Ethnic Differences in Juvenile Idiopathic Arthritis in the Circumpolar Region
Highlights
- Sakha children have a unique profile of JIA: higher prevalence, entesitis-related arthritis predominance, late access to biologic therapy, and lower probability to obtain remission with the first biological drug.
- The optimization of the healthcare system: contemporary web-service technologies, including artificial intelligence, may shorten the gap to specialists’ consultation, and administration of the treatment, allowing entry into the window of opportunity and improving the disease’s outcomes (increasing the probability of remission).
Abstract
1. Introduction
Objectives
2. Materials and Methods
2.1. Design of the Study and Selection of Participants
2.1.1. Inclusion Criteria
- (1)
- Diagnosis of JIA
- (2)
- Minors, or those under 18 years of age.
2.1.2. Exclusion Criteria
2.2. Data Collection
- (a)
- Clinical and demographic information: Detailed patient information includes gender, date of birth, year of diagnosis, region of residence, ethnicity, family history, and the triggering factor of the illness. JIA subtype, age of JIA onset, number of active joints at onset, and presence of uveitis. The number of mixed families in the Sakha population is minimal, only 3%. According to the socio-cultural features, the question about ethnicities in mixed Sakha families is based on the mother’s opinion. The term “Russians” is more difficult, because it includes all white Caucasian peoples with self-identifications as Russian.
- (b)
- Laboratory features: baseline clinical blood count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP); presence of HLA-B27 antigen, antinuclear antibodies (ANA), rheumatoid factor (RF), antibodies against cyclic citrullinated peptides (anti-CCP), and levels of immunoglobulin classes A (IgA), M (Ig M), and G (Ig G).
- (c)
- Treatment options: We evaluated various antirheumatic treatments, including non-steroidal anti-inflammatory drugs (NSAIDs), systemic (oral and intravenous) and local (intra-articular injections) glucocorticosteroid therapy, non-biologic disease-modifying antirheumatic drugs (nbDMARDs) and biologic disease-modifying antirheumatic drugs (bDMARDs), as well as treatment duration.
- (d)
- Outcomes: attainment of juvenile idiopathic arthritis (JIA) remission according to C. Wallace criteria [26], the specific date of remission, any occurrences of JIA flare-ups, and the duration until the next flare-up.
2.3. Subgroup Analysis
3. Results
3.1. Differences in the Course of JIA Between Sakha and Caucasian Populations
3.2. Treatment of JIA in Studied Populations
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ravelli, A.; Martini, A. Juvenile idiopathic arthritis. Lancet 2007, 369, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Lovell, D.J.; Albani, S.; Brunner, H.I.; Hyrich, K.L.; Thompson, S.D.; Ruperto, N. Juvenile idiopathic arthritis. Nat. Rev. Dis. Primers 2022, 8, 5. [Google Scholar] [CrossRef]
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.-M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar] [PubMed]
- Saurenmann, R.K.; Rose, J.B.; Tyrrell, P.; Feldman, B.M.; Laxer, R.M.; Schneider, R.; Silverman, E.D. Epidemiology of juvenile idiopathic arthritis in a multiethnic cohort: Ethnicity as a risk factor. Arthritis Rheum. 2007, 56, 1974–1984. [Google Scholar] [CrossRef]
- Manners, P.J.; Diepeveen, D.A. Prevalence of juvenile chronic arthritis in a population of 12-year-old children in urban Australia. Pediatrics 1996, 98, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Arkachaisri, T.; Tang, S.P.; Daengsuwan, T.; Phongsamart, G.; Vilaiyuk, S.; Charuvanij, S.; Hoh, S.F.; Tan, J.H.; Das, L.; Ang, E.; et al. Pediatric rheumatology clinic population in Southeast Asia: Are we different? Rheumatology 2017, 56, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, K.; Jiang, S.; Venema, R.; Christie, V.; Boughtwood, T.; Ritha, M.; Skinner, J.; Ali, N.; Rambaldini, B.; Calma, T. Genomics and inclusion of Indigenous peoples in high income countries. Hum. Genet. 2023, 142, 1407–1416. [Google Scholar] [CrossRef]
- Dawe, R.; Penashue, J.; Knight, J.C.; Pike, A.; Benuen, M.P.; Qupee, A.; Pollock, N.J. Mortality in Innu communities in Labrador, 1993–2018: A cross-sectional study of causes and location of death. Int. J. Circumpolar Health 2024, 83, 2378581. [Google Scholar] [CrossRef] [PubMed]
- Amson, A.; Zhang, J.; Frehlich, L.; Ji, Y.; Checholik, C.; Doyle-Baker, P.; Crowshoe, L.; McBrien, K.; Wicklum, S. Nutritional interventions for indigenous adults in Canada—Opportunities to sustain health and cultural practices: A scoping review. Int. J. Circumpolar Health 2024, 83, 2418152. [Google Scholar] [CrossRef]
- Komar, D.; Denic, N.; Marsh, B.; Rumbolt, N. Hangings in Newfoundland and Labrador: A 40-year retrospective analysis of medical examiner data. J. Forensic Sci. 2022, 67, 1557–1564. [Google Scholar] [CrossRef]
- Waits, A.; Emelyanova, A.; Oksanen, A.; Abass, K.; Rautio, A. Human infectious diseases and the changing climate in the Arctic. Environ. Int. 2018, 121, 703–713. [Google Scholar] [CrossRef]
- Huang, G.; Martin, I.; Tsang, R.S.; Demczuk, W.H.; Tyrrell, G.J.; Li, Y.A.; Dickson, C.; Reyes-Domingo, F.; Squires, S.G. Invasive bacterial diseases in northern Canada, 1999 to 2018. Can. Commun. Dis. Rep. 2021, 47, 491–499. [Google Scholar] [CrossRef]
- van Doren, T.P.; Brown, R.A.; Chi, G.; Cochran, P.; Cueva, K.; Eichelberger, L.; Fried, R.; Fritz, S.; Hahn, M.B.; Heintz, R.; et al. Beyond COVID: Towards a transdisciplinary synthesis for understanding responses and developing pandemic preparedness in Alaska. Int. J. Circumpolar Health 2024, 83, 2404273. [Google Scholar] [CrossRef]
- Tvermosegaard, M.; Dahl-Petersen, I.K.; Nielsen, N.O.; Bjerregaard, P.; Jørgensen, M.E. Cardiovascular Disease Susceptibility and Resistance in Circumpolar Inuit Populations. Can. J. Cardiol. 2015, 31, 1116–1123. [Google Scholar] [CrossRef]
- Koller, K.R.; Nash, S.H.; Beans, J.A.; Day, G.M.; Hiratsuka, V.Y.; Lin, A.-L.; Narayanan, M.; A Patten, C.; Hammock, S.A.; Howard, B.V.; et al. Evidence-based screening, clinical care and health education recommendations for Alaska Native peoples with prediabetes living in southcentral Alaska: Findings from the Alaska EARTH follow-up study. Int. J. Circumpolar Health 2024, 83, 2343143. [Google Scholar] [CrossRef] [PubMed]
- Little, M.; Hagar, H.; Zivot, C.; Dodd, W.; Skinner, K.; Kenny, T.-A.; Caughey, A.; Gaupholm, J.; Lemire, M. Drivers and health implications of the dietary transition among Inuit in the Canadian Arctic: A scoping review. Public Health Nutr. 2021, 24, 2650–2668. [Google Scholar] [CrossRef]
- Boyer, G.S.; Benevolenskaya, L.I.; Templin, D.W.; Erdesz, S.; Bowler, A.; Alexeeva, L.I.; Goring, W.P.; Krylov, M.Y.; Mylov, N.M. Prevalence of rheumatoid arthritis in circumpolar native populations. J. Rheumatol. 1998, 25, 23–29. [Google Scholar] [PubMed]
- Templin, D. One year in an Alaskan arthritis clinic. Int. J. Circumpolar Health 1999, 58, 242–247. [Google Scholar] [PubMed]
- Seidler, I.K.; Hansen, N.L.; Bloch, A.P.; Larsen, C.V.L. A systematic review on risk and protective factors for suicide and suicidal behaviour among Greenland Inuit. Int. J. Circumpolar Health 2023, 82, 2226284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klockmann, L.L.; Tøttenborg, E.M.K.; Backe, M.B.; Jørgensen, M.E.; Pedersen, M.L. Prevalence of cardiovascular and other selected diseases among Greenlanders with and without type 2 diabetes. Int. J. Circumpolar Health 2024, 83, 2421052. [Google Scholar] [CrossRef]
- Burtseva, T.E.; Uvarova, T.E.; Tomsky, M.I.; Odland, J.Ø. The health of populations living in the indigenous minority settlements of northern Yakutia. Int. J. Circumpolar Health 2014, 73, 25758. [Google Scholar] [CrossRef]
- Burtseva, T.; Uvarova, T.; Savvina, M.; Shadrin, V.; Avrusin, S.; Chasnyk, V. Health status of Native people living in the Republic of Sakha (Yakutia). Int. J. Circumpolar Health 2013, 72, 21166. [Google Scholar] [CrossRef]
- Boeskorova, S.; Afonskaya, M.; Argunova, V.; Burtseva, T.; Raupov, R.; Kostik, M. Clinical and epidemiological characteristics of systemic lupus erythematosus in the Republic of Sakha (Yakutia). Yakut. Med. J. 2024, 86, 26–30. [Google Scholar] [CrossRef]
- Boeskorova, S.; Afonskaya, M.; Argunova, V.; Sleptsova, P.; Leonteva, L.; Burtseva, T.; Kostik, M.M. Ethnic heterogeneity of juvenile arthritis in the Republic of Sakha (Yakutia) related to a high human leukocyte antigen B27 frequency. World J. Clin. Pediatr. 2025, 14, 101873. [Google Scholar] [CrossRef] [PubMed]
- Khodra, B.; Stevens, A.M.; Ferucci, E.D. Prevalence of Juvenile Idiopathic Arthritis in the Alaska Native Population. Arthritis Care Res. 2020, 72, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.A.; Giannini, E.H.; Huang, B.; Itert, L.; Ruperto, N. Childhood Arthritis Rheumatology Research Alliance (CARRA), Pediatric Rheumatology Collaborative Study Group (PRCSG), Paediatric Rheumatology International Trials Organisation (PRINTO), 2011. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res. 2011, 63, 929–936. [Google Scholar] [CrossRef]
- Uinuk-Ool, T.S.; Takezaki, N.; Sukernik, R.I.; Nagl, S.; Klein, J. Origin and affinities of indigenous Siberian populations as revealed by HLA class II gene frequencies. Hum. Genet. 2002, 110, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.F.; Fuhlbrigge, R.C.; von Scheven, E.; Lovell, D.J.; Colbert, R.A.; Brunner, H.I.; PRCSG Advisory Council; The CARRA Executive Committee. Children with Enthesitis-Related Arthritis and Possible Benefits from Treatments for Adults with Spondyloarthritis. Arthritis Care Res. 2022, 74, 1058–1064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bach, J.F. The hygiene hypothesis in autoimmunity: The role of pathogens and commensals. Nat. Rev. Immunol. 2018, 18, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Shedlock, A.; Langefeld, C. Genetics of autoimmune diseases: Insights from population genetics. J. Hum. Genet. 2015, 60, 657–664. [Google Scholar] [CrossRef]
- Parkinson, A.J.; Evengard, B.; Semenza, J.C.; Ogden, N.; Børresen, M.L.; Berner, J.; Brubaker, M.; Sjöstedt, A.; Evander, M.; Hondula, D.M.; et al. Climate change and infectious diseases in the Arctic: Establishment of a circumpolar working group. Int. J. Circumpolar Health 2014, 73, 25163. [Google Scholar] [CrossRef]
- Bisaccia, M.; Berini, F.; Marinelli, F.; Binda, E. Emerging Trends in Antimicrobial Resistance in Polar Aquatic Ecosystems. Antibiotics 2025, 14, 394. [Google Scholar] [CrossRef]
- Zhou, H.; Tang, L.; A Fenton, K.; Song, X. Exploring and Evaluating Microbiome Resilience in the Gut. FEMS Microbiol. Ecol. 2025, 101, fiaf046. [Google Scholar] [CrossRef]
- Smolik, I.; Robinson, D.; El-Gabalawy, H.S. Periodontitis and rheumatoid arthritis: Epidemiologic, clinical, and immunologic associations. Compend. Contin. Educ. Dent. 2009, 30, 188–210. [Google Scholar]
- Mahdi, H.; A Fisher, B.; Källberg, H.; Plant, D.; Malmström, V.; Rönnelid, J.; Charles, P.; Ding, B.; Alfredsson, L.; Padyukov, L.; et al. Specific interaction between genotype, smoking, and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat. Genet. 2009, 41, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Adlard, B.; Bonefeld-Jørgensen, E.C.; Dudarev, A.A.; Olafsdottir, K.; Abass, K.; Ayotte, P.; Caron-Beaudoin, É.; Drysdale, M.; Garcia-Barrios, J.; Gyllenhammar, I.; et al. Levels and trends of metals in human populations living in the Arctic. Int. J. Circumpolar Health 2024, 83, 2386140. [Google Scholar] [CrossRef] [PubMed]
- Adlard, B.; Bonefeld-Jørgensen, E.C.; Dudarev, A.A.; Olafsdottir, K.; Abass, K.; Averina, M.; Ayotte, P.; Berner, J.; Byrne, S.; Caron-Beaudoin, É.; et al. Levels and trends of persistent organic pollutants in human populations living in the Arctic. Int. J. Circumpolar Health 2024, 83, 2392405. [Google Scholar] [CrossRef] [PubMed]
- Aker, A.M.; Ayotte, P.; Gaudreau, É.; Lemire, M. Current-use pesticide exposures in remote Inuit communities. Int. J. Circumpolar Health 2024, 83, 2421048. [Google Scholar] [CrossRef]
- Andersen, L.; Corazon, S.S.S.; Stigsdotter, U.K.K. Nature Exposure and Its Effects on Immune System Functioning: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1416. [Google Scholar] [CrossRef]
- Hitchon, C.A.; Khan, S.; Elias, B.; Lix, L.M.; Peschken, C.A. Prevalence and incidence of rheumatoid arthritis in Canadian First Nations and Non-First Nations People: A Population-Based Study. J. Clin. Rheumatol. 2020, 26, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Boyer, G.S.; Templin, D.W.; Lanier, A.P. Rheumatic diseases in Alaskan Indians of the southeast coast: High prevalence of rheumatoid arthritis and systemic lupus erythematosus. J. Rheumatol. 1991, 18, 1477–1484. [Google Scholar]
- Barnabe, C.; Jones, C.A.; Bernatsky, S.; Peschken, C.A.; Voaklander, D.; Homik, J.; Crowshoe, L.F.; Esdaile, J.M.; El-Gabalawy, H.; Hemmelgarn, B. Inflammatory arthritis prevalence and health services use in the First Nations and Non-First Nations Populations of Alberta, Canada. Arthritis Care Res. 2017, 69, 467–474. [Google Scholar] [CrossRef]
- Ferucci, E.D.; Templin, D.W.; Lanier, A.P. Rheumatoid arthritis in American Indians and Alaska Natives: A review of the literature. Semin. Arthritis Rheum. 2005, 34, 662e7. [Google Scholar] [CrossRef]
- Scally, S.W.; Law, S.-C.; Ting, Y.T.; van Heemst, J.; Sokolove, J.; Deutsch, A.J.; Clemens, E.B.; Moustakas, A.K.; Papadopoulos, G.K.; van der Woude, D.; et al. Molecular basis for increased susceptibility of Indigenous North Americans to seropositive rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 1915–1923. [Google Scholar] [CrossRef]
- Peschken, C.A.; Hitchon, C.A.; Robinson, D.B.; Smolik, I.; Barnabe, C.R.; Prematilake, S.; El-Gabalawy, H.S. Rheumatoid arthritis in a North American native population: Longitudinal follow-up and comparison with a white population. J. Rheumatol. 2010, 37, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Tanner, S.; Dufault, B.; Smolik, I.; Meng, X.; Anaparti, V.; Hitchon, C.; Robinson, D.B.; Robinson, W.; Sokolove, J.; Lahey, L.; et al. A prospective study of the development of inflammatory arthritis in the family members of indigenous North American people with rheumatoid arthritis. Arthritis Rheumatol. 2019, 71, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Peschken, C.A.; Robinson, D.B.; Hitchon, C.A.; Smolik, I.; Hart, D.; Bernstein, C.N.; El-Gabalawy, H.S. Pregnancy and the risk of rheumatoid arthritis in a highly predisposed North American Native population. J. Rheumatol. 2012, 39, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Hitchon, C.A.; ONeil, L.; Peschken, C.A.; Robinson, D.B.; Fowler-Woods, A.; El-Gabalawy, H.S. Disparities in rheumatoid arthritis outcomes for North American Indigenous populations. Int. J. Circumpolar Health 2023, 82, 2166447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McVicar, J.A.; Poon, A.; Caron, N.R.; Bould, M.D.; Nickerson, J.W.; Ahmad, N.; Kimmaliardjuk, D.M.; Sheffield, C.; Champion, C.; McIsaac, D.I. Postoperative outcomes for Indigenous peoples in Canada: A systematic review. Can. Med. Assoc. J. 2021, 193, E713–E722. [Google Scholar] [CrossRef]
- Hurd, K.; Barnabe, C. Mortality causes and outcomes in Indigenous populations of Canada, the USA, and Australia with rheumatic disease: A systematic review. Semin. Arthritis Rheum. 2018, 47, 586–592. [Google Scholar] [CrossRef]
- Ferucci, E.D.; Donnithorne, K.J.; Koller, K.R.; Swango-Wilson, A.; Pflaum, J.; Lanier, A.P. Performance on rheumatoid arthritis quality indicators in an Alaska Native healthcare system. Qual. Saf. Health Care. 2010, 19, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Boyer, G.S.; Templin, D.W.; Cornoni-Huntley, J.C.; Everett, D.F.; Lawrence, R.C.; Heyse, S.F.; Miller, M.M.; Goring, W.P. Prevalence of spondyloarthropathies in Alaskan Eskimos. J. Rheumatol. 1994, 21, 2292–2297. [Google Scholar] [PubMed]
- Dean, L.E.; Jones, G.T.; MacDonald, A.G.; Downham, C.; Sturrock, R.D.; Macfarlane, G.J. Global prevalence of ankylosing spondylitis. Rheumatology 2014, 53, 650–657. [Google Scholar] [CrossRef]
- Hegde, A.; Acharya, S.; Singh, K.; Kovilapu, U.B. Clinical Profile of Juvenile Idiopathic Arthritis from a Tertiary Care Hospital in Northern India. Indian J. Rheumatol. 2020, 15, 310–316. [Google Scholar] [CrossRef]
- Tanya, M.; Teh, K.L.; Das, L.; Hoh, S.F.; Gao, X.; Arkachaisri, T. Juvenile idiopathic arthritis in Southeast Asia: The Singapore experience over two decades. Clin. Rheumatol. 2020, 39, 3455–3464. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Bosan, K.; Khurshid, S.; Rasheed, U.; Zeb, A.; Zammurrad, S. Prevalence of Depression in Patients with Juvenile Idiopathic Arthritis Presenting at a Tertiary Care Hospital. Cureus 2020, 12, e6807. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vinokurova, F.; Argunova, V.; Sleptsova, P.; Nikolaev, V.; Rumyantsev, E.; Burtseva, T.; Chasnyk, V.; Kostik, M. Prevalence and structure of juvenile idiopathic arthritis in children in the Republic of Sakha (Yakutia). Yakut Med. J. 2020, 4, 6–9. [Google Scholar] [CrossRef]
- Fefelova, V.V. Participation of Indo-European tribes in ethnogeny of the mongoloid population of Siberia: Analysis of the HLA antigen distribution in mongoloids of Siberia. Am. J. Hum. Genet. 1990, 47, 294–301. [Google Scholar] [PubMed] [PubMed Central]
- Sherilee, L. Harper, Carlee Wright, Stephanie Masina, Shaugn Coggins. Climate change, water, and human health research in the Arctic. Water Secur. 2020, 10, 100062. [Google Scholar] [CrossRef]
- Horneff, G.; Borchert, J.; Heinrich, R.; Kock, S.; Klaus, P.; Dally, H.; Hagemann, C.; Diesing, J.; Schönfelder, T. Incidence, prevalence, and comorbidities of juvenile idiopathic arthritis in Germany: A retrospective observational cohort health claims database study. Pediatr. Rheumatol. 2022, 20, 100. [Google Scholar] [CrossRef]
- Al-Mayouf, S.M.; Al Mutairi, M.; Bouayed, K.; Habjoka, S.; Hadef, D.; Lotfy, H.M.; Scott, C.; Sharif, E.M.; Tahoun, N. Epidemiology and demographics of juvenile idiopathic arthritis in Africa and Middle East. Pediatr. Rheumatol. Online J. 2021, 19, 166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yener, G.O.; Tekin, Z.E.; Girisgen, I.; Çetin, E.N.; Akdağ, B.; Yüksel, S. Juvenile idiopathic arthritis in a center in the Western Anatolia region in Turkey. Turk. Pediatri Ars. 2020, 55, 157–165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Çakan, M.; Aktay-Ayaz, N.; Keskindemirci, G.; Ekinci, D.Y.; Karadağ, Ş.G. Subtype frequencies, demographic features, and remission rates in juvenile idiopathic arthritis—265 cases from a Turkish center. Turk. J. Pediatr. 2017, 59, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Demirkaya, E.; Ozen, S.; Sozeri, B.; Ayaz, N.A.; Kasapçopur, O.; Unsal, E.; Makay, B.B.; Barut, K.; Fidanci, B.E.; Simsek, D.; et al. The Turkish version of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR). Rheumatol. Int. 2018, 38 (Suppl. S1), 395–402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kasapçopur, O.; Yologlu, N.; Ozyazgan, Y.; Ercan, G.; Caliskan, S.; Sever, L.; Ozdogan, H.; Arisoy, N. Uveitis and anti nuclear antibody positivity in children with juvenile idiopathic arthritis. Indian Pediatr. 2004, 41, 1035–1039. [Google Scholar] [PubMed]
- Şen, V.; Ece, A.; Uluca, Ü.; Güneş, A.; Yel, S.; Tan, I.; Karabel, D.; Yıldırım, B.; Haspolat, K. Evaluation of children with juvenile idiopathic arthritis in southeastern Turkey: A single center experience. Hippokratia 2015, 19, 63–68. [Google Scholar] [PubMed] [PubMed Central]
- Ozen, S.; Karaaslan, Y.; Ozdemir, O.; Saatci, U.; Bakkaloglu, A.; Koroglu, E.; Tezcan, S. Prevalence of juvenile chronic arthritis and familial Mediterranean fever in Turkey: A field study. J. Rheumatol. 1998, 25, 2445–2449. [Google Scholar] [PubMed]
- Takei, S.; Yamashita, S.; Kato, T. Nation-wide survey for patients with juvenile idiopathic arthritis in Japan. In Annual Report on Children with Chronic Refractory Diseases from the Japanese Ministry of Health, Labor and Welfare; The Japanese Ministry of Health, Labor and Welfare: Tokyo, Japan, 2008; pp. 102–113. [Google Scholar]
- Srivastava, R.; Phatak, S.; Yadav, A.; Bajpai, P.; Aggarwal, A. HLA B27 typing in 511 children with juvenile idiopathic arthritis from India. Rheumatol. Int. 2016, 36, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Beesley, R.P.; Hyrich, K.L.; Humphreys, J.H. The incidence and prevalence of juvenile idiopathic arthritis differs between ethnic groups in England. Rheumatology 2023, 64, 296–302. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Yang, Y.-H.; Lin, C.-Y.; Chang, C.-L.; Chiang, B.-L. Enthesitis-related arthritis is the most common category of juvenile idiopathic arthritis in Taiwan and presents persistent active disease. Pediatr. Rheumatol. 2019, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.M.; Claw, K.G.; Woodahl, E.L.; Robinson, R.F.; Boyer, B.B.; Burke, W.; Thummel, K.E. P450 Pharmacogenetics in Indigenous North American Populations. J. Pers. Med. 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Temirbulatov, I.; Ivashchenko, D.; Rudykh, Z.; Popova, N.; Tayurskaya, K.; Chertovskikh, Y.; Sychev, D. The prevalence of CYP2C19*2, *3 polymorphisms among russians and yakuts with peptic ulcer disease, living in Sakha Republic (Yakutia). Mol. Meditsina 2018, 16, 34–38. (In Russian) [Google Scholar] [CrossRef]
- Vilaiyuk, S.; Lerkvaleekul, B.; Thammanichanond, D. Associations between HLA-B27 subtypes and outcomes in Thai children with enthesitis-related arthritis. Clin. Rheumatol. 2022, 41, 203–212. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Sakha Children, n = 168 | Russian Children, n = 48 | p-Value |
|---|---|---|---|
| Sex, male, n (%) | 85 (50.6) | 19 (38.8) | 0.198 |
| Age at onset * | 9.0 [6.0–12.0] | 6.0 [4.0–11.0] | 0.024 |
| Asian ethnicity, n (%) | 168 (100.0) | 0 (0.0) | |
| Place of residence, urban, n (%) | 87 (51.8) | 30 (61.2) | 0.259 |
| JIA category, n (%) | 0.0002 | ||
| -Oligoarthritis | 47 (27.9) | 26 (53.1) | |
| -RF-negative polyarthritis | 25 (14.9) | 8 (16.3) | |
| -RF-positive polyarthritis | 2 (1.2) | 0 (0.0) | |
| -Enthesitis-related arthritis | 86 (51.2) | 9 (18.3) | |
| -Systemic arthritis | 3 (1.8) | 4 (8.2) | |
| -Psoriatic arthritis | 5 (3.0) | 2 (4.1) | |
| Presence of enthesitis, n (%) | 32 (19.0) | 1 (2.0) | 0.003 |
| Presence of sacroiliitis, n (%) | 40 (23.8) | 1 (2.0) | 0.0003 |
| Presence of uveitis, n (%) | 18 (10.7) | 4 (8.2) | 0.790 |
| Presence of psoriasis, n (%) | 5 (3.0) | 2 (4.1) | 0.658 |
| Active joints * | 4.0 [1.0–24.0] | 3.0 [1.0–24.0] | 0.328 |
| RF positivity, n (%) | 1 (0.6) | 0 (0.0) | 1.0 |
| HLA-B27 positivity, n (%) | 76/164 (46.3) | 7/48 (14.6) | 0.00005 |
| ANA positivity, n (% among tested) | 24/36 (66.7) | 5/8 (62.5) | 1.0 |
| Hemoglobin, g/L * | 119.0 [107.5–128.5] | 122.0 [111.0–127.0] | 0.322 |
| Leukocytes, 109/L * | 7.8 [6.1–10.0] | 7.6 [6.0–9.7] | 0.744 |
| Platelets, 109/L * | 379.0 [325.0–469.0] | 350.0 [291.0–455.0] | 0.081 |
| ESR at onset, mm/h * | 27.0 [1.0–117.0] | 20.0 [0.0–65.0] | 0.304 |
| CRP at onset, mg/L * | 4.4 [0.0–303.0] | 3.0 [0.0–59.0] | 0.645 |
| Parameter | Sakha Children, n = 168 | Russian Children, n = 48 | p-Value |
|---|---|---|---|
| Therapy | |||
| -No corticosteroids | 132 (78.6) | 41 (83.7) | 0.886 |
| -Intravenous corticosteroids | 9 (5.4) | 2 (4.1) | |
| -Oral corticosteroids | 3 (1.8) | 1 (2.0) | |
| -Intra-articular corticosteroids | 18 (10.7) | 3 (6.1) | |
| -Intravitreal corticosteroids | 6 (3.6) | 2 (4.1) | |
| Methotrexate | 153 (91.6) | 45 (91.8) | 1.0 |
| Methotrexate discontinuation | 24 (15.7) | 4 (8.9) | 0.333 |
| Patients treated with bDMARD | 70 (41.7) | 18 (36.7) | 0.621 |
| First bDMARD, n (%) | 0.027 | ||
| -Adalimumab | 18 (25.7) | 1 (5.6) | |
| -Abatacept | 1 (1.4) | 0 (0.0) | |
| -Secukinumab | 1 (1.4) | 1 (5.6) | |
| -Tocilizumab | 3 (4.3) | 4 (22.2) | |
| -Etanercept | 47 (67.2) | 12 (66.6) | |
| Time to bDMARD initiation, months * | 8.0 [0–60.0] | 4.0 [0–60.0] | 0.153 |
| The frequency of bDMARD administration, according to JIA categories | 0.0003 | ||
| -Oligoarthritis | 8/47 (17.0) | 7/24 (29.2) | |
| -RF-negative polyarthritis | 9/25 (36.0) | 4/8 (50.0) | |
| -RF-positive polyarthritis | 0/2 (0.0) | 0/0 (0.0) | |
| -Enthesitis-related arthritis | 45/86 (52.3) | 3/9 (33.3) | |
| -Systemic arthritis | 3/3 (100.0) | 4/4 (100.0) | |
| -Psoriatic arthritis | 4/5 (80.0) | 0/2 (0.0) | |
| JIA outcomes | |||
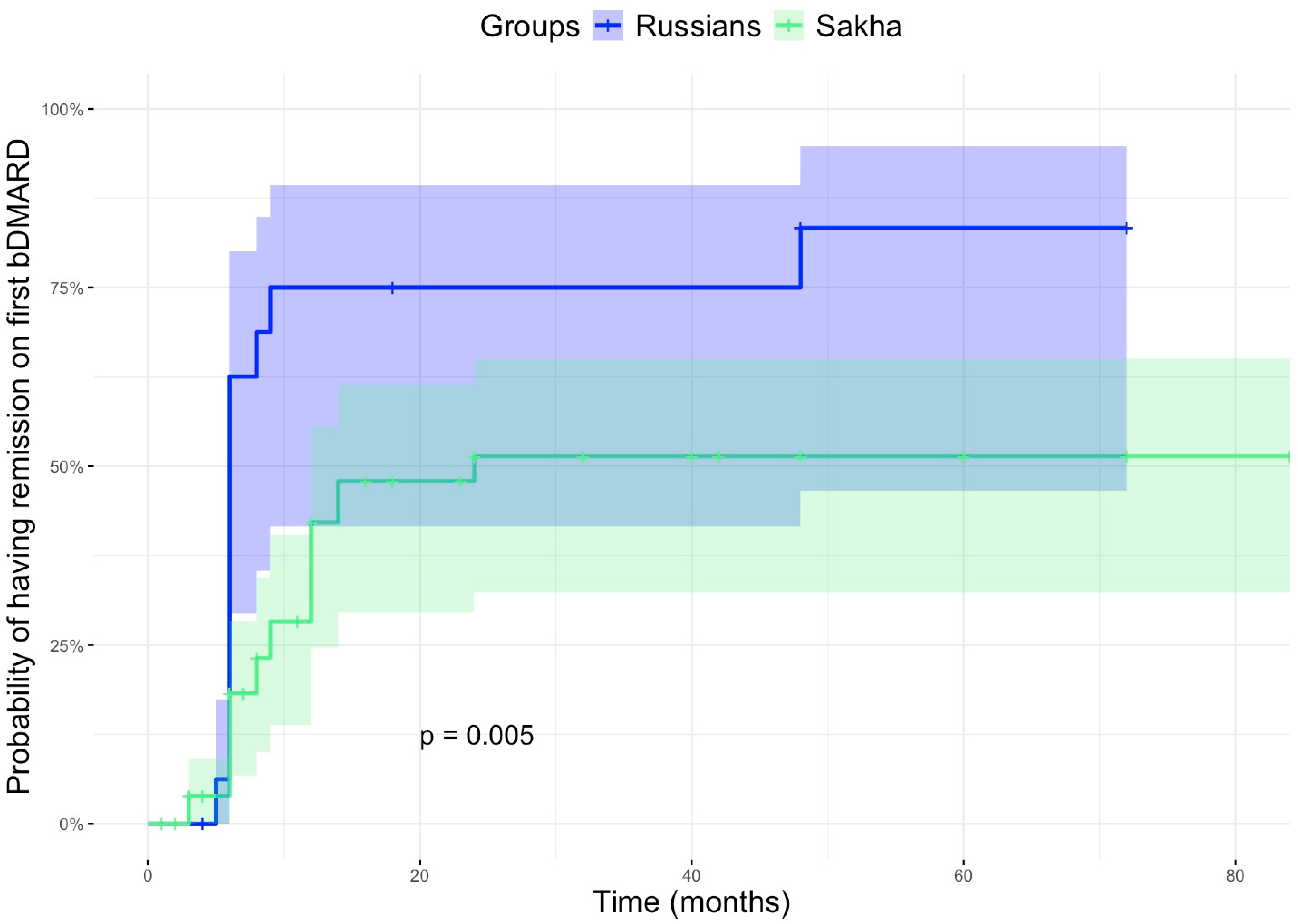
| Remission on first bDMARD, n (%) | 19 (29.2) | 13 (72.2) | 0.002 |
| The achievement of remission on first bDMARD, according to JIA categories | 0.008 | ||
| -Oligoarthritis | 1/7 (14.3) | 4/7 (57.1) | |
| -RF-negative polyarthritis | 3/8 (37.5) | 3/4 (75) | |
| -RF-positive polyarthritis | 0/0 (0.0) | 0/0 (0.0) | |
| -Enthesitis-related arthritis | 13/43 (30.2) | 2/3 (66.6) | |
| -Systemic arthritis | 1/3 (33.3) | 4/4 (100) | |
| -Psoriatic arthritis | 1/3 (33.3) | 0/0 (0.0) | |
| Time to remission on the first bDMARD * | 11.0 [1.0–84.0] | 6.0 [4.0–72.0] | 0.171 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boeskorova, S.G.; Afonskaya, M.V.; Argunova, V.M.; Sleptsova, P.A.; Leonteva, L.V.; Nikiforova, V.V.; Chikova, I.A.; Yakovlev, A.A.; Burtseva, T.E.; Kostik, M.M. Ethnic Differences in Juvenile Idiopathic Arthritis in the Circumpolar Region. Children 2025, 12, 1525. https://doi.org/10.3390/children12111525
Boeskorova SG, Afonskaya MV, Argunova VM, Sleptsova PA, Leonteva LV, Nikiforova VV, Chikova IA, Yakovlev AA, Burtseva TE, Kostik MM. Ethnic Differences in Juvenile Idiopathic Arthritis in the Circumpolar Region. Children. 2025; 12(11):1525. https://doi.org/10.3390/children12111525
Chicago/Turabian StyleBoeskorova, Sargylana G., Marina V. Afonskaya, Vera M. Argunova, Polina A. Sleptsova, Liudmila V. Leonteva, Vasilina V. Nikiforova, Irina A. Chikova, Alexandr A. Yakovlev, Tatiana E. Burtseva, and Mikhail M. Kostik. 2025. "Ethnic Differences in Juvenile Idiopathic Arthritis in the Circumpolar Region" Children 12, no. 11: 1525. https://doi.org/10.3390/children12111525
APA StyleBoeskorova, S. G., Afonskaya, M. V., Argunova, V. M., Sleptsova, P. A., Leonteva, L. V., Nikiforova, V. V., Chikova, I. A., Yakovlev, A. A., Burtseva, T. E., & Kostik, M. M. (2025). Ethnic Differences in Juvenile Idiopathic Arthritis in the Circumpolar Region. Children, 12(11), 1525. https://doi.org/10.3390/children12111525







