Res. 2023, 66, 749–756. [Google Scholar] [CrossRef]
| Normal (n = 30) | Overweight (n = 30) p | Obese (n = 30) p | p c | |
|---|---|---|---|---|
| Age (years) | 11.3 ± 2.56 | 11.7 ± 3.14 0.530 a | 11.6 ± 3.40 0.639 a | 0.797 |
| Gender Male/Female-n (%) | 14 (46.7)/16 (53.3) | 14 (46.7)/16 (53.3) 1 b | 12 (40)/18 (60) 0.610 b | 0.840 |
| BMI (kg/m2) | 18.6 ±1.98 | 23.3 ± 1.92 <0.001 a | 27.2 ± 1.93 <0.001 a | <0.001 |
| WC (cm) | 66 ± 9.55 | 103 ± 16.7 <0.001 a | 111 ± 11 <0.001 a | <0.001 |
| IOP (mmHg) | 13.6 ± 1.52 | 15.8 ± 2.35 <0.001 a | 16.5 ± 2.19 <0.001 a | <0.001 |
| Normal (n = 30) | Overweight (n = 30) p a | Obese (n = 30) p a | p b | |
|---|---|---|---|---|
| CCT (μm) | 537 ± 23.9 | 549 ± 21.7 0.046 | 561 ± 22.5 <0.001 | <0.001 |
| K flat (D) | 42.4 ± 0.808 | 42.3 ± 1.33 0.652 | 42.2 ± 1.01 0.763 | 0.751 |
| K steep (D) | 43 ± 0.808 | 43.4 ± 1.49 0.724 | 43.1 ± 1.17 0.563 | 0.445 |
| Kmax (D) | 42.8 ± 0.837 | 42.9 ± 1.39 0.263 | 42.7 ± 1.06 0.283 | 0.722 |
| CV (mm3) | 59.1 ± 2.32 | 59.3 ± 3.59 0.779 | 59.5 ± 2.68 0.310 | 0.833 |
| ACV (mm3) | 199 ± 29.9 | 201 ± 28.2 0.218 | 203 ± 29 0.622 | 0.201 |
| ACD (mm) | 3.13 ± 0.254 | 3.14 ± 0.238 0.896 | 3.20 ± 0.267 0.376 | 0.544 |
| ACA (°) | 39.8 ± 5.10 | 40.1 ± 5.26 0.830 | 40.2 ± 5.37 0.765 | 0.186 |
| Normal (n = 30) | Overweight (n = 30) p a | Obese (n = 30) p a | p b | |
|---|---|---|---|---|
| ECD (cells/mm2) | 3128 ± 257 | 3123 ± 270 0.753 | 3121 ± 265 0.664 | 0.903 |
| CV | 28.6 ± 3.54 | 28.5 ± 4.52 0.919 | 28.2 ± 4.71 0.591 | 0.852 |
| HEX (%) | 69.7 ± 9.91 | 67.8 ± 10.15 0.106 | 67.4 ± 10.61 0.074 | 0.162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzun, İ.; Colak, E.; Atlıhan, Z.; Mutaf, Ç.; Reyhan, A.H.; Yüksekyayla, F. Evaluation of the Effect of Body Mass Index and Waist Circumference on Ocular Health Parameters in Children and Adolescents. Children 2025, 12, 413. https://doi.org/10.3390/children12040413
Uzun İ, Colak E, Atlıhan Z, Mutaf Ç, Reyhan AH, Yüksekyayla F. Evaluation of the Effect of Body Mass Index and Waist Circumference on Ocular Health Parameters in Children and Adolescents. Children. 2025; 12(4):413. https://doi.org/10.3390/children12040413
Chicago/Turabian StyleUzun, İrfan, Enes Colak, Zeliha Atlıhan, Çağrı Mutaf, Ali Hakim Reyhan, and Funda Yüksekyayla. 2025. "Evaluation of the Effect of Body Mass Index and Waist Circumference on Ocular Health Parameters in Children and Adolescents" Children 12, no. 4: 413. https://doi.org/10.3390/children12040413
APA StyleUzun, İ., Colak, E., Atlıhan, Z., Mutaf, Ç., Reyhan, A. H., & Yüksekyayla, F. (2025). Evaluation of the Effect of Body Mass Index and Waist Circumference on Ocular Health Parameters in Children and Adolescents. Children, 12(4), 413. https://doi.org/10.3390/children12040413







