The Pediatric Microbiota–Gut–Brain Axis: Implications for Neuropsychiatric Development and Intervention
Highlights
- Alterations in the pediatric gut microbiota are linked to neurodevelopmental and psychiatric conditions such as ASD, ADHD, and mood disorders.
- Mechanistic pathways include immune signaling, microbial metabolites, and neural communication via the vagus nerve and enteric nervous system.
- Early-life modulation of the gut microbiota may reduce the risk or severity of neuropsychiatric disorders in children.
- Interventions such as probiotics, diet, and psychobiotics show promise but require more pediatric-specific clinical research.
Abstract
1. Introduction
2. Methods
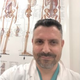
3. Early-Life Development of the Gut Microbiota
3.1. Neonatal Colonization: Vaginal Delivery vs. Cesarean Section
3.2. Breastfeeding vs. Formula Feeding
3.3. Dietary and Antibiotic Effects on the Gut Microbiota
4. The Microbiota and Brain Development
4.1. Influence on Neurobiological Development
4.2. Interaction with the Central Nervous System
4.3. Role in Blood–Brain Barrier Development, Neuroinflammation, and Neurotransmitters
5. Correlation Between Microbiota and Neuropsychiatric Disorders in Developmental Age
5.1. Gut Microbiota and Autism Spectrum Disorder (ASD)
5.2. Gut Microbiota and ADHD
Possible Mechanisms Linking Gut Microbiota Dysbiosis and ADHD
5.3. Gut Microbiota and Bipolar Disorder
5.4. Gut Microbiota and Major Depressive Disorder
5.5. Gut Microbiota and Anorexia Nervosa
5.6. Gut Microbiota and Social Anxiety
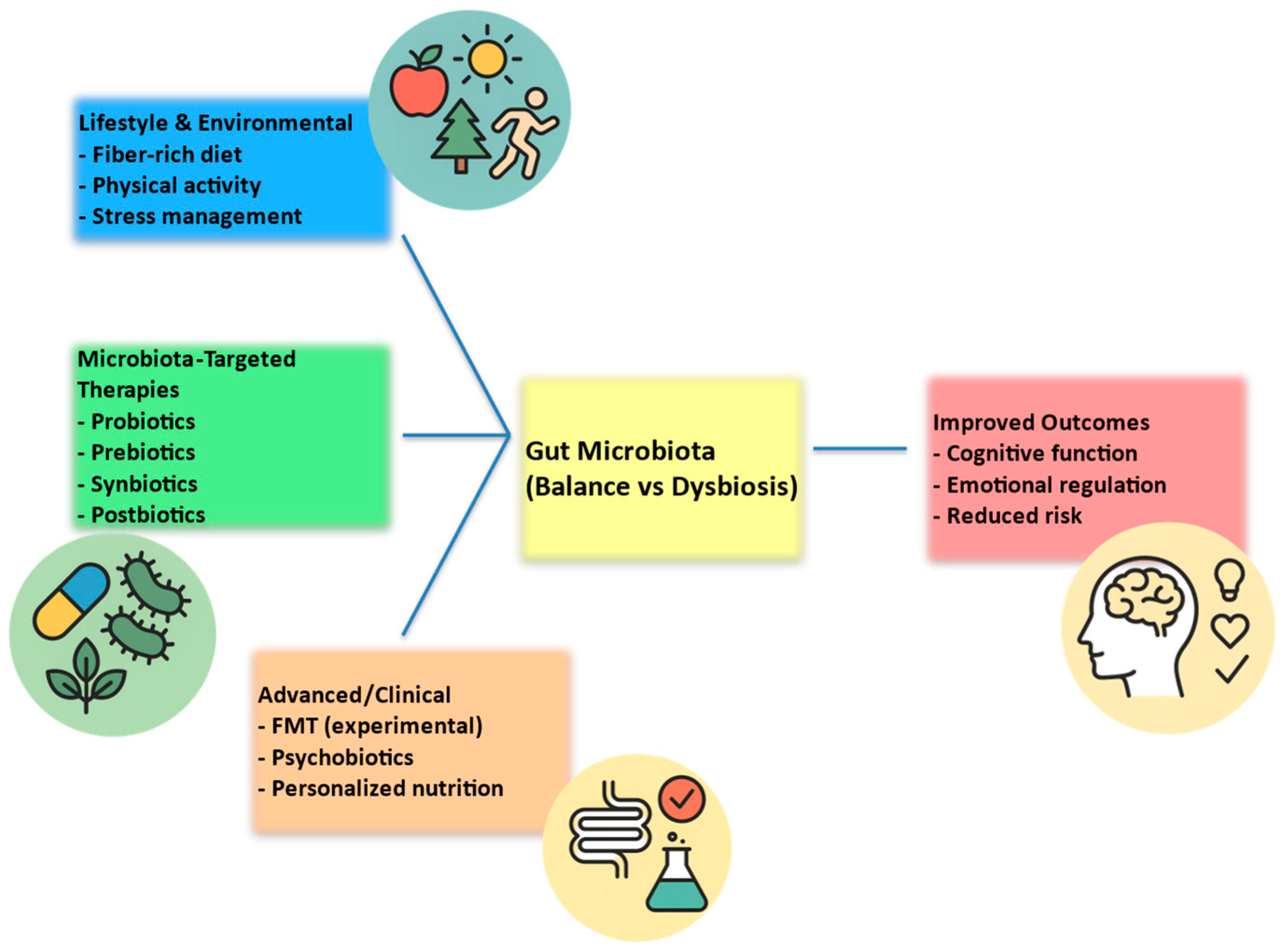
6. Possible Interventions and Therapeutic Implications
6.1. Probiotics/Psychobiotics and Mental Health
6.2. Diet and Nutritional Interventions
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCFAs | short-chain fatty acids |
| HPA | hypothalamic–pituitary–adrenal |
| ANS | autonomic nervous system |
| ENS | enteric nervous system |
| BBB | blood–brain barrier |
| ASD | autism spectrum disorder |
| ADHD | attention-deficit/hyperactivity disorder |
| MDD | major depressive disorder |
| BD | bipolar disorder |
| FMT | fecal microbiota transplantation |
| MAMPs | microbe-associated molecular patterns |
References
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’RIordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Dy, A.B.C.; Hagerman, R.J. The gut microbiota and autism spectrum disorders. Front. Cell. Neurosci. 2022, 16, 817438. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Laue, H.E.; Coker, M.O.; Madan, J.C. The Developing Microbiome From Birth to 3 Years: The Gut-Brain Axis and Neurodevelopmental Outcomes. Front. Pediatr. 2022, 10, 815885. [Google Scholar] [CrossRef]
- Tirone, C.; Pezza, L.; Paladini, A.; Tana, M.; Aurilia, C.; Lio, A.; D’IPpolito, S.; Tersigni, C.; Posteraro, B.; Sanguinetti, M.; et al. Gut and lung microbiota in preterm infants: Immunological modulation and implication in neonatal outcomes. Front. Immunol. 2019, 10, 2910. [Google Scholar] [CrossRef] [PubMed]
- Reyman, M.; Van Houten, M.A.; Van Baarle, D.; Bosch, A.A.T.M.; Man, W.H.; Chu, M.L.J.N.; Arp, K.; Watson, R.L.; Sanders, E.A.M.; Fuentes, S.; et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun. 2019, 10, 4997. [Google Scholar] [CrossRef]
- Adlerberth, I.; Lindberg, E.; Åberg, N.; Hesselmar, B.; Saalman, R.; Strannegård, I.L.; Wold, A.E. Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: An effect of hygienic lifestyle? Pediatr. Res. 2006, 59, 96–101. [Google Scholar] [CrossRef]
- Stokholm, J.; Thorsen, J.; Chawes, B.L.; Schjørring, S.; Krogfelt, K.A.; Bønnelykke, K.; Bisgaard, H. Cesarean section changes neonatal gut colonization. J. Allergy Clin. Immunol. 2016, 138, 881–889.e2. [Google Scholar] [CrossRef]
- Low, J.; Soh, S.-E.; Lee, Y.; Kwek, K.; Holbrook, J.; Van der Beek, E.; Shek, L.; Goh, A.; Teoh, O.; Godfrey, K.; et al. Ratio of Klebsiella/Bifidobacterium in early life correlates with later development of paediatric allergy. Benef. Microbes 2017, 8, 681–695. [Google Scholar] [CrossRef]
- Kumar, H.; du Toit, E.; Kulkarni, A.; Aakko, J.; Linderborg, K.M.; Zhang, Y.; Nicol, M.P.; Isolauri, E.; Yang, B.; Collado, M.C.; et al. Distinct patterns in human milk microbiota and fatty acid profiles across specific geographic locations. Front. Microbiol. 2016, 7, 1619. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017, 171, 647. [Google Scholar] [CrossRef]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical mother neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 2014, 16, 2891–2904. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Ksiazyk, J.; Lagström, H.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.-M.; Picaud, J.-C.; Possner, M.; et al. Human milk oligosaccharides: 2′-fucosyllactose (2′ FL) and lacto-N-neotetraose (LNnT) in infant formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-specificity and disease specificity of probiotic efficacy: A systematic review and metaanalysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Bazanella, M.; Maier, T.V.; Clavel, T.; Lagkouvardos, I.; Lucio, M.; Maldonado-Gòmez, M.X.; Autran, C.; Walter, J.; Bode, L.; Schmitt-Kopplin, P.; et al. Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am. J. Clin. Nutr. 2017, 106, 1274–1286. [Google Scholar] [CrossRef]
- Guarner, F. Studies with inulin-type fructans on intestinal infections, permeability, and inflammation. J. Nutr. 2007, 137, 2568S–2571S. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F.; Andersen, L.B.B.; Michaelsen, K.F.; Mølgaard, C.; Trolle, E.; Bahl, M.I.; Licht, T.R. Infant gut microbiota development is driven by transition to family foods independent of maternal obesity. mSphere 2016, 1, e00069-15. [Google Scholar] [CrossRef]
- Jeong, S. Factors influencing development of the infant microbiota: From prenatal period to early infancy. Clin. Exp. Pediatr. 2022, 65, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Duong, Q.A.; Pittet, L.F.; Curtis, N.; Zimmermann, P. Antibiotic exposure and adverse long-term health outcomes in children: A systematic review and meta analysis. J. Infect. 2022, 85, 213–300. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The effect of antibiotics on the composition of the intestinal microbiota-a systematic review. J. Infect. 2019, 79, 471–489. [Google Scholar] [CrossRef]
- Wurm, J.; Curtis, N.; Zimmermann, P. The effect of antibiotics on the intestinal microbiota in children—A systematic review. Front. Allergy. 2024, 5, 1458688. [Google Scholar] [CrossRef]
- Szajewska, H.; Berni Canani, R.; Domellöf, M.; Guarino, A.; Hojsak, I.; Indrio, F.; Lo Vecchio, A.; Mihatsch, W.A.; Mosca, A.; Orel, R.; et al. Probiotics for the management of pediatric gastrointestinal disorders: Position paper of the ESPGHAN special interest group on gut Microbiota and modifications. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Westaway, J.A.F.; Huerlimann, R.; Kandasamy, Y.; Miller, C.M.; Norton, R.; Watson, D.; Infante-Vilamil, S.; Rudd, D. To probiotic or not to probiotic: A metagenomic comparison of the discharge gut microbiome of infants supplemented with probiotics in NICU and those who are not. Front. Pediatr. 2022, 10, 838559. [Google Scholar] [CrossRef]
- DiazHeijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Tau, G.Z.; Peterson, B.S. Normal Development of Brain Circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef]
- Silbereis, J.C.; Pochareddy, S.; Zhu, Y.; Li, M.; Sestan, N. The cellular and molecularlandscapes of the developing human central nervous system. Neuron 2016, 89, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.I. Sensitive periods in the development of the brain and behavior. J. Cogn. Neurosci. 2004, 16, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B. Nested sensitive periods: How plasticity across the microbiota-gut-brainaxis interacts to affect the development of learning and memory. Curr. Opin. Behav. Sci. 2020, 36, 55–62. [Google Scholar] [CrossRef]
- Cowan, C.S.M.; Dinan, T.G.; Cryan, J.F. Annual research review: Critical windows—Themicrobiota-gut-brain axis in neurocognitive development. J. Child Psychol. Psychiatry 2020, 61, 353–371. [Google Scholar] [CrossRef]
- Zhou, R.; Qian, S.; Cho, W.C.S.; Zhou, J.; Jin, C.; Zhong, Y.; Wang, J.; Zhang, X.; Xu, Z.; Tian, M.; et al. Microbiota-microglia connections in age-related cognition decline. Aging Cell 2022, 21, e13599. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Zinselmeyer, B.H.; Corps, K.N.; McGavern, D.B. In vivo dynamics of innate immune sentinels in the CNS. Intravital 2012, 1, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the Human Infant Intestinal Microbiota. PLoS Biol. 2007, 5, 1556–1573. [Google Scholar] [CrossRef]
- Turroni, F.; Milani, C.; Duranti, S.; Lugli, G.A.; Bernasconi, S.; Margolles, A.; Di Pierro, F.; van Sinderen, D.; Ventura, M. The Infant Gut Microbiome as a Microbial Organ Influencing Host Well-Being. Ital. J. Pediatr. 2020, 46, 16. [Google Scholar] [CrossRef]
- Sakamoto, M.; Ohkuma, M. Bacteroides sartorii is an earlier heterotypic synonym of Bacteroides chinchillae and has priority. Int. J. Syst. Evol. Microbiol. 2012, 62 Pt 6, 1241–1244. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luck, B.; Visuthranukul, C.; Ihekweazu, F.D.; Engevik, A.C.; Shi, Z.; Danhof, H.A.; Chang-Graham, A.L.; Hall, A.; Endres, B.T.; et al. Human-Derived Bifidobacterium dentium Modulates the Mammalian Serotonergic System and Gut–Brain Axis. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 221–248. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Kadosh, K.C.; Basso, M.; Knytl, P.; Johnstone, N.; Lau, J.Y.F.; Gibson, G.R. Psychobiotic interventions for anxiety in young people: Asystematic review and meta-analysis, with youth consultation. Transl. Psychiatry 2021, 11, 352. [Google Scholar] [CrossRef]
- Erny, D.; de Angelis, A.L.H.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Matt, S.M.; Allen, J.M.; Lawson, M.A.; Mailing, L.J.; Woods, J.A.; Johnson, R.W. Butyrate and Dietary Soluble Fiber Improve Neuroinflammation Associated with Aging in Mice. Front. Immunol. 2018, 9, 1832. [Google Scholar] [CrossRef] [PubMed]
- DeCastro, M.; Nankova, B.B.; Shah, P.; Patel, P.; Mally, P.V.; Mishra, R.; La Gamma, E.F. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Mol. Brain Res. 2005, 142, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhong, X.; He, Y.; Shi, Y. Butyrate, but not propionate, reverses maternal diet-induced neurocognitive deficits in offspring. Pharmacol. Res. 2020, 160, 105082. [Google Scholar] [CrossRef] [PubMed]
- Hoyles, L.; Snelling, T.; Umlai, U.K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome-host systems interactions: Protective effects of propionate upon the blood-brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef]
- Navarro, F.; Liu, Y.; Rhoads, J.M. Can probiotics benefit children with autism spectrum disorders? World J. Gastroenterol. 2016, 22, 10093–10102. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Bibbo, S.; Gasbarrini, A. Gut microbiota modulation: Probiotics, antibiotics or fecal microbiota transplantation? Intern. Emerg. Med. 2014, 9, 365–373. [Google Scholar] [CrossRef]
- Gorrindo, P.; Williams, K.C.; Lee, E.B.; Walker, L.S.; McGrew, S.G.; Levitt, P. Gastrointestinal dysfunction in autism: Parental report, clinical evaluation, and associated factors. Autism Res. 2012, 5, 101–108. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Arneson, C.L.; Levy, S.E.; Kirby, R.S.; Nicholas, J.S.; Durkin, M.S. Brief report: Association between behavioral features and gastrointestinal problems among children with autism spectrum disorder. J. Autism Dev. Disord. 2012, 42, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Srikantha, P.; Mohajeri, M.H. The possible role of the microbiota-gutbrain-axis in autism spectrum disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef]
- Ding, H.T.; Taur, Y.; Walkup, J.T. Gut microbiota and autism: Key concepts and findings. J. Autism Dev. Disord. 2017, 47, 480–489. [Google Scholar] [CrossRef]
- Nikolov, R.N.; Bearss, K.E.; Lettinga, J.; Erickson, C.; Rodowski, M.; Aman, M.G.; McCracken, J.T.; McDougle, C.J.; Tierney, E.; Vitiello, B.; et al. Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. J. Autism Dev. Disord. 2009, 39, 405–413. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Luna, R.A.; Oezguen, N.; Balderas, M.; Venkatachalam, A.; Runge, J.K.; Versalovic, J.; Veenstra-VanderWeele, J.; Anderson, G.M.; Savidge, T.; Williams, K.C. Distinct microbiome-neuroimmune signatures correlate with functional abdominal pain in children with autism spectrum disorder. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 218–230. [Google Scholar] [CrossRef]
- Iovene, M.R.; Bombace, F.; Maresca, R.; Sapone, A.; Iardino, P.; Picardi, A.; Marotta, R.; Schiraldi, C.; Siniscalco, D.; Serra, N.; et al. Intestinal dysbiosis and yeast isolation in stool of subjects with autism spectrum disorders. Mycopathologia 2017, 182, 349–363. [Google Scholar] [CrossRef]
- Kushak, R.I.; Winter, H.S.; Buie, T.M.; Cox, S.B.; Phillips, C.D.; Ward, N.L. Analysis of the duodenal microbiome in autistic individuals: Association with carbohydrate digestion. J. Pediatr. Gastroenterol. Nutr. 2017, 64, e110–e116. [Google Scholar] [CrossRef]
- Kong, X.; Liu, J.; Cetinbas, M.; Sadreyev, R.; Koh, M.; Huang, H.; Adeseye, A.; He, P.; Zhu, J.; Russell, H.; et al. New and preliminary evidence on altered oral and gut microbiota in individuals with autism spectrum disorder (ASD): Implications for ASD diagnosis and subtyping based on microbial biomarkers. Nutrients 2019, 11, 2128. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.L.; Hornig, M.; Buie, T.; Bauman, M.L.; Paik, M.C.; Wick, I.; Bennett, A.; Jabado, O.; Hirschberg, D.L.; Lipkin, W.I. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS ONE 2011, 6, e24585. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Pivina, L.; Dadar, M.; Meguid, N.A.; Semenova, Y.; Anwar, M.; Chirumbolo, S. Gastrointestinal Alterations in Autism Spectrum Disorder: What Do We Know? Neurosci. Biobehav. Rev. 2020, 118, 111–120. [Google Scholar] [CrossRef]
- Kang, D.-W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef]
- Parracho, H.M.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005, 54, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Pärtty, A.; Kalliomäki, M.; Wacklin, P.; Salminen, S.; Isolauri, E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatr. Res. 2015, 77, 823–828. [Google Scholar] [CrossRef]
- Cassidy-Bushrow, A.E.; Sitarik, A.R.; Johnson, C.C.; Johnson-Hooper, T.M.; Kassem, Z.; Levin, A.M.; Lynch, S.V.; Ownby, D.R.; Phillips, J.M.; Yong, G.J.M.; et al. Early-life gut microbiota and attention deficit hyperactivity disorder in preadolescents. Pediatr. Res. 2023, 93, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Aarts, E.; Ederveen, T.H.A.; Naaijen, J.; Zwiers, M.P.; Boekhorst, J.; Timmerman, H.M.; Smeekens, S.P.; Netea, M.G.; Buitelaar, J.K.; Franke, B.; et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS ONE 2017, 12, e0183509. [Google Scholar] [CrossRef]
- Prehn-Kristensen, A.; Zimmermann, A.; Tittmann, L.; Lieb, W.; Schreiber, S.; Baving, L.; Fischer, A. Reduced microbiome alpha diversity in young patients with ADHD. PLoS ONE 2018, 13, e0200728. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-Y.; Zhou, Y.-Y.; Zhou, G.-L.; Li, Y.-C.; Yuan, J.; Li, X.-H.; Ruan, B. Gut microbiota profiles in treatment-naïve children with attention deficit hyperactivity disorder. Behav. Brain Res. 2018, 347, 408–413. [Google Scholar] [CrossRef]
- Stevens, A.J.; Purcell, R.V.; Darling, K.A.; Eggleston, M.J.F.; Kennedy, M.A.; Rucklidge, J.J. Human gut microbiome changes during a 10 week Randomised Control Trial for micronutrient supplementation in children with attention deficit hyperactivity disorder. Sci. Rep. 2019, 9, 10128. [Google Scholar] [CrossRef]
- Wang, L.-J.; Yang, C.-Y.; Chou, W.-J.; Lee, M.-J.; Chou, M.-C.; Kuo, H.-C.; Yeh, Y.-M.; Lee, S.-Y.; Huang, L.-H.; Li, S.-C. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2020, 29, 287–297. [Google Scholar] [CrossRef]
- Szopinska-Tokov, J.; Dam, S.; Naaijen, J.; Konstanti, P.; Rommelse, N.; Belzer, C.; Buitelaar, J.; Franke, B.; Bloemendaal, M.; Aarts, E.; et al. Correction: Szopinska-Tokov et al. Investigating the Gut Microbiota Composition of Individuals with Attention-Deficit/Hyperactivity Disorder and Association with Symptoms. Microorganisms. 2020, 8, 406. [Google Scholar] [CrossRef]
- Wang, L.-J.; Li, S.-C.; Li, S.-W.; Kuo, H.-C.; Lee, S.-Y.; Huang, L.-H.; Chin, C.-Y.; Yang, C.-Y. Gut microbiota and plasma cytokine levels in patients with attention-deficit/hyperactivity disorder. Trans. Psychiatry 2022, 12, 76. [Google Scholar] [CrossRef]
- Lee, M.J.; Lai, H.C.; Kuo, Y.L.; Chen, V.C. Association between gut microbiota and emotional-behavioral symptoms in children with attention-deficit/hyperactivity disorder. J. Pers. Med. 2022, 12, 1634. [Google Scholar] [CrossRef]
- Bundgaard-Nielsen, C.; Lauritsen, M.B.; Knudsen, J.K.; Rold, L.S.; Larsen, M.H.; Hindersson, P.; Villadsen, A.B.; Leutscher, P.D.C.; Hagstrøm, S.; Nyegaard, M.; et al. Children and adolescents with attention deficit hyperactivity disorder and autism spectrum disorder share distinct microbiota compositions. Gut Microbes 2023, 15, 2211923. [Google Scholar] [CrossRef]
- Wan, L.; Ge, W.R.; Zhang, S.; Sun, Y.L.; Wang, B.; Yang, G. Case-control study of the effects of gut microbiota composition on neurotransmitter metabolic pathways in children with attention deficit hyperactivity disorder. Front. Neurosci. 2020, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.; Huang, Y.; Yin, A.; Zhang, L.; Han, J.; Lyu, Y.; Xu, X.; Zhai, Y.; Sun, H.; et al. Gut metagenomic characteristics of ADHD reveal low Bacteroides ovatus-associated host cognitive impairment. Gut Microbes 2022, 14, 2125747. [Google Scholar] [CrossRef] [PubMed]
- Stiernborg, M.; Debelius, J.; Yang, L.L.; Skott, E.; Millischer, V.; Giacobini, M.; Melas, P.A.; Boulund, F.; Lavebratt, C. Bacterial gut microbiome differences in adults with ADHD and in children with ADHD on psychostimulant medication. Brain Behav. Immun. 2023, 110, 310–321. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Yeh, Y.; Lee, S.; Kuo, H.; Yang, C. Gut mycobiome dysbiosis and its impact on intestinal permeability in attention-deficit/hyperactivity disorder. J. Child Psychol Psychiatry Allied Discip. 2023, 64, 1280–1291. [Google Scholar] [CrossRef]
- Wang, N.; Wang, H.; Bai, Y.; Zhao, Y.; Zheng, X.; Gao, X.; Zhang, Z.; Yang, L. Metagenomic analysis reveals difference of gut microbiota in ADHD. J. Atten. Disord. 2024, 28, 872–879. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Tillisch, K. The effects of gut microbiota on CNS function in humans. Gut Microbes 2014, 5, 404–410. [Google Scholar] [CrossRef]
- Douglas-Escobar, M.; Elliott, E.; Neu, J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013, 167, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmakh, M.; Anuar, F.; Zadjali, F.; Rafter, J.; Pettersson, S. Gut microbial communities modulating brain development and function. Gut Microbes 2012, 3, 366–373. [Google Scholar] [CrossRef]
- Belzer, C.; De Vos, W.M. Microbes inside–from diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Hida, H. The importance of vagus nerve afferent in the formation of emotions in attention-deficit hyperactivity disorder model rat. Brain Nerve = Shinkei kenkyu No Shinpo 2016, 68, 633–639. [Google Scholar] [CrossRef]
- Lyte, M.; Li, W.; Opitz, N.; Gaykema, R.P.; Goehler, L.E. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 2006, 89, 350–357. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Wang, X.; Tang, R.; Wei, Z.; Zhan, Y.; Lu, J.; Li, Z. The enteric nervous system deficits in autism spectrum disorder. Front. Neurosci. 2023, 17, 1101071. [Google Scholar] [CrossRef] [PubMed]
- Montanari, M.; Imbriani, P.; Bonsi, P.; Martella, G.; Peppe, A. Beyond the microbiota: Understanding the role of the enteric nervous system in Parkinson’s disease from mice to human. Biomedicines 2023, 11, 1560. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocr. 2019, 31, e12684. [Google Scholar] [CrossRef]
- Hyland, N.P.; Cryan, J.F. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev. Biol. 2016, 417, 182–187. [Google Scholar] [CrossRef]
- Caputi, V.; Marsilio, I.; Filpa, V.; Cerantola, S.; Orso, G.; Bistoletti, M. Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. Br. J. Pharmacol. 2017, 174, 3623–3639. [Google Scholar] [CrossRef] [PubMed]
- Mcvey Neufeld, K.A.; Mao, Y.K.; Bienenstock, J.; Foster, J.A.; Kunze, W.A. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol. Motil. 2013, 25, 183-e88. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.A.; Keenan, C.M.; Wallace, L.E.; Woods, C.; Cavin, J.-B.; Flockton, A.R.; Macklin, W.B.; Belkind-Gerson, J.; Hirota, S.A.; Sharkey, K.A. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome 2021, 9, 210. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Del Campo, N.; Chamberlain, S.R.; Sahakian, B.J.; Robbins, T.W. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attentiondeficit/hyperactivity disorder. Biol. Psychiatry 2011, 69, e145–e157. [Google Scholar] [CrossRef]
- Hayes, D.J.; Jupp, B.; Sawiak, S.J.; Merlo, E.; Caprioli, D.; Dalley, J.W. Brain gaminobutyric acid: A neglected role in impulsivity. Eur. J. Neurosci. 2014, 39, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, E.; Nandagopal, K. Does serotonin deficit mediate susceptibility to ADHD? Neurochem. Int. 2015, 82, 52–68. [Google Scholar] [CrossRef]
- Plichta, M.M.; Scheres, A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: A meta-analytic review of the fMRI literature. Neurosci. Biobehav. Rev. 2014, 38, 125–134. [Google Scholar] [CrossRef]
- Makris, N.; Biederman, J.; Monuteaux, M.C.; Seidman, L.J. Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Dev. Neurosci. 2009, 31, 36–49. [Google Scholar] [CrossRef]
- da Silva, B.S.; Grevet, E.H.; Silva, L.C.F.; Ramos, J.K.N.; Rovaris, D.L.; Bau, C.H.D. An overview on neurobiology and therapeutics of attention-deficit/hyperactivity disorder. Discov. Ment. Health 2023, 3, 2. [Google Scholar] [CrossRef]
- Oades, R.D.; Lasky-Su, J.; Christiansen, H.; Faraone, S.V.; Sonuga-Barke, E.J.; Banaschewski, T.; Chen, W.; Anney, R.J.; Buitelaar, J.K.; Ebstein, R.P.; et al. The influence of serotoninand other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/ hyperactivity disorder (ADHD): Findings from a family-based association test (FBAT) analysis. Behav. Brain Funct. 2008, 4, 48. [Google Scholar] [CrossRef]
- Worbe, Y.; Savulich, G.; Voon, V.; Fernandez-Egea, E.; Robbins, T.W. Serotonin depletion induces ‘waiting impulsivity’ on the human four-choice serial reaction time task: Cross-species translational significance. Neuropsychopharmacology 2014, 39, 1519–1526. [Google Scholar] [CrossRef]
- Edden, R.A.E.; Crocetti, D.; Zhu, H.; Gilbert, D.L.; Mostofsky, S.H. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 2012, 69, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Stiernborg, M.; Skott, E.; Gillberg, T.; Landberg, R.; Giacobini, M.; Lavebratt, C. Lower plasma concentrations of short-chain fatty acids (SCFAs) in patients with ADHD. J. Psychiatr. Res. 2022, 156, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.J.; Shin, D.W.; Lee, E.H.; Oh, Y.H.; Noh, K.S. Hypothalamic-pituitary-adrenal reactivity in boys with attention deficit hyperactivity disorder. Yonsei Med J. 2003, 44, 608–614. [Google Scholar] [CrossRef]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef]
- Cichoń, L.; Janas-Kozik, M.; Siwiec, A.; Rybakowski, J.K. Clinical picture and treatment of bipolar affective disorder in children and adolescents. Psychiatr. Pol. 2020, 54, 35–50. [Google Scholar] [CrossRef]
- Post, R.M.; Grunze, H. The challenges of children with bipolar disorder. Medicina 2021, 57, 601. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.H.; Vecera, C.M.; Pinjari, O.F.; Machado-Vieira, R. Inflammatory signaling mechanisms in bipolar disorder. J. Biomed. Sci. 2021, 28, 45. [Google Scholar] [CrossRef]
- Huang, T.T.; Lai, J.B.; Du, Y.L.; Xu, Y.; Ruan, L.M.; Hu, S.H. Current Understanding of Gut Microbiota in Mood Disorders: An Update of Human Studies. Front. Genet. 2019, 10, 98. [Google Scholar] [CrossRef]
- Evans, S.J.; Bassis, C.M.; Hein, R.; Assari, S.; Flowers, S.A.; Kelly, M.B.; Young, V.B.; Ellingrod, V.E.; McInnis, M.G. The gut microbiome composition associates with bipolar disorder and illness severity. J. Psychiatr. Res. 2017, 87, 23–29. [Google Scholar] [CrossRef]
- Painold, A.; Mörkl, S.; Kashofer, K.; Halwachs, B.; Dalkner, N.; Bengesser, S.; Birner, A.; Fellendorf, F.; Platzer, M.; Queissner, R.; et al. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019, 21, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.; Adamos, M.; Katsafanas, E.; Khushalani, S.; Origoni, A.; Savage, C.; Schweinfurth, L.; Stallings, C.; Sweeney, K.; Goga, J.; et al. Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: A randomized controlled trial. Bipolar Disord. 2018, 20, 614–621. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Wetzlmair, L.C.; Fellendorf, F.T.; Platzer, M.; Queissner, R.; Birner, A.; Pilz, R.; Hamm, C.; Maget, A.; Rieger, A.; et al. Probiotic treatment in individuals with euthymic bipolar disorder: A pilot-study on clinical changes and compliance. Neuropsychobiology 2020, 79, 71–79. [Google Scholar] [CrossRef]
- Bharwani, A.; Szamosi, J.C.; Taylor, V.H.; Lee, Y.; Bala, A.; Mansur, R.; Subramaniapillai, M.; Surette, M.; McIntyre, R.S. Changes in the gut microbiome associated with infliximab in patients with bipolar disorder. Brain Behav. 2021, 11, e2259. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, G.V.; Salum, G.A.; Sugaya, L.S.; Caye, A.; Rohde, L.A. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry 2015, 56, 345–365. [Google Scholar] [CrossRef]
- Salk, R.H.; Hyde, J.S.; Abramson, L.Y. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol. Bull. 2017, 143, 783–822. [Google Scholar] [CrossRef] [PubMed]
- Lagges, A.M.; Dunn, D.W. Depression in children and adolescents. Neurol. Clin. 2003, 21, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered composition of gut microbiota in depression: A systematic review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z.; et al. Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Khalid, S.; Williams, C.M.; Reynolds, S.A. Is there an association between diet and depression in children and adolescents? A systematic review. Br. J. Nutr. 2016, 116, 2097–2108. [Google Scholar] [CrossRef]
- Marano, G.; Rossi, S.; Sfratta, G.; Traversi, G.; Lisci, F.M.; Anesini, M.B.; Pola, R.; Gasbarrini, A.; Gaetani, E.; Mazza, M. Gut Microbiota: A New Challenge in Mood Disorder Research. Life 2025, 15, 593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marano, G.; Rossi, S.; Sfratta, G.; Acanfora, M.; Anesini, M.B.; Traversi, G.; Lisci, F.M.; Rinaldi, L.; Pola, R.; Gasbarrini, A.; et al. Gut Microbiota in Women with Eating Disorders: A New Frontier in Pathophysiology and Treatment. Nutrients. 2025, 17, 2316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neale, J.; Hudson, L.D. Anorexia nervosa in adolescents. Br. J. Hosp. Med. 2020, 81, 1–8. [Google Scholar] [CrossRef]
- World Health Organization. ICD-11: International Classi—Cation of Diseases (11th Revision). 2018. Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 30 September 2025).
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry 2015, 2, 1099–1111. Available online: https://www.thelancet.com/journals/lanpsy/article/PIIS2215-0366(15)00356-9/abstract (accessed on 30 September 2025). [CrossRef] [PubMed]
- Kleiman, S.C.; Watson, H.J.; Bulik-Sullivan, E.C.; Huh, E.Y.; Taranti-no, L.M.; Bulik, C.M.; Carroll, I.M. The intestinal microbiota in acute anorexia nervosa and during renourishment: Relationship to depression, anxiety, and eating disorder psychopathology. Psychosom. Med. 2015, 77, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Hanachi, M.; Manichanh, C.; Schoenenberger, A.; Pascal, V.; Leve-nez, F.; Cournède, N.; Doré, J.; Melchior, J.C. Altered host-gut microbes symbiosis in severely malnourished anorexia nervosa (AN) patients undergoing enteral nutrition: An explicative factor of functional intestinal disorders? Clin. Nutr. 2019, 38, 2304–2310. [Google Scholar] [CrossRef]
- Borgo, F.; Riva, A.; Benetti, A.; Casiraghi, M.C.; Bertelli, S.; Garbossa, S.; Anselmetti, S.; Scarone, S.; Pontiroli, A.E.; Morace, G.; et al. Microbiota in anorexia nervosa: The triangle between bacterial species, metabolites and psychological tests. PLoS ONE 2017, 12, e0179739. [Google Scholar] [CrossRef]
- Prochazkova, P.; Roubalova, R.; Dvorak, J.; Kreisinger, J.; Hill, M.; Tlaskalova-Hogenova, H.; Tomasova, P.; Pelantova, H.; Cermako-va, M.; Kuzma, M.; et al. The intestinal microbiota and metabolites in patients with anorexia nervosa. Gut Microbes 2021, 13, 1902771. [Google Scholar] [CrossRef]
- Monteleone, A.M.; Troisi, J.; Serena, G.; Fasano, A.; Dalle Grave, R.; Cascino, G.; Marciello, F.; Calugi, S.; Scala, G.; Corrivetti, G.; et al. The gut microbiome and metabolomics pro les of restricting and binge-purging type anorexia nervosa. Nutrients 2021, 13, 507. [Google Scholar] [CrossRef]
- Schulz, N.; Belheouane, M.; Dahmen, B.; Ruan, V.A.; Specht, H.E.; Dempe, A.; Herpertz-Dahlmann, B.; Baines, J.F.; Seitz, J. Gut microbiota alteration in adolescent anorexia nervosa does not normalize with short-term weight restoration. Int. J. Eat. Disord. 2021, 54, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Gröbner, E.M.; Zeiler, M.; Fischmeister, F.P.S.; Kollndorfer, K.; Schmelz, S.; Schneider, A.; Haid-Stecher, N.; Sevecke, K.; Wagner, G.; Keller, L.; et al. The effects of probiotics administration on the gut microbiome in adolescents with anorexia nervosa: A study protocol for a longitudinal, double-blind, randomized, placebo-controlled trial. Eur. Eat. Disord. Rev. 2022, 30, 61–74. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The microbiota-gutbrain axis: From motility to mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Bai, J.; Wu, D.; Yu, S.-F.; Qiang, X.-L.; Bai, H.; Wang, H.-N.; Peng, Z.-W. Association between fecal microbiota and generalized anxiety disorder: Severity and early treatment response. J. Affect. Disord. 2019, 259, 56–66. [Google Scholar] [CrossRef]
- Kim, C.-S.; Shin, G.-E.; Cheong, Y.; Shin, J.; Shin, D.-M.; Chun, W.Y. Experiencing social exclusion changes gut microbiota composition. Transl. Psychiatry 2022, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Abbaspour, A.; Mkoma, G.F.; Bulik, C.M.; Rück, C.; Djurfeldt, D. Gut microbiota in psychiatric disorders: A systematic review. Psychosom 2021, 83, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Leylabadlo, H.E.; Ghotaslou, R.; Feizabadi, M.M.; Farajnia, S.; Moaddab, S.Y.; Ganbarov, K.; Khodadadi, E.; Tanomand, A.; Sheykhsaran, E.; Yousefi, B.; et al. The critical role of Faecalibacterium prausnitzii in human health: An overview. Microb. Pathog. 2020, 149, 104344. [Google Scholar] [CrossRef] [PubMed]
- O’RIordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Marano, G.; Mazza, M.; Lisci, F.M.; Ciliberto, M.; Traversi, G.; Kotzalidis, G.D.; De Berardis, D.; Laterza, L.; Sani, G.; Gasbarrini, A.; et al. The Microbiota-Gut-Brain Axis: Psychoneuroimmunological Insights. Nutrients 2023, 15, 1496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marano, G.; Traversi, G.; Gaetani, E.; Gasbarrini, A.; Mazza, M. Gut microbiota in women: The secret of psychological and physical well-being. World J. Gastroenterol. 2023, 29, 5945–5952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marano, G.; Anesini, M.B.; Milintenda, M.; Acanfora, M.; d’Abate, C.; Lisci, F.M.; Pirona, I.; Traversi, G.; Pola, R.; Gaetani, E.; et al. Discovering a new paradigm: Gut microbiota as a central modulator of sexual health. World J. Gastrointest. Pathophysiol. 2025, 16, 107823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Kodikara, S.; Ellul, S.; Lê Cao, K.-A. Statistical challenges in longitudinal microbiome data analysis. Brief. Bioinform. 2022, 23, bbac273. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Misra, S.; Mohanty, D. Psychobiotics: A new approach for treating mental illness? Crit. Rev. Food Sci. Nutr. 2019, 59, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H.; et al. PROVIT: Supplementary Probiotic Treatment and Vitamin B7 in Depression-A Randomized Controlled Trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef] [PubMed]
- Gruenwald, J.; Graubaum, H.J.; Harde, A. Effect of a probiotic multivitamin compound on stress and exhaustion. Adv. Ther. 2002, 19, 141–150. [Google Scholar] [CrossRef]
- Marcos, A.; Wärnberg, J.; Nova, E.; Gómez, S.; Alvarez, A.; Alvarez, R.; Mateos, J.A.; Cobo, J.M. The effect of milk fermented by yogurt cultures plus Lactobacillus casei DN-114001 on the immune response of subjects under academic examination stress. Eur. J. Nutr. 2004, 43, 381–389. [Google Scholar] [CrossRef]
- Diop, L.; Guillou, S.; Durand, H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: A double-blind, placebo-controlled, randomized trial. Nutr. Res. 2008, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Long-Smith, C.M.; Carbia, C.; Bastiaanssen, T.F.S.; van de Wouw, M.; Wiley, N.; Strain, C.R.; Fouhy, F.; Stanton, C.; Cryan, J.F.; et al. A specific dietary fibre supplementation improves cognitive performance-an exploratory randomised, placebo-controlled, crossover study. Psychopharmacology 2021, 238, 149–163. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, X.; Tang, S.; Huang, H.; Zhao, X.; Ning, Z.; Fu, X.; Zhang, C. Probiotics reduce psychological stress in patients before laryngeal cancer surgery. Asia-Pac. J. Clin. Oncol. 2016, 12, e92–e96. [Google Scholar] [CrossRef]
- Hou, R.; Garner, M.; Holmes, C.; Osmond, C.; Teeling, J.; Lau, L.; Baldwin, D.S. Peripheral inflammatory cytokines and immune balance in Generalised Anxiety Disorder: Case-controlled study. Brain Behav. Immun. 2017, 62, 212–218. [Google Scholar] [CrossRef]
- Gualtieri, P.; Marchetti, M.; Cioccoloni, G.; De Lorenzo, A.; Romano, L.; Cammarano, A.; Colica, C.; Condò, R.; Di Renzo, L. Psychobiotics Regulate the Anxiety Symptoms in Carriers of Allele A of IL-1β Gene: A Randomized, Placebo-Controlled Clinical Trial. Mediat. Inflamm. 2020, 2020, 2346126. [Google Scholar] [CrossRef]
- Logan, A.C.; Katzman, M. Major depressive disorder: Probiotics may be an adjuvant therapy. Med. Hypotheses 2005, 64, 533–538. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. New Zealand J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Nazari, S.; Etesam, F.; Nourimajd, S.; Ahmadpanah, M.; Jahromi, S.R. The Effect of Synbiotic as an Adjuvant Therapy to Fluoxetine in Moderate Depression: A Randomized Multicenter Trial. Arch. Neurosci. 2018, 5, e60507. [Google Scholar] [CrossRef]
- Publisher, F.S. Effect of coordinated probiotic/prebiotic/phytobiotic supplementation on microbiome balance and psychological mood state in healthy stressed adults. Funct. Foods Heal. Dis. 2019, 9, 265–275. [Google Scholar] [CrossRef]
- Heidarzadeh-Rad, N.; Gökmen-Özel, H.; Kazemi, A.; Almasi, N.; Djafarian, K. Effects of a Psychobiotic Supplement on Serum Brain-derived Neurotrophic Factor Levels in Depressive Patients: A Post Hoc Analysis of a Randomized Clinical Trial. J. Neurogastroenterol. Motil. 2020, 26, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Doll, J.P.K.; Schweinfurth, N.; Kettelhack, C.; Schaub, A.C.; Yamanbaeva, G.; Varghese, N.; Mählmann, L.; Brand, S.; Eckert, A.; et al. Effect of short-term, high-dose probiotic supplementation on cognition, related brain functions and BDNF in patients with depression: A secondary analysis of a randomized controlled trial. J. Psychiatry Neurosci. JPN 2023, 48, E23–E33. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, L.S.; Kelly, S.; Isaev, D.; Aleman, A.; Aftanas, L.I.; Bauer, J.; Baune, B.T.; Brak, I.V.; Carballedo, A.; Connolly, C.G.; et al. White matter disturbances in major depressive disorder: A coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol. Psychiatry 2020, 25, 1511–1525. [Google Scholar] [CrossRef]
- Reiter, A.; Bengesser, S.A.; Hauschild, A.C.; Birkl-Töglhofer, A.M.; Fellendorf, F.T.; Platzer, M.; Färber, T.; Seidl, M.; Mendel, L.M.; Unterweger, R.; et al. Interleukin-6 Gene Expression Changes after a 4-Week Intake of a Multispecies Probiotic in Major Depressive Disorder-Preliminary Results of the PROVIT Study. Nutrients 2020, 12, 2575. [Google Scholar] [CrossRef] [PubMed]
- Yamanbaeva, G.; Schaub, A.C.; Schneider, E.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Mählmann, L.; Brand, S.; Beglinger, C.; Borgwardt, S.; et al. Effects of a probiotic add-on treatment on fronto-limbic brain structure, function, and perfusion in depression: Secondary neuroimaging findings of a randomized controlled trial. J. Affect. Disord. 2023, 324, 529–538. [Google Scholar] [CrossRef]
- Ma, T.; Jin, H.; Kwok, L.Y.; Sun, Z.; Liong, M.T.; Zhang, H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol. Stress 2021, 14, 100294. [Google Scholar] [CrossRef]
- Lew, L.C.; Hor, Y.Y.; Yusoff, N.A.A.; Choi, S.B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Venkataraman, R.; Madempudi, R.S.; Neelamraju, J.; Ahire, J.J.; Vinay, H.R.; Lal, A.; Thomas, G.; Stephen, S. Effect of Multi-strain Probiotic Formulation on Students Facing Examination Stress: A Double-Blind, Placebo-Controlled Study. Probiotics Antimicrob. Proteins 2021, 13, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, K.; Hu, J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef]
- Borre, Y.E.; Moloney, R.D.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The impact of microbiota on brain and behavior: Mechanisms & therapeutic potential. Adv. Exp. Med. Biol. 2014, 817, 373–403. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Jazayeri, S.; Khosravi-Darani, K.; Solati, Z.; Mohammadpour, N.; Asemi, Z.; Adab, Z.; Djalali, M.; Tehrani-Doost, M.; Hosseini, M.; et al. Effects of Probiotics on Biomarkers of Oxidative Stress and Inflammatory Factors in Petrochemical Workers: A Randomized, Double-Blind, Placebo-Controlled Trial. Int. J. Prev. Med. 2015, 6, 82. [Google Scholar] [CrossRef]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Borkent, J.; Ioannou, M.; Laman, J.D.; Haarman, B.C.M.; Sommer, I.E.C. Role of the Gut Microbiome in Three Major Psychiatric Disorders. Psychol. Med. 2022, 52, 1222–1242. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.S.D.; Vieira-Coelho, M.A. Probiotics and prebiotics: Focus on psychiatric disorders a systematic review. Nutr. Rev. 2020, 78, 437–450. [Google Scholar] [CrossRef]
- Dickerson, F.B.; Stallings, C.; Origoni, A.; Katsafanas, E.; Savage, C.L.; Schweinfurth, L.A.; Goga, J.; Khushalani, S.; Yolken, R.H. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: A randomized, placebo-controlled trial. Prim. Care Companion CNS Disord. 2014, 16, PCC.13m01579. [Google Scholar] [CrossRef]
- Tomasik, J.; Yolken, R.H.; Bahn, S.; Dickerson, F.B. Immunomodulatory Effects of Probiotic Supplementation in Schizophrenia Patients: A Randomized, Placebo-Controlled Trial. Biomark. Insights 2015, 10, 47–54. [Google Scholar] [CrossRef]
- Bui, T.A.; O’Croinin, B.R.; Dennett, L.; Winship, I.R.; Greenshaw, A.J. Pharmaco-Psychiatry and Gut Microbiome: A Systematic Review of the Effects of Psychotropic Drugs for Bipolar Disorder. Microbiology 2025, 171, 001568. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Majeed, S.; Ali, F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: A randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 2018, 62, 10–29219. [Google Scholar] [CrossRef]
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejía, J.L.; Hansen, L.H.; Leigh Gibson, E.; Nielsen, D.S.; Costabile, A. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome 2018, 6, 133. [Google Scholar] [CrossRef]
- Santocchi, E.; Guiducci, L.; Fulceri, F.; Billeci, L.; Buzzigoli, E.; Apicella, F.; Calderoni, S.; Grossi, E.; Morales, M.A.; Muratori, F. Gut to Brain Interaction in Autism Spectrum Disorders: A Randomized Controlled Trial on the Role of Probiotics on Clinical, Biochemical and Neurophysiological Parameters. BMC Psychiatry 2016, 16, 183. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Krajmalnik-Brown, R.; Porazinska, D.L.; Weiss, S.J.; Knight, R. Toward effective probiotics for autism and other neurodevelopmental disorders. Cell 2013, 155, 1446–1448. [Google Scholar] [CrossRef] [PubMed]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Lv, F.; Chen, S.; Wang, L.; Jiang, R.; Tian, H.; Li, J.; Yao, Y.; Zhuo, C. The role of microbiota in the pathogenesis of schizophrenia and major depressive disorder and the possibility of targeting microbiota as a treatment option. Oncotarget 2017, 8, 100899–100907. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Moles, L.; Otaegui, D. The Impact of Diet on Microbiota Evolution and Human Health. Is Diet an Adequate Tool for Microbiota Modulation? Nutrients 2020, 12, 1654. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Vangay, P.; Johnson, A.J.; Ward, T.L.; Al-Ghalith, G.A.; Shields-Cutler, R.R.; Hillmann, B.M.; Lucas, S.K.; Beura, L.K.; Thompson, E.A.; Till, L.M.; et al. US Immigration Westernizes the Human Gut Microbiome. Cell 2018, 175, 962–972.e10. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef]
- Han, Y.; Xiao, H. Whole Food-Based Approaches to Modulating Gut Microbiota and Associated Diseases. Annu. Rev. Food Sci. Technol. 2020, 11, 119–143. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S.; Walker, R.E.; Xiao, X.; Saha, P.; Golonka, R.M.; Cai, J.; Bretin, A.C.A.; Cheng, X.; Liu, Q.; et al. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut 2019, 68, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, J.; Ji, K.; Zhang, P. Bamboo shoot fiber prevents obesity in mice by modulating the gut microbiota. Sci. Rep. 2016, 6, 32953. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef]
- Windey, K.; De Preter, V.; Verbeke, K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 2012, 56, 184–196. [Google Scholar] [CrossRef]
- Wu, L.; Tang, Z.; Chen, H.; Ren, Z.; Ding, Q.; Liang, K.; Sun, Z. Mutual interaction between gut microbiota and protein/amino acid metabolism for host mucosal immunity and health. Anim. Nutr. 2021, 7, 11–16. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Butteiger, D.N.; Hibberd, A.A.; McGraw, N.J.; Napawan, N.; Hall-Porter, J.M.; Krul, E.S. Soy Protein Compared with Milk Protein in a Western Diet Increases Gut Microbial Diversity and Reduces Serum Lipids in Golden Syrian Hamsters. J. Nutr. 2016, 146, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, L.; Yu, L.; Sun, L.; Li, K.; Tong, C.; Xu, W.; Cui, G.; Long, M.; Li, P. Selenium-enriched yeast reduces caecal pathological injuries and intervenes changes of the diversity of caecal microbiota caused by Ochratoxin-A in broilers. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 137, 111139. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Waliullah, S.; Godfrey, V.; Khan, M.A.W.; Ramachandran, R.A.; Cantarel, B.L.; Behrendt, C.; Peng, L.; Hooper, L.V.; Zaki, H. Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Sci. Transl. Med. 2020, 12, eaay6218. [Google Scholar] [CrossRef]
- Jin, Q.; Black, A.; Kales, S.N.; Vattem, D.; Ruiz-Canela, M.; Sotos-Prieto, M. Metabolomics and Microbiomes as Potential Tools to Evaluate the Effects of the Mediterranean Diet. Nutrients 2019, 11, 207. [Google Scholar] [CrossRef]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Carruth, B.R.; Ziegler, P.J.; Gordon, A.; Barr, S.I. Prevalence of picky eaters among infants and toddlers and their caregivers’ decisions about offering a new food. J. Am. Diet. Assoc. 2004, 104 (Suppl. S1), s57–s64. [Google Scholar] [CrossRef]
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am. J. Occup. Ther. Off. Publ. Am. Occup. Ther. Assoc. 2007, 61, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.; Faccioli, L.S.; Baronio, D.; Gottfried, C.; Perry, I.D.S.; Riesgo Rdos, S. Effect of a ketogenic diet on autism spectrum disorder: A systematic review. Res. Autism Spectr. Disord. 2015, 20, 31–38. [Google Scholar] [CrossRef]
- Ma, N.S.; Thompson, C.; Weston, S. Brief Report: Scurvy as a Manifestation of Food Selectivity in Children with Autism. J. Autism Dev. Disord. 2016, 46, 1464–1470. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- de Clercq, N.C.; Groen, A.K.; Romijn, J.A.; Nieuwdorp, M. Gut Microbiota in Obesity and Undernutrition. Adv. Nutr. 2016, 7, 1080–1089. [Google Scholar] [CrossRef]
- Nistal, E.; Caminero, A.; Herrán, A.R.; Arias, L.; Vivas, S.; de Morales, J.M.; Calleja, S.; de Miera, L.E.; Arroyo, P.; Casqueiro, J. Differences of small intestinal bacteria populations in adults and children with/without celiac disease: Effect of age, gluten diet, and disease. Inflamm. Bowel Dis. 2012, 18, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Ghalichi, F.; Ghaemmaghami, J.; Malek, A.; Ostadrahimi, A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: A randomized clinical trial. World J. Pediatr. WJP 2016, 12, 436–442. [Google Scholar] [CrossRef]
- Newell, C.; Bomhof, M.R.; Reimer, R.A.; Hittel, D.S.; Rho, J.M.; Shearer, J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol. Autism 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Obih, C.; Wahbeh, G.; Lee, D.; Braly, K.; Giefer, M.; Shaffer, M.L.; Nielson, H.; Suskind, D.L. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition 2016, 32, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Štefan, L.; Prosoli, R.; Juranko, D.; Čule, M.; Milinović, I.; Novak, D.; Sporiš, G. The Reliability of the Mediterranean Diet Quality Index (KIDMED) Questionnaire. Nutrients 2017, 9, 419. [Google Scholar] [CrossRef]
- Ríos-Hernández, A.; Alda, J.A.; Farran-Codina, A.; Ferreira-García, E.; Izquierdo-Pulido, M. The Mediterranean Diet and ADHD in Children and Adolescents. Pediatrics 2017, 139, e20162027. [Google Scholar] [CrossRef]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Personalized Microbiome Class Students Walter, J.; et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 2019, 25, 789–802.e5. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef]
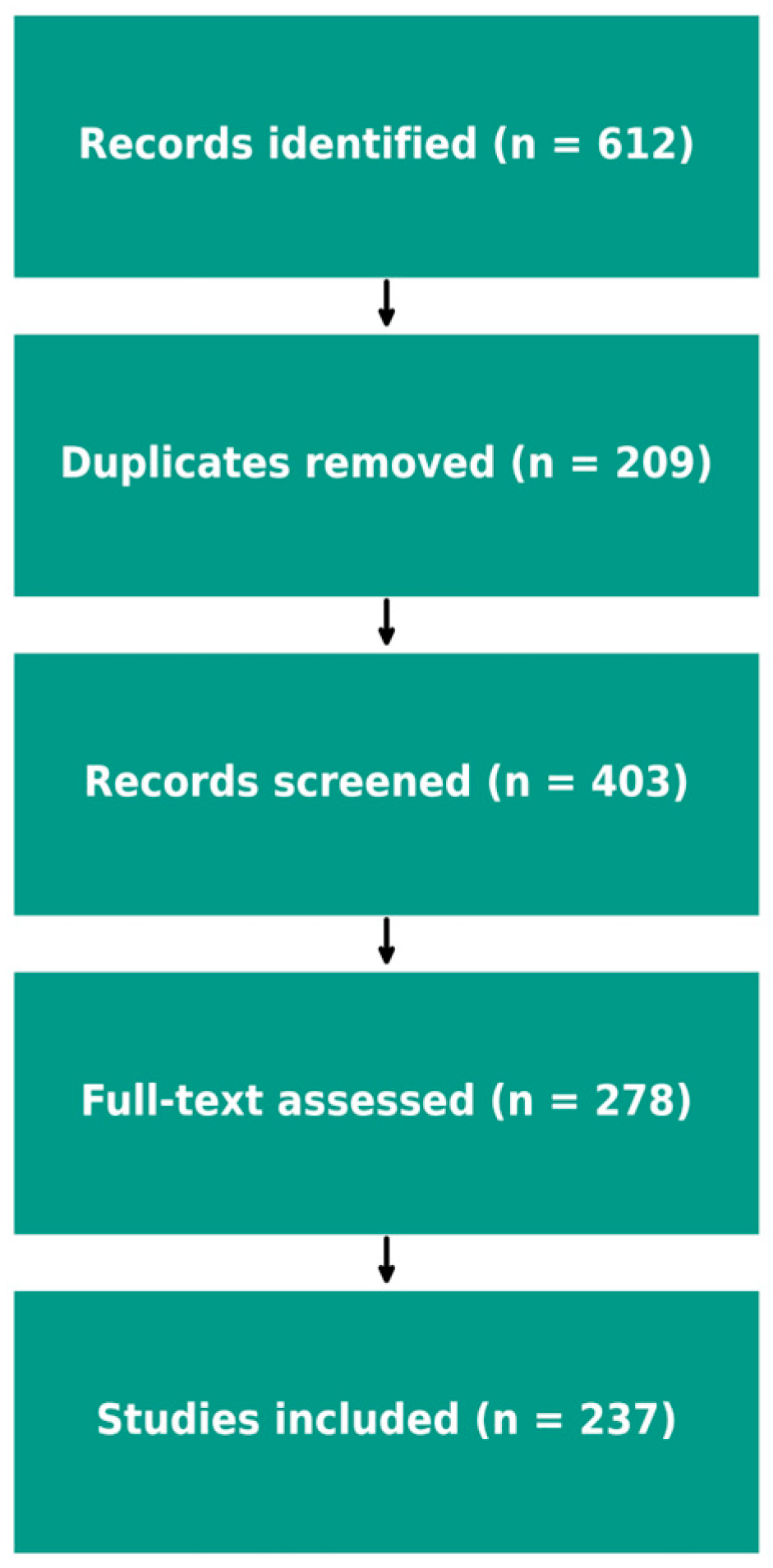
| Factor | Characteristics | Impact on the Microbiota |
|---|---|---|
| Mode of delivery | Vaginal birth vs. cesarean section | Vaginal birth → higher microbial diversity; Cesarean section → reduced exposure to maternal microbes |
| Feeding | Breastfeeding vs. formula feeding | Breast milk → promotes Bifidobacteria dominance; Formula → more heterogeneous composition |
| Antibiotics | Early-life exposure | Reduced diversity, risk of persistent dysbiosis |
| Introduction of solids | Timing and diversity of complementary feeding | Increased microbial diversity, progressive maturation of the microbiota |
| Environment | Rural vs. urban settings | Rural exposure → richer microbial diversity; Urban/sterile environments → lower diversity |
| Disorder | Microbiota Alterations | Key Findings/Associations |
|---|---|---|
| Autism Spectrum Disorder (ASD) | ↑ Clostridium, Escherichia/Shigella; ↓ Faecalibacterium, Lactobacillus | Reduced microbial diversity; GI symptoms frequently correlated with severity of ASD behaviors |
| Attention-Deficit/Hyperactivity Disorder (ADHD) | ↓ Faecalibacterium, Lactobacillales; ↑ Actinobacteria, Bacteroidaceae | Altered short-chain fatty acids; links with cognitive and behavioral regulation |
| Bipolar Disorder (BD) | ↑ Escherichia coli, Bifidobacterium adolescentis; ↓ Faecalibacterium | Pro-inflammatory microbiota signature; potential role in mood instability |
| Major Depressive Disorder (MDD) | Reduced beta diversity; variable taxa changes | Impaired serotonin pathway metabolites; associated with altered gut–brain signaling |
| Anorexia Nervosa (AN) | ↓ Roseburia, Ruminococcus; ↑ Methanobrevibacter smithii | Reduced SCFAs production; microbial shifts may contribute to weight regulation difficulties |
| Social Anxiety Disorder | ↓ Faecalibacterium | Lower abundance linked with increased anxiety and altered stress response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marano, G.; Sfratta, G.; Marzo, E.M.; Cozzo, G.; Abate, F.; Traversi, G.; Mazza, O.; Capristo, E.; Gaetani, E.; Mazza, M. The Pediatric Microbiota–Gut–Brain Axis: Implications for Neuropsychiatric Development and Intervention. Children 2025, 12, 1561. https://doi.org/10.3390/children12111561
Marano G, Sfratta G, Marzo EM, Cozzo G, Abate F, Traversi G, Mazza O, Capristo E, Gaetani E, Mazza M. The Pediatric Microbiota–Gut–Brain Axis: Implications for Neuropsychiatric Development and Intervention. Children. 2025; 12(11):1561. https://doi.org/10.3390/children12111561
Chicago/Turabian StyleMarano, Giuseppe, Greta Sfratta, Ester Maria Marzo, Giorgia Cozzo, Francesca Abate, Gianandrea Traversi, Osvaldo Mazza, Esmeralda Capristo, Eleonora Gaetani, and Marianna Mazza. 2025. "The Pediatric Microbiota–Gut–Brain Axis: Implications for Neuropsychiatric Development and Intervention" Children 12, no. 11: 1561. https://doi.org/10.3390/children12111561
APA StyleMarano, G., Sfratta, G., Marzo, E. M., Cozzo, G., Abate, F., Traversi, G., Mazza, O., Capristo, E., Gaetani, E., & Mazza, M. (2025). The Pediatric Microbiota–Gut–Brain Axis: Implications for Neuropsychiatric Development and Intervention. Children, 12(11), 1561. https://doi.org/10.3390/children12111561