Characteristics of Studies Focusing on Vaccine Series Completion Among Children Aged 12–23 Months in Sub-Saharan Africa: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategies
2.2. Eligibility Criteria
- Primary research studies (quantitative, qualitative, or mixed-methods) and the gray literature conducted in SSA, whether covering part of a country, an entire country, or multiple countries.
- Focused on children aged 12–23 months.
- Reported the VSC rates, as defined in this study.
- Published in English between January 2000 and December 2023, coinciding with the introduction of pentavalent, pneumococcal conjugate (PCV), and rotavirus (RV) vaccines in most SSA countries.
- Lacked a clear definition of VSC or relevant concepts.
- Did not provide specific VSC rates or related metrics.
- Focused exclusively on one or several types of vaccines rather than the full immunization schedule or included vaccines not part of the national EPI.
- Were systematic reviews, study protocols, journal commentaries, or conference papers.
- Were duplicate studies or lacked access to full texts.
2.3. Data Extraction and Analysis
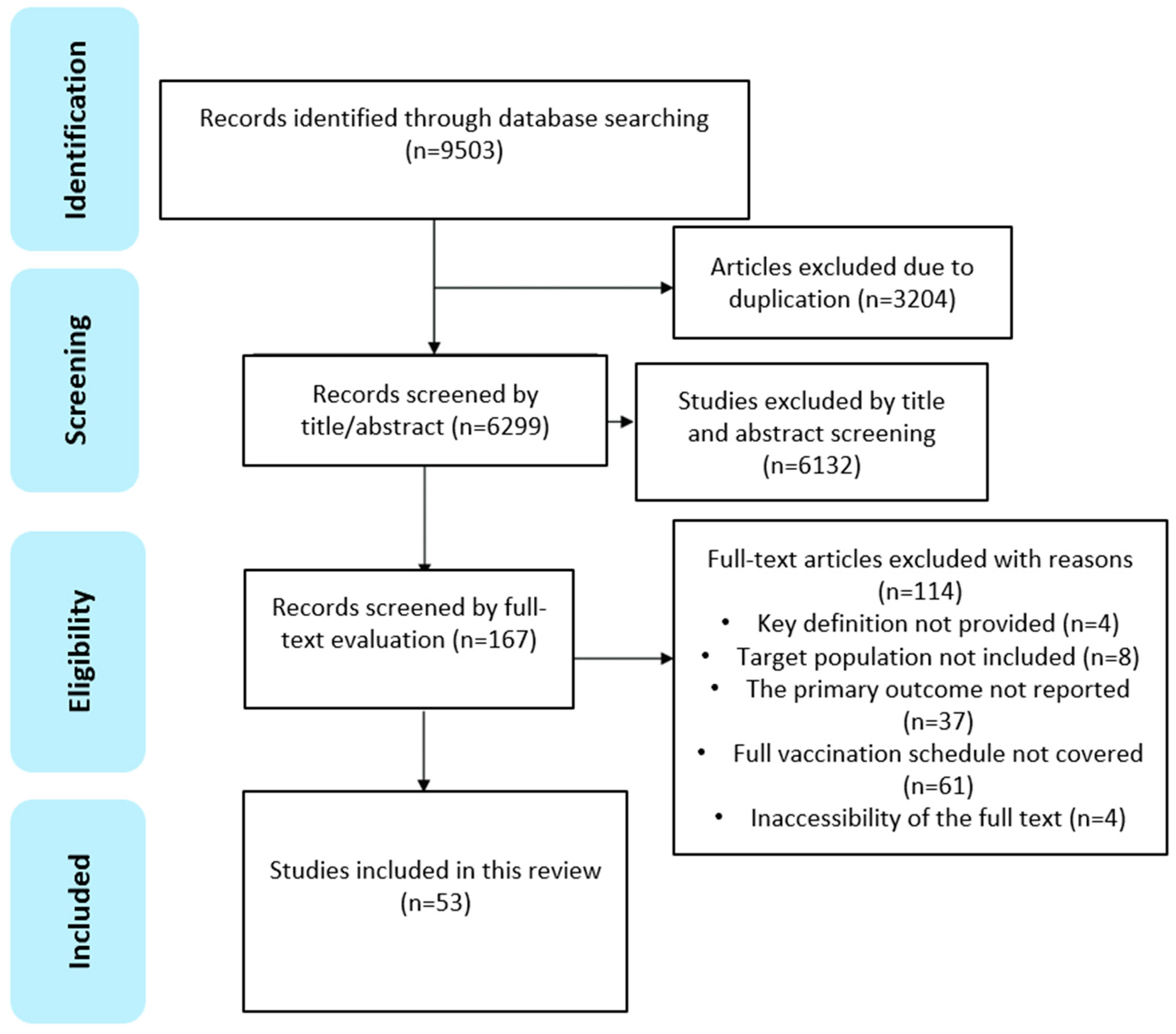
3. Results
3.1. Overview of Included Studies
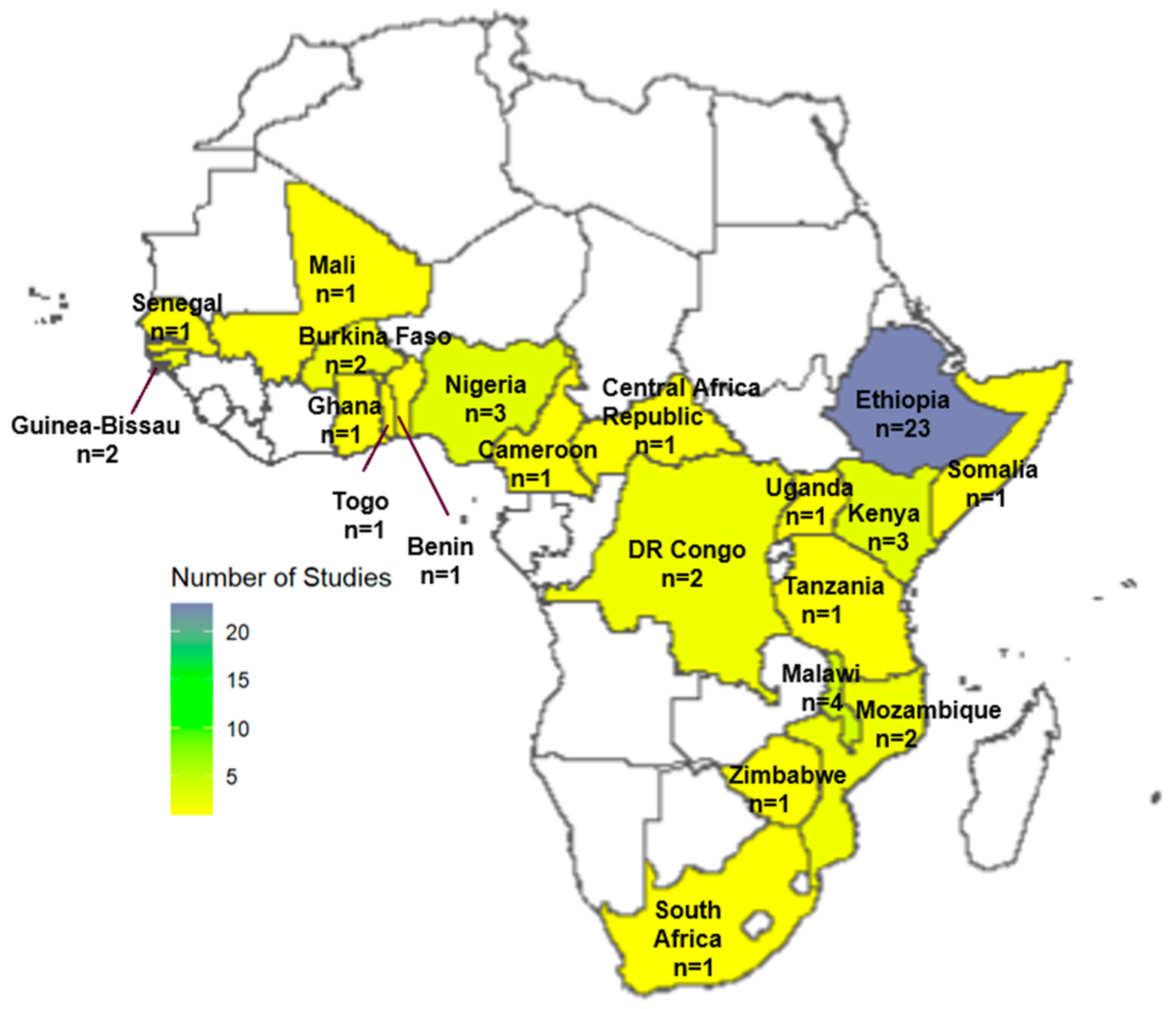
3.2. Study Settings
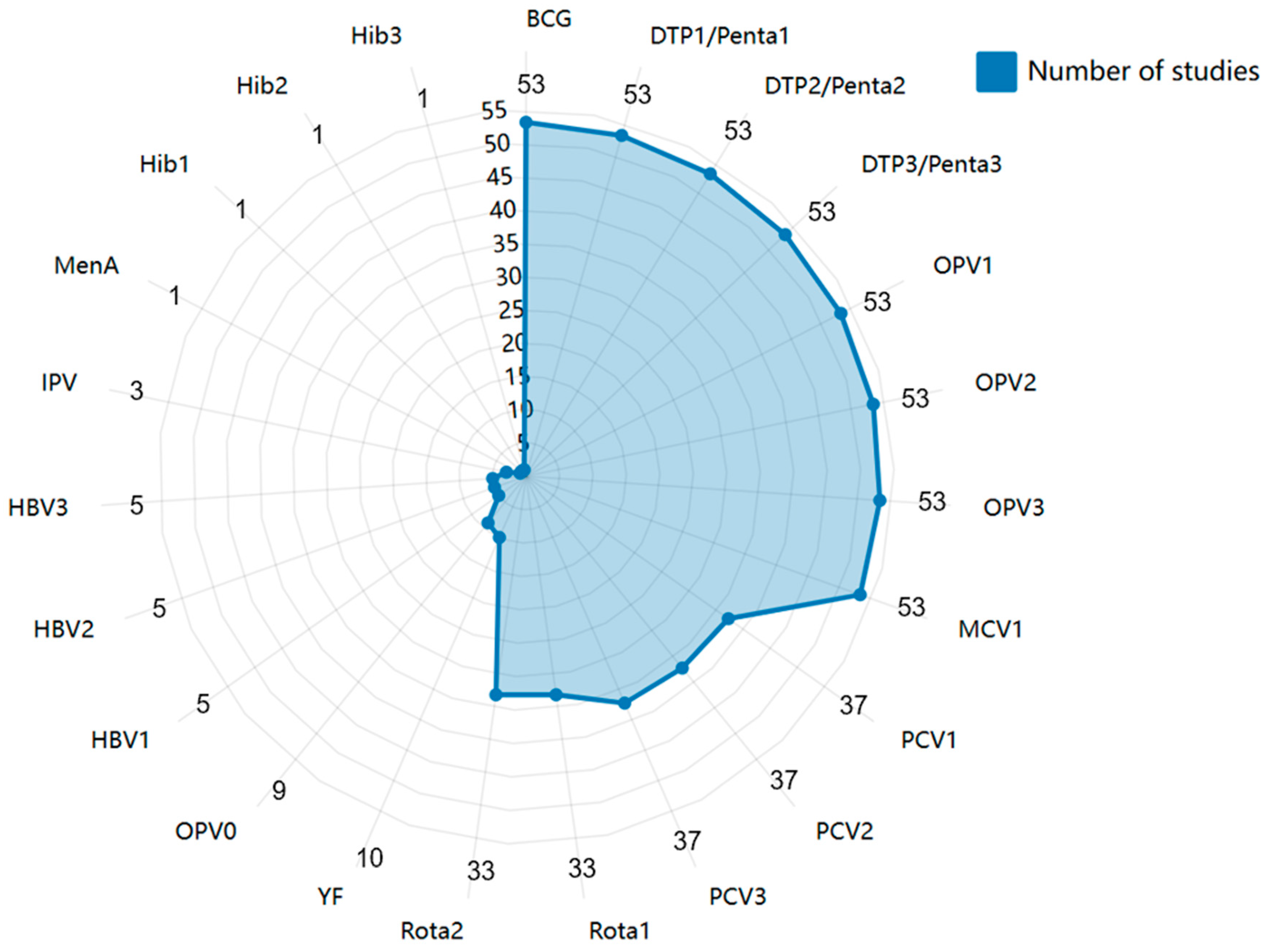
3.3. Vaccines Assessed in the Published Literature
3.3.1. Prevalence of VSC in SSA
3.3.2. Trends of VSC in SSA
3.3.3. Inequality of VSC in SSA
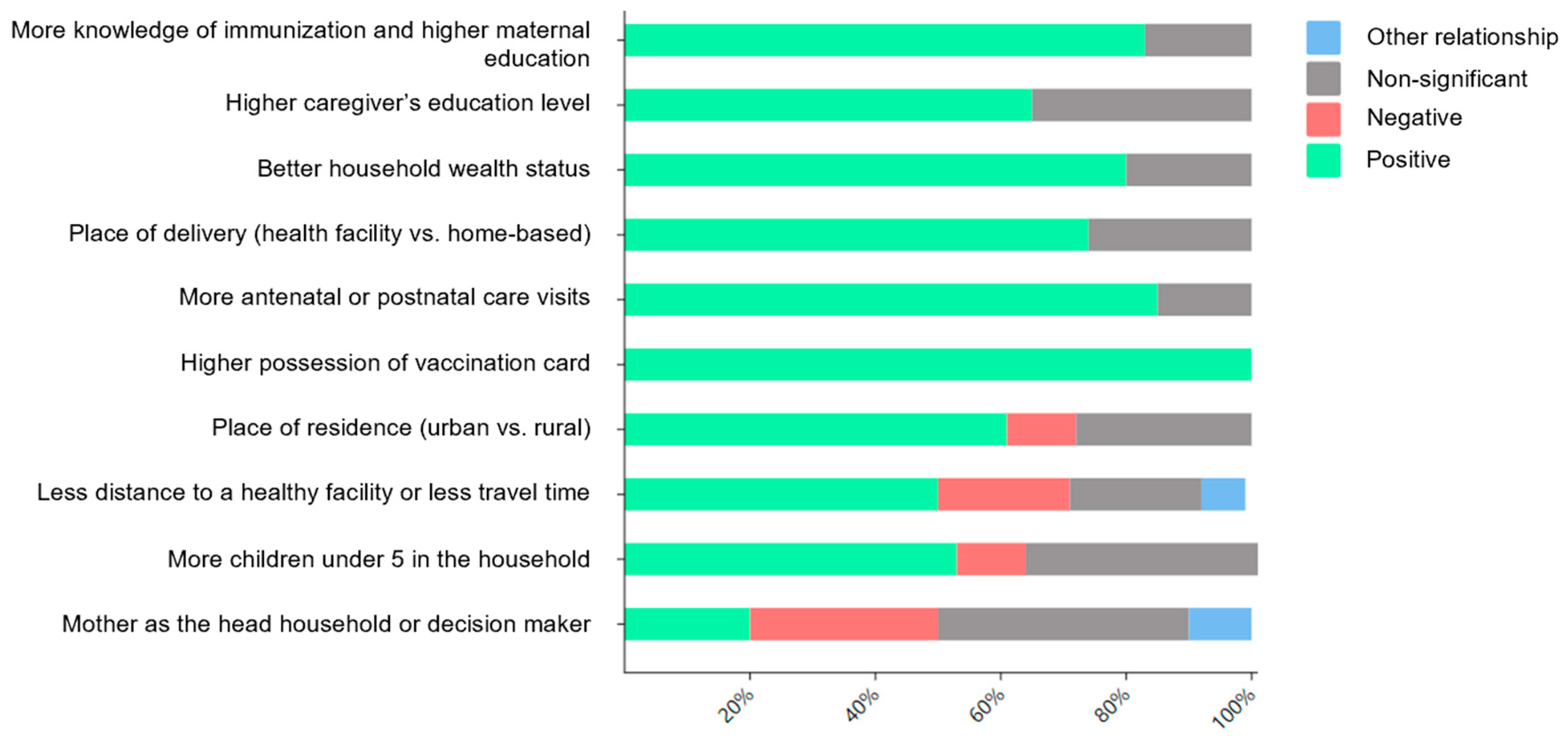
3.3.4. Factors Associated with VSC in SSA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vaccines: The Powerful Innovations Bringing WHO’s Mission to Life Every Day. Available online: https://www.who.int/news-room/commentaries/detail/vaccines-the-powerful-innovations-bringing-who-s-mission-to-life-every-day (accessed on 3 May 2024).
- Vaccination and Immunization Statistics—UNICEF DATA. Available online: https://data.unicef.org/topic/child-health/immunization/#more (accessed on 3 May 2024).
- Sharrow, D.; Hug, L.; You, D.; Alkema, L.; Black, R.; Cousens, S.; Croft, T.; Gaigbe-Togbe, V.; Gerland, P.; Guillot, M.; et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: A systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet Glob. Health 2022, 10, e195–e206. [Google Scholar] [CrossRef]
- Rapid Measles Outbreak Response Critical to Protect Millions of Vulnerable Children. Available online: https://www.who.int/europe/news/item/22-02-2024-rapid-measles-outbreak-response-critical-to-protect-millions-of-vulnerable-children (accessed on 3 May 2024).
- WHO Vaccine-Preventable Diseases: Monitoring System: 2010 Global Summary. Available online: https://iris.who.int/handle/10665/70535 (accessed on 3 May 2024).
- Lindstrand, A.; Cherian, T.; Chang-Blanc, D.; Feikin, D.; O’brien, K. The World of Immunization: Achievements, Challenges, and Strategic Vision for the Next Decade. J. Infect. Dis. 2021, 224, S452–S467. [Google Scholar] [PubMed]
- Phillips, D.E.; Dieleman, J.L.; Lim, S.S.; Shearer, J. Determinants of effective vaccine coverage in low and middle-income countries: A systematic review and interpretive synthesis. BMC Health Serv. Res. 2017, 17, 681. [Google Scholar]
- Bangura, J.B.; Xiao, S.; Qiu, D.; Ouyang, F.; Chen, L. Barriers to childhood immunization in sub-Saharan Africa: A systematic review. BMC Public Health 2020, 20, 1108. [Google Scholar]
- Fenta, S.M.; Biresaw, H.B.; Fentaw, K.D.; Gebremichael, S.G. Determinants of full childhood immunization among children aged 12–23 months in sub-Saharan Africa: A multilevel analysis using Demographic and Health Survey Data. Trop. Med. Health 2021, 49, 29. [Google Scholar]
- Budu, E.; Ahinkorah, B.O.; Aboagye, R.G.; Armah-Ansah, E.K.; Seidu, A.-A.; Adu, C.; Ameyaw, E.K.; Yaya, S. Maternal healthcare utilsation and complete childhood vaccination in sub-Saharan Africa: A cross-sectional study of 29 nationally representative surveys. BMJ Open 2021, 11, e045992. [Google Scholar] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [PubMed]
- Sako, S.; Gilano, G.; Hailegebreal, S. Determinants of childhood vaccination among children aged 12–23 months in Ethiopia: A community-based cross-sectional study. BMJ Open 2023, 13, e069278. [Google Scholar] [CrossRef]
- Gelagay, A.A.; Worku, A.G.; Bashah, D.T.; Tebeje, N.B.; Gebrie, M.H.; Yeshita, H.Y.; Cherkose, E.A.; Ayana, B.A.; Lakew, A.M.; Bitew, D.A.; et al. Complete childhood vaccination and associated factors among children aged 12–23 months in Dabat demographic and health survey site, Ethiopia, 2022. BMC Public Health 2023, 23, 802. [Google Scholar] [CrossRef]
- Worku, A.; Alemu, H.; Belay, H.; Mohammedsanni, A.; Denboba, W.; Mulugeta, F.; Omer, S.; Abate, B.; Mohammed, M.; Ahmed, M.; et al. Contribution of health information system to child immunization services in Ethiopia: Baseline study of 33 woredas. BMC Med. Inform. Decis. Mak. 2022, 22, 64. [Google Scholar] [CrossRef]
- Muluye, M.; Oljira, L.; Eyeberu, A.; Getachew, T.; Debella, A.; Deressa, A.; Dheresa, M. Partial vaccination and associated factors among children aged 12–23 months in eastern Ethiopia. BMC Pediatr. 2022, 22, 268. [Google Scholar]
- Darebo, T.D.; Oshe, B.B.; Diro, C.W. Full vaccination coverage and associated factors among children aged 12 to 23 months in remote rural area of Demba Gofa District, Southern Ethiopia. PeerJ 2022, 10, e13081. [Google Scholar] [PubMed]
- Asmare, G.; Madalicho, M.; Sorsa, A. Disparities in full immunization coverage among urban and rural children aged 12–23 months in southwest Ethiopia: A comparative cross-sectional study. Hum. Vaccines Immunother. 2022, 18, 2101316. [Google Scholar]
- Mekonnen, Z.A.; Gelaye, K.A.; Were, M.; Tilahun, B. Effect of Mobile Phone Text Message Reminders on the Completion and Timely Receipt of Routine Childhood Vaccinations: Superiority Randomized Controlled Trial in Northwest Ethiopia. JMIR mHealth uHealth 2021, 9, e27603. [Google Scholar] [PubMed]
- Miretu, D.G.; Asfaw, Z.A.; Addis, S.G. Impact of COVID-19 pandemic on vaccination coverage among children aged 15 to 23 months at Dessie town, Northeast Ethiopia, 2020. Hum. Vaccines Immunother. 2021, 17, 2427–2436. [Google Scholar]
- Jimma, M.S.; GebreEyesus, F.A.; Chanie, E.S.; Delelegn, M.W. Full Vaccination Coverage and Associated Factors Among 12-to-23-Month Children at Assosa Town, Western Ethiopia, 2020. Pediatr. Health Med. Ther. 2021, 12, 279–288. [Google Scholar]
- Gelagay, A.A.; Geremew, A.B.; Teklu, A.; Mekonnen, Z.A.; Gera, R.; Ba-Nguz, A.; Tilahun, B. Full immunization coverage and its determinants among children aged 12–23 months in Wogera district, Northwest Ethiopia. Ethiop. J. Health Dev. 2021, 35, 16–27. [Google Scholar]
- Mekonnen, Z.A.; Gelaye, K.A.; Were, M.C.; Tilahun, B. Timely completion of vaccination and its determinants among children in northwest, Ethiopia: A multilevel analysis. BMC Public Health 2020, 20, 908. [Google Scholar]
- Geweniger, A.; Abbas, K.M. Childhood vaccination coverage and equity impact in Ethiopia by socioeconomic, geographic, maternal, and child characteristics. Vaccine 2020, 38, 3627–3638. [Google Scholar]
- Debie, A.; Lakew, A.M.; Tamirat, K.S.; Amare, G.; Tesema, G.A. Complete vaccination service utilization inequalities among children aged 12–23 months in Ethiopia: A multivariate decomposition analyses. Int. J. Equity Health 2020, 19, 1–16. [Google Scholar]
- Debie, A.; Amare, G.; Handebo, S.; Mekonnen, M.E.; Tesema, G.A. Individual- and Community-Level Determinants for Complete Vaccination among Children Aged 12–23 Months in Ethiopia: A Multilevel Analysis. BioMed Res. Int. 2020, 2020, 6907395. [Google Scholar] [CrossRef]
- Porth, J.M.; Wagner, A.L.; Tefera, Y.A.; Boulton, M.L. Childhood Immunization in ethiopia: Accuracy of maternal recall compared to vaccination cards. Vaccines 2019, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Tamirat, K.S.; Sisay, M.M. Full immunization coverage and its associated factors among children aged 12–23 months in Ethiopia: Further analysis from the 2016 Ethiopia demographic and health survey. BMC Public Health 2019, 19, 1019. [Google Scholar]
- Mekonnen, A.G.; Bayleyegn, A.D.; Ayele, E.T. Immunization coverage of 12-23 months old children and its associated factors in Minjar-Shenkora district, Ethiopia: A community-based study. BMC Pediatr. 2019, 19, 198. [Google Scholar]
- Tesfaye, T.D.; Temesgen, W.A.; Kasa, A.S. Vaccination coverage and associated factors among children aged 12–23 months in Northwest Ethiopia. Hum. Vaccines Immunother. 2018, 14, 2348–2354. [Google Scholar]
- Tefera, Y.A.; Wagner, A.L.; Mekonen, E.B.; Carlson, B.F.; Boulton, M.L. Predictors and barriers to full vaccination among children in ethiopia. Vaccines 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Animaw, W.; Taye, W.; Merdekios, B.; Tilahun, M.; Ayele, G. Expanded program of immunization coverage and associated factors among children age 12-23 months in Arba Minch town and Zuria District, Southern Ethiopia, 2013. BMC Public Health 2014, 14, 464. [Google Scholar]
- Moyer, C.A.; Tadesse, L.; Fisseha, S. The relationship between facility delivery and infant immunization in Ethiopia. Int. J. Gynaecol. Obstet. 2013, 123, 217–220. [Google Scholar]
- Etana, B.; Deressa, W. Factors associated with complete immunization coverage in children aged 12–23 months in Ambo Woreda, Central Ethiopia. BMC Public Health 2012, 12, 566. [Google Scholar] [CrossRef]
- Luman, E.T.; Worku, A.; Berhane, Y.; Martin, R.; Cairns, L. Comparison of two survey methodologies to assess vaccination coverage. Int. J. Epidemiol. 2007, 36, 633–641. [Google Scholar]
- Mmanga, K.; Mwenyenkulu, T.E.; Nkoka, O.; Ntenda, P.A.M. Tracking immunization coverage, dropout and equity gaps among children ages 12-23 months in Malawi–bottleneck analysis of the Malawi Demographic and Health Survey. Int. Health 2022, 14, 250–259. [Google Scholar]
- E Johns, N.; Hosseinpoor, A.R.; Chisema, M.; Danovaro-Holliday, M.C.; Kirkby, K.; Schlotheuber, A.; Shibeshi, M.; Sodha, S.V.; Zimba, B. Association between childhood immunisation coverage and proximity to health facilities in rural settings: A cross-sectional analysis of Service Provision Assessment 2013–2014 facility data and Demographic and Health Survey 2015–2016 individual data in Malawi. BMJ Open 2022, 12, e061346. [Google Scholar]
- Ntenda, P.A.M. Factors associated with non- and under- vaccination among children aged 12-23 months in Malawi. A multinomial analysis of the population-based sample. Pediatr. Neonatol. 2019, 60, 623–633. [Google Scholar] [PubMed]
- Tsega, A.; Hausi, H.; Chriwa, G.; Steinglass, R.; Smith, D.; Valle, M. Vaccination coverage and timely vaccination with valid doses in Malawi. Vaccine Rep. 2016, 6, 8–12. [Google Scholar] [CrossRef]
- Fatiregun, A.A.; Etukiren, E.E. Determinants of uptake of third doses of oral polio and DTP vaccines in the Ibadan North Local Government Area of Nigeria. Int. Health 2014, 6, 213–224. [Google Scholar]
- Fatiregun, A.A.; Okoro, A.O. Maternal determinants of complete child immunization among children aged 12–23 months in a southern district of Nigeria. Vaccine 2012, 30, 730–736. [Google Scholar] [CrossRef]
- Sadoh, A.E.; Eregie, C.O. Timeliness and completion rate of immunization among nigerian children attending a clinic-based immunization service. J. Health Popul. Nutr. 2009, 27, 391–395. [Google Scholar]
- Allan, S.; Adetifa, I.M.O.; Abbas, K. Inequities in childhood immunisation coverage associated with socioeconomic, geographic, maternal, child, and place of birth characteristics in Kenya. BMC Infect. Dis. 2021, 21, 553. [Google Scholar]
- Maina, L.C.; Karanja, S.; Kombich, J. Immunization coverage and its determinants among children aged 12–23 months in a peri-urban area of Kenya. Pan Afr. Med. J. 2013, 14, 3. [Google Scholar] [CrossRef]
- Kawakatsu, Y.; Honda, S. Individual-, family- and community-level determinants of full vaccination coverage among children aged 12–23 months in western Kenya. Vaccine 2012, 30, 7588–7593. [Google Scholar]
- Koulidiati, J.L.; Kaboré, R.; INebié, E.; Sidibé, A.; Lohmann, J.; Brenner, S.; Badolo, H.; Hamadou, S.; Ouédraogo, N.; De Allegri, M. Timely completion of childhood vaccination and its predictors in Burkina Faso. Vaccine 2022, 40, 3356–3365. [Google Scholar] [PubMed]
- Sia, D.; Fournier, P.; Kobiané, J.-F.; Sondo, B.K. Rates of coverage and determinants of complete vaccination of children in rural areas of Burkina Faso (1998–2003). BMC Public Health 2009, 9, 416. [Google Scholar]
- Mukanda, S. Effect of COVID-19 on Uptake of Routine Immunization in Goma, Democratic Republic of Congo. Ph.D. Thesis, Kenya Methodist University, Nairobi, Kenya, 2022. [Google Scholar]
- Lu, X.; Fu, C.; Wang, Q.; He, Q.; Hee, J.; Takesue, R.; Tang, K. Women’s Empowerment and Children’s Complete Vaccination in the Democratic Republic of the Congo: A Cross-Sectional Analysis. Vaccines 2021, 9, 1117. [Google Scholar] [CrossRef]
- Shemwell, S.A.; Peratikos, M.B.; González-Calvo, L.; Renom-Llonch, M.; Boon, A.; Martinho, S.; Cherry, C.B.; Green, A.F.; Moon, T.D. Determinants of full vaccination status in children aged 12–23 months in Gurue, and Milange districts, Mozambique: Results of a population-based cross-sectional survey. Int. Health 2017, 9, 234–242. [Google Scholar] [PubMed]
- Jani, J.V.; De Schacht, C.; Jani, I.V.; Bjune, G. Risk factors for incomplete vaccination and missed opportunity for immunization in rural Mozambique. BMC Public Health 2008, 8, 161. [Google Scholar]
- Fisker, A.B.; Hornshøj, L.; Rodrigues, A.; Balde, I.; Fernandes, M.; Benn, C.S.; Aaby, P. Effects of the introduction of new vaccines in Guinea-Bissau on vaccine coverage, vaccine timeliness, and child survival: An observational study. Lancet Glob. Health 2014, 2, e478–e487. [Google Scholar]
- Hornshøj, L.; Benn, C.S.; Fernandes, M.; Rodrigues, A.; Aaby, P.; Fisker, A.B. Vaccination coverage and out-of-sequence vaccinations in rural Guinea-Bissau: An observational cohort study. BMJ Open 2012, 2, e001509. [Google Scholar]
- Budu, E.; Darteh, E.K.M.; Ahinkorah, B.O.; Seidu, A.-A.; Dickson, K.S. Trend and determinants of complete vaccination coverage among children aged 12–23 months in Ghana: Analysis of data from the 1998 to 2014 Ghana Demographic and Health Surveys. PLoS ONE 2020, 15, e0239754. [Google Scholar]
- Batalingaya, F.X. Internal Population Displacement and Vaccination Completion, Malnutrition, and Respiratory Infections Among Children in the Central African Republic. Ph.D. Thesis, Walden University, Minneapolis, MN, USA, 2023. [Google Scholar]
- Sangaré, S.; Sangho, O.; Doumbia, L.; Marker, H.; Sarro, Y.D.S.; Dolo, H.; Tel-Ly, N.; Ben Zakour, I.; Ndiaye, H.M.; Sanogo, M.; et al. Concordance of vaccination status and associated factors with incomplete vaccination: A household survey in the health district of Segou, Mali, 2019. Pan Afr. Med. J. 2021, 40, 102. [Google Scholar]
- Montwedi, D.N.; Meyer, J.C.; Nkwinika, V.V.; Burnett, R.J. Health facility obstacles result in missed vaccination opportunities in tshwane region 5, gauteng province. SAJCH S. Afr. J. Child Health 2021, 15, 159–164. [Google Scholar]
- Budu, E.; Seidu, A.-A.; Agbaglo, E.; Armah-Ansah, E.K.; Dickson, K.S.; Hormenu, T.; Hagan, J.E.; Adu, C.; Ahinkorah, B.O. Maternal healthcare utilization and full immunization coverage among 12–23 months children in Benin: A cross sectional study using population-based data. Arch. Public Health 2021, 79, 34. [Google Scholar] [PubMed]
- Jama, A.A. Determinants of Complete Immunization Coverage among Children Aged 11–24 Months in Somalia. Int. J. Pediatr. 2020, 2020, 5827074. [Google Scholar]
- Cortaredona, S.; Diop, R.; Seror, V.; Sagaon-Teyssier, L.; Peretti-Watel, P. Regional variations of childhood immunisations in Senegal: A multilevel analysis. Trop. Med. Int. Health 2020, 25, 1122–1130. [Google Scholar]
- Ekouevi, D.K.; Gbeasor-Komlanvi, F.A.; Yaya, I.; Zida-Compaore, W.I.; Boko, A.; Sewu, E.; Lacle, A.; Ndibu, N.; Toke, Y.; Landoh, D.E. Incomplete immunization among children aged 12–23 months in Togo: A multilevel analysis of individual and contextual factors. BMC Public Health 2018, 18, 952. [Google Scholar]
- Russo, G.; Miglietta, A.; Pezzotti, P.; Biguioh, R.M.; Mayaka, G.B.; Sobze, M.S.; Stefanelli, P.; Pezzotti, P.; Rezza, G. Vaccine coverage and determinants of incomplete vaccination in children aged 12–23 months in Dschang, West Region, Cameroon: A cross-sectional survey during a polio outbreak. BMC Public Health 2015, 15, 85. [Google Scholar]
- Kruger, C.; E Olsen, O.; Mighay, E.; Ali, M. Immunisation coverage and its associations in rural Tanzanian infants. Rural Remote Health 2013, 13, 2457. [Google Scholar] [PubMed]
- Babirye, J.N.; Engebretsen, I.M.S.; Makumbi, F.; Fadnes, L.T.; Wamani, H.; Tylleskar, T.; Nuwaha, F. Timeliness of Childhood Vaccinations in Kampala Uganda: A Community-Based Cross-Sectional Study. PLoS ONE 2012, 7, e35432. [Google Scholar]
- Mukungwa, T. Factors Associated with full Immunization Coverage amongst children aged 12–23 months in Zimbabwe. Afr. Popul. Stud. 2015, 29. [Google Scholar] [CrossRef]
- Global Vaccine Action Plan—2019 Regional Reports on Progress Towards GVAP-RVAP Goals. Available online: https://www.who.int/publications/m/item/global-vaccine-action-plan-2019-regional-reports-on-progress-towards-gvap-rvap-goals (accessed on 5 May 2024).
- UNAIDS. In Danger: UNAIDS Global AIDS Update 2022; United Nations: Manhattan, NY, USA, 2022. [Google Scholar]
- Donfouet, H.P.P.; Agesa, G.; Mutua, M.K. Trends of inequalities in childhood immunization coverage among children aged 12–23 months in Kenya, Ghana, and Côte d’Ivoire. BMC Public Health 2019, 19, 988. [Google Scholar]
- Yibeltal, K.; Tsegaye, S.; Zelealem, H.; Worku, W.; Demissie, M.; Worku, A.; Berhane, Y. Trends, projection and inequalities in full immunization coverage in Ethiopia: In the period 2000–2019. BMC Pediatr. 2022, 22, 193. [Google Scholar]
- Lubanga, A.F.; Bwanali, A.N.; Munthali, L.; Mphepo, M.; Chumbi, G.D.; Kangoma, M.; Khuluza, C. Malawi vaccination drive: An integrated immunization campaign against typhoid, measles, rubella, and polio; health benefits and potential challenges. Hum. Vaccines Immunother. 2023, 19, 2233397. [Google Scholar] [CrossRef] [PubMed]
- Okeibunor, J.; Nsubuga, P.; Salla, M.; Mihigo, R.; Mkanda, P. Coordination as a best practice from the polio eradication initiative: Experiences from five member states in the African region of the World Health Organization. Vaccine 2016, 34, 5203–5207. [Google Scholar] [CrossRef]
- Bobo, F.T.; Asante, A.; Woldie, M.; Dawson, A.; Hayen, A. Child vaccination in sub-Saharan Africa: Increasing coverage addresses inequalities. Vaccine 2022, 40, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ameyaw, E.K.; Kareem, Y.O.; Ahinkorah, B.O.; Seidu, A.-A.; Yaya, S. Decomposing the rural–urban gap in factors associated with childhood immunisation in sub-Saharan Africa: Evidence from surveys in 23 countries. BMJ Glob. Health 2021, 6, e003773. [Google Scholar] [CrossRef]
- Mihigo, R.; Okeibunor, J.; Anya, B.; Mkanda, P.; Zawaira, F. Challenges of immunization in the African Region. Pan Afr. Med. J. 2017, 27 (Suppl. 3), 12. [Google Scholar] [CrossRef] [PubMed]
- Adamu, A.A.; Uthman, O.A.; Sambala, E.Z.; Ndwandwe, D.; Wiyeh, A.B.; Olukade, T.; Bishwajit, G.; Yaya, S.; Okwo-Bele, J.-M.; Wiysonge, C.S. Rural-urban disparities in missed opportunities for vaccination in sub-Saharan Africa: A multi-country decomposition analyses. Hum. Vaccines Immunother. 2019, 15, 1191–1198. [Google Scholar] [CrossRef]
- Abdelmagid, N.; Southgate, R.J.; Alhaffar, M.; Ahmed, M.; Bani, H.; Mounier-Jack, S.; Dahab, M.; Checchi, F.; Sabahelzain, M.M.; Nor, B.; et al. The Governance of Childhood Vaccination Services in Crisis Settings: A Scoping Review. Vaccines 2023, 11, 1853. [Google Scholar] [CrossRef]
- Decouttere, C.; De Boeck, K.; Vandaele, N. Advancing sustainable development goals through immunization: A literature review. Glob. Health 2021, 17, 95. [Google Scholar] [CrossRef]
- Rainey, J.J.; Watkins, M.; Ryman, T.K.; Sandhu, P.; Bo, A.; Banerjee, K. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: Findings from a systematic review of the published literature, 1999–2009. Vaccine 2011, 29, 8215–8221. [Google Scholar] [CrossRef]
- Wilson, L.; Rubens-Augustson, T.; Murphy, M.; Jardine, C.; Crowcroft, N.; Hui, C.; Wilson, K. Barriers to immunization among newcomers: A systematic review. Vaccine 2018, 36, 1055–1062. [Google Scholar] [CrossRef]
- Kagoné, M.; Yé, M.; Nébié, E.; Sie, A.; Schoeps, A.; Becher, H.; Muller, O.; Fisker, A.B. Vaccination coverage and factors associated with adherence to the vaccination schedule in young children of a rural area in Burkina Faso. Glob. Health Action 2017, 10, 1399749. [Google Scholar] [PubMed]
- El-Halabi, S.; Khader, Y.S.; Abu Khdeir, M.; Hanson, C.; Alfvén, T.; El-Khatib, Z. Children Immunization App (CIMA): A Non-randomized Controlled Trial Among Syrian Refugees in Zaatari Camp, Jordan. J. Prev. 2023, 44, 239–252. [Google Scholar]
- Ngah, Y.E.; Raoufi, G.; Amirkhani, M.; Esmaeili, A.; Nikooifard, R.; Mood, S.G.; Rahmanian, A.; Boltena, M.T.; Aga, E.; Neogi, U.; et al. Testing the Impact of Phone Texting Reminders for Children’s Immunization Appointments in Rural Cameroon: Protocol for a Nonrandomized Controlled Trial. JMIR Res. Protoc. 2023, 12, e47018. [Google Scholar] [PubMed]
| Characteristics | N (%) |
|---|---|
| Publication year | |
| 2000–2010 | 4 (7.5%) |
| 2011–2023 | 49 (92.5%) |
| Publication delay † | |
| Secondary analysis | 4.0 (3.0, 6.0) years |
| Primary studies | 2.0 (1.0, 3.0) years |
| Study design | |
| Cross-sectional | 31 (60.4%) |
| Secondary analysis | 19 (34.0%) |
| Cohort | 2 (3.8%) |
| Randomized control trial | 1 (1.9%) |
| Data sources | |
| Demographic Health Survey | 29 (54.7%) |
| Multiple Indicator Cluster Survey | 2 (3.8%) |
| Primary data collection | 22 (41.5%) |
| Primary objective of study | |
| Prevalence | 26 (49.1%) |
| Inequalities | 4 (7.5%) |
| Trends | 3 (5.7%) |
| Associated factors | 43 (81.1%) |
| Others | 8 (15.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Sewolo, F.; Aoun, A.; Boltena, M.T.; Musad, A.; Lindstrand, A.; Alfvén, T.; Hanson, C.; El-Khatib, Z. Characteristics of Studies Focusing on Vaccine Series Completion Among Children Aged 12–23 Months in Sub-Saharan Africa: A Scoping Review. Children 2025, 12, 415. https://doi.org/10.3390/children12040415
Li W, Sewolo F, Aoun A, Boltena MT, Musad A, Lindstrand A, Alfvén T, Hanson C, El-Khatib Z. Characteristics of Studies Focusing on Vaccine Series Completion Among Children Aged 12–23 Months in Sub-Saharan Africa: A Scoping Review. Children. 2025; 12(4):415. https://doi.org/10.3390/children12040415
Chicago/Turabian StyleLi, Weiqi, Fabrice Sewolo, Andrew Aoun, Minyahil Tadesse Boltena, Amro Musad, Ann Lindstrand, Tobias Alfvén, Claudia Hanson, and Ziad El-Khatib. 2025. "Characteristics of Studies Focusing on Vaccine Series Completion Among Children Aged 12–23 Months in Sub-Saharan Africa: A Scoping Review" Children 12, no. 4: 415. https://doi.org/10.3390/children12040415
APA StyleLi, W., Sewolo, F., Aoun, A., Boltena, M. T., Musad, A., Lindstrand, A., Alfvén, T., Hanson, C., & El-Khatib, Z. (2025). Characteristics of Studies Focusing on Vaccine Series Completion Among Children Aged 12–23 Months in Sub-Saharan Africa: A Scoping Review. Children, 12(4), 415. https://doi.org/10.3390/children12040415








