Significant Response to Denosumab Yet with Severe Rebound Hypercalcemia in a 9-Year-Old Boy with Aneurysmal Bone Cyst: A Case Report
Abstract
1. Introduction
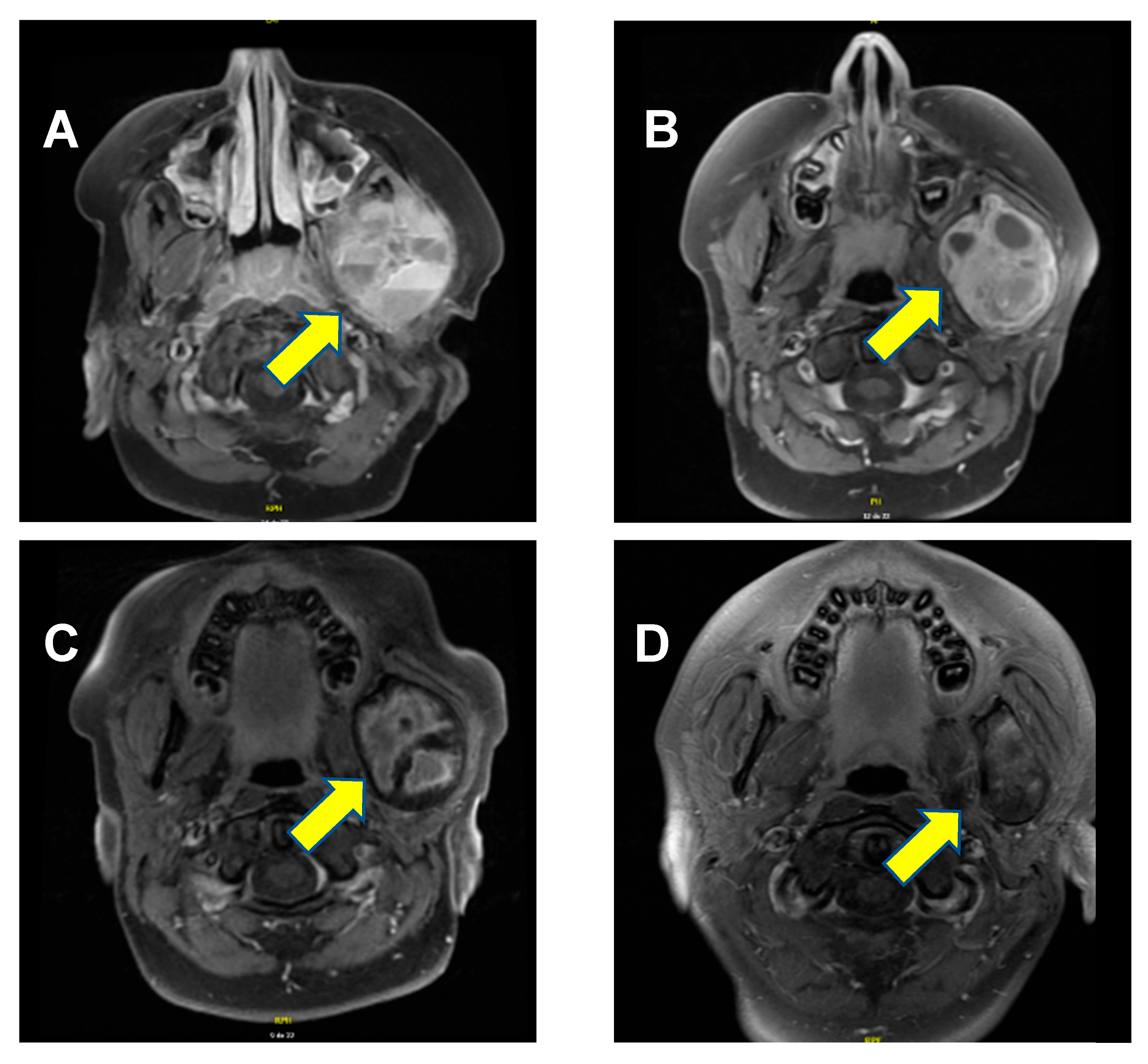
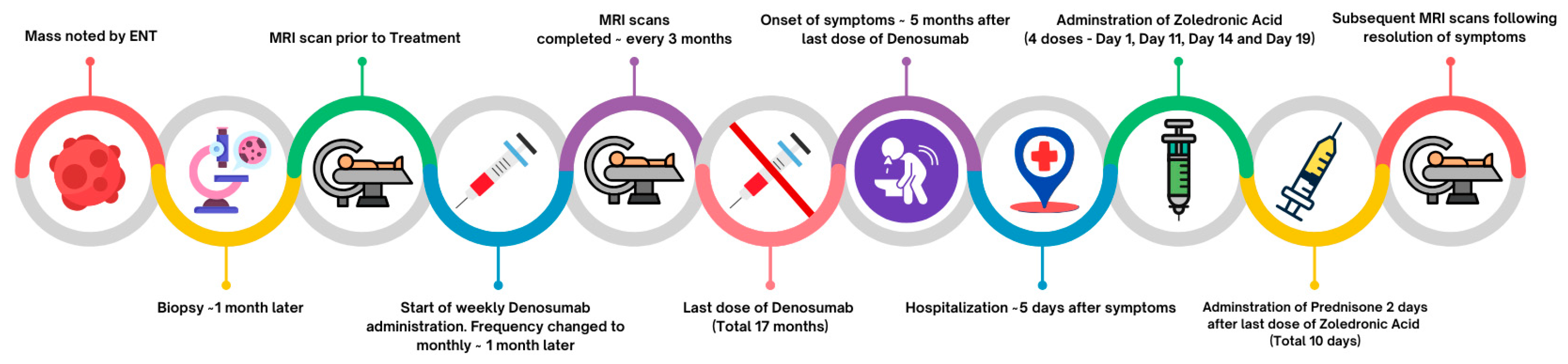
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | Aneurysmal bone cysts |
| GCTB | Giant cell tumors of the bone |
| MRI | Magnetic resonance imaging |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
References
- Vergel De Dios, A.M.; Bond, J.R.; Shives, T.C.; McLeod, R.A.; Unni, K.K. Aneurysmal bone cyst: A clinicopathologic study of 238 cases. Cancer 1992, 69, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Boubbou, M.; Atarraf, K.; Chater, L.; Afifi, A.; Tizniti, S. Aneurysmal bone cyst primary: About eight pediatric cases: Radiological aspects and review of the literature. Pan Afr. Med. J. 2013, 15, 111. [Google Scholar] [CrossRef]
- Schreuder, H.W.B.; Veth, R.P.H.; Pruszczynski, M.; Lemmens, J.A.M.; Koops, H.S.; Molenaar, W.M. Aneurysmal bone cysts treated by curettage, cryotherapy and bone grafting. J. Bone Jt. Surg. Br. 1997, 79, 20–25. [Google Scholar] [CrossRef]
- Pelo, S.; Gasparini, G.; Boniello, R.; Moro, A.; Amoroso, P.F. Aneurysmal bone cyst located in the mandibular condyle. Head Face Med. 2009, 5, 8. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Shi, J. Clinicopathology and recurrence analysis of 44 jaw aneurysmal bone cyst cases: A literature review. Front. Surg. 2021, 8, 678696. [Google Scholar] [CrossRef]
- Restrepo, R.; Zahrah, D.; Pelaez, L.; Temple, H.T.; Murakami, J.W. Update on aneurysmal bone cyst: Pathophysiology, histology, imaging and treatment. Pediatr. Radiol. 2022, 52, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Dürr, H.R.; Grahneis, F.; Baur-Melnyk, A.; Knösel, T.; Birkenmaier, C.; Jansson, V.; Klein, A. Aneurysmal bone cyst: Results of an off label treatment with denosumab. BMC Musculoskelet. Disord. 2019, 20, 456. [Google Scholar] [CrossRef]
- Gupta, A.; Durocher-Allen, L.; Popovic, S.; Tozer, R.; Yao, X.; Ghert, M. The role of denosumab for surgical outcomes in patients with giant cell tumour of bone: A systematic review. Curr. Oncol. 2021, 28, 1302–1313. [Google Scholar] [CrossRef]
- Kurucu, N.; Akyuz, C.; Ergen, F.B.; Yalcin, B.; Kosemehmetoglu, K.; Ayvaz, M.; Varan, A.; Aydin, B.; Kutluk, T. Denosumab treatment in aneurysmal bone cyst: Evaluation of nine cases. Pediatr. Blood Cancer 2018, 65, e26926. [Google Scholar] [CrossRef]
- Chawla, S.; Henshaw, R.; Seeger, L.; Choy, E.; Blay, J.Y.; Ferrari, S.; Kroep, J.; Grimer, R.; Reichardt, P.; Rutkowski, P.; et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: Interim analysis of an open-label, parallel group, phase 2 study. Lancet Oncol. 2013, 14, 901–908. [Google Scholar] [CrossRef]
- Naidu, A.; Malmquist, M.P.; Denham, C.A.; Schow, S.R. Management of central giant cell granuloma with subcutaneous denosumab therapy. J. Oral Maxillofac. Surg. 2014, 72, 2469–2484. [Google Scholar] [CrossRef]
- Palmerini, E.; Ruggieri, P.; Angelini, A.; Boriani, S.; Campanacci, D.; Milano, G.M.; Cesari, M.; Paioli, A.; Longhi, A.; Abate, M.E.; et al. Denosumab in patients with aneurysmal bone cysts: A case series with preliminary results. Tumori J. 2018, 104, 344–351. [Google Scholar] [CrossRef]
- Renaghan, A.D.; Rosner, M.H. Hypercalcemia: Etiology and management. Nephrol. Dial. Transplant. 2018, 33, 549–551. [Google Scholar] [CrossRef]
- Tebben, P.J.; Singh, R.J.; Kumar, R. Vitamin D-Mediated hypercalcemia: Mechanisms, diagnosis, and treatment. Endocr. Rev. 2016, 37, 521–547. [Google Scholar] [CrossRef]
- Vanderniet, J.A.; Szymczuk, V.; Högler, W.; Beck-Nielsen, S.S.; Uday, S.; Merchant, N.; Crane, J.L.; Ward, L.M.; Boyce, A.M.; Munns, C.F. Management of RANKL-Mediated disorders with denosumab in children and adolescents: A global expert guidance document. J. Clin. Endocrinol. Metab. 2024, 109, 1371–1382. [Google Scholar] [CrossRef]
- Wang, D.; Tang, X.; Shi, Q.; Wang, R.; Ji, T.; Tang, X.; Guo, W. Denosumab in pediatric bone disorders and the role of RANKL blockade: A narrative review. Transl. Pediatr. 2023, 12, 470–486. [Google Scholar] [CrossRef] [PubMed]
- van Geloven, T.P.G.; van de Sande, M.A.J.; van der Heijden, L. The treatment of aneurysmal bone cysts. Curr. Opin. Pediatr. 2023, 35, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Cottalorda, J.; Louahem Sabah, D.; Joly Monrigal, P.; Jeandel, C.; Delpont, M. minimally invasive treatment of aneurysmal bone cysts: Systematic literature review. Orthop. Traumatol. Surg. Res. 2022, 108, 103272. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, G.K.; Patel, P.; Kasi, A. Denosumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535388/ (accessed on 5 November 2025).
- Medicines and Healthcare Products Regulatory Agency (MHRA). Denosumab 60 mg (Prolia): Should not be used in patients under 18 years due to the risk of serious hypercalcaemia. Drug Saf. Update 2022, 15, 1. [Google Scholar]
- Sydlik, C.; Dürr, H.R.; Pozza, S.B.; Weißenbacher, C.; Roeb, J.; Schmidt, H. Hypercalcaemia after treatment with denosumab in children: Bisphosphonates as an option for therapy and prevention? World J. Pediatr. 2020, 16, 520–527. [Google Scholar] [CrossRef]
- Setsu, N.; Kobayashi, E.; Asano, N.; Yasui, N.; Kawamoto, H.; Kawai, A.; Horiuchi, K. Severe hypercalcemia following denosumab treatment in a juvenile patient. J. Bone Miner. Metab. 2016, 34, 118–122. [Google Scholar] [CrossRef]
- Boyce, A.M.; Chong, W.H.; Yao, J.; Gafni, R.I.; Kelly, M.H.; Chamberlain, C.E.; Bassim, C.; Cherman, N.; Ellsworth, M.; Kasa-Vubu, J.Z.; et al. Denosumab treatment for fibrous dysplasia. J. Bone Miner. Res. 2012, 27, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Gossai, N.; Hilgers, M.V.; Polgreen, L.E.; Greengard, E.G. Critical hypercalcemia following discontinuation of denosumab therapy for metastatic giant cell tumor of bone. Pediatr. Blood Cancer 2015, 62, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Grasemann, C.; Schündeln, M.M.; Hövel, M.; Schweiger, B.; Bergmann, C.; Herrmann, R.; Wieczorek, D.; Zabel, B.; Wieland, R.; Hauffa, B.P. Effects of RANK-ligand antibody (denosumab) treatment on bone turnover markers in a girl with juvenile Paget’s disease. J. Clin. Endocrinol. Metab. 2013, 98, 3121–3126. [Google Scholar] [CrossRef]
- Uday, S.; Gaston, C.L.; Rogers, L.; Parry, M.; Joffe, J.; Pearson, J.; Sutton, D.; Grimer, R.; Högler, W. Osteonecrosis of the jaw and rebound hypercalcemia in young people treated with denosumab for giant cell tumor of bone. J. Clin. Endocrinol. Metab. 2018, 103, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, K.; Kobayashi, E.; Mizuno, T.; Susa, M.; Chiba, K. Hypercalcemia following discontinuation of denosumab therapy: A systematic review. Bone Rep. 2021, 15, 101148. [Google Scholar] [CrossRef]
- Schmitt, L.; Theiler-Schwetz, V.; Sadoghi, P.; Trummer, C.; Pilz, S. Rebound hypercalcemia after denosumab cessation during follow-up after surgical treatment for parathyroid carcinoma: Case report and literature review. Arch. Endocrinol. Metab. 2024, 68, e240035. [Google Scholar] [CrossRef]
- Roux, S.; Massicotte, M.H.; Huot Daneault, A.; Brazeau-Lamontagne, L.; Dufresne, J. Acute hypercalcemia and excessive bone resorption following anti-RANKL withdrawal: Case report and brief literature review. Bone 2019, 120, 482–486. [Google Scholar] [CrossRef]
- Gandolfi, A.; Shaaban, S. Denosumab-Induced rebound hypercalcemia treated with bisphosphonates in a pediatric patient. JCEM Case Rep. 2023, 1, luad133. [Google Scholar] [CrossRef]
- Evangelisti, G.; Altorfer, F.C.S.; Falzetti, L.; Palmerini, E.; Griffoni, C.; Ghermandi, R.; Boriani, S.; Monetta, A.; Cesari, M.; Ibrahim, T.; et al. Denosumab re-challenge and long-term efficacy for aneurysmal bone cyst of the spine: Enhanced treatment algorithm. J. Clin. Med. 2024, 13, 4522. [Google Scholar] [CrossRef]
- Maximen, J.; Robin, F.; Tronchot, A.; Rossetti, A.; Ropars, M.; Guggenbuhl, P. Denosumab in the management of aneurysmal bone cyst. Jt. Bone Spine 2022, 89, 105260. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Lee, Y.J.; Kim, J.; Jeong, C.; Lim, Y.; Lee, J.; Baek, K.H.; Catholic Medical Center Bone Research Group. Long-Term efficacy and safety of denosumab: Insights beyond 10 years of use. Endocrinol. Metab. 2025, 40, 47–56. [Google Scholar] [CrossRef] [PubMed]
| Assessed Parameter (Normal Range) | Pre-Denosumab | During Treatment | At Presentation with Hypercalcemia | After Hospitalization |
|---|---|---|---|---|
| Calcium (2.20–2.65 mmol/L) | 2.52 mmol/L | Between 2.10 mmol/L and 2.57 mmol/L | 3.17 mmoL/L | Between 2.35 mmol/L and 2.95 mmol/L |
| Ionized calcium corrected for 7.4 pH (1.14–1.32 mmol/L) | - | 1.35 mmol/L | 2.01 mmol/L and 2.07 mmol/L | Between 1.28 mmol/L and 1.49 mmol/L |
| Albumin (37–47 g/L) | 39 g/L | Between 38 g/L and 41 g/L | 32 g/L (day after presentation) | Between 38 g/L and 40 g/L |
| Phosphate (1.30–1.80 mmol/L) | 1.57 mmol/L | Between 0.66 mmol/L and 1.50 mmol/L | 1.09, 0.96, and 0.76 | Between 0.87 and 1.37 |
| Magnesium (0.70–1.05 mmol/L) | 0.97 mmol/L | Between 0.88 mmol/L and 1.07 mmol/L | 0.67 mmol/L and 0.63 mmol/L | Between 0.81 mmol/L and 0.94 mmol/L |
| 25-hydroxy vitamin D (50–125 nmol/L) | 56.4 nmol/L | - | 60.6 nmol/L | Between 52.9 nmol/L and 75.4 nmol/L |
| PTH I-84 (15–68 ng/L) | 51 ng/L | - | <5 ng/L | - |
| Creatinine (25–75 µmol/L) | 43 µmol/L | Between 29 µmol/L and 43 µmol/L | 84 µmol/L, 88 µmol/L, and 93 µmol/L | Between 35 µmol/L and 68 µmol/L |
| Urinary calcium/urinary creatinine (0.04–0.70 mmol/mmol) | - | - | - | 0.64 |
| Zoledronic Acid Dose Administration | Calcium (2.20–2.65 mmol/L) Before Zoledronic Acid Dose Administration | Calcium, Approximately 2 Days After Dose Administration | Ionized Calcium Corrected for pH (1.14–1.32 mmol/L) Before Zoledronic Acid Dose Administration | Ionized Calcium Corrected for pH (1.14–1.32 mmol/L) Approximately 2 Days After Zoledronic Acid Dose Administration |
|---|---|---|---|---|
| Day 1 | 3.17 mmol/L | - | 2.07 mmol/L | 1.42 mmol/L |
| Day 11 | 3.08 mmol/L | 3.17 mmol/L | 1.60 mmol/L | 1.84 mmol/L |
| Day 14 | 3.17 mmol/L | 2.77 mmol/L | 1.84 mmol/L | 1.64 mmol/L |
| Day 19 | 3.19 mmol/L | 3.04 mmol/L | 1.62 mmol/L | - |
| Date | Calcium (2.20–2.65 mmol/L) | Ionized Calcium Corrected for pH 7.4 (1.14–1.32 mmol/L) |
|---|---|---|
| Pre-prednisone | 3.04 mmol/L | 1.62 mmol/L |
| During prednisone | Between 2.28 mmol/L and 2.95 mmol/L | 1.32 mmol/L |
| Approximately one month post-prednisone | 2.62 mmol/L | 1.43 mmol/L |
| Approximately two months post-prednisone | 2.52 mmol/L | 1.33 mmol/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allain, L.; Elbaz, S.; Sathyakumar, S.; Le Gallou, W.; Coleman, C.; Coltin, H.; Eugène, D.; Gupta, A.; Lava, S.A.G.; Renzi, S. Significant Response to Denosumab Yet with Severe Rebound Hypercalcemia in a 9-Year-Old Boy with Aneurysmal Bone Cyst: A Case Report. Children 2025, 12, 1524. https://doi.org/10.3390/children12111524
Allain L, Elbaz S, Sathyakumar S, Le Gallou W, Coleman C, Coltin H, Eugène D, Gupta A, Lava SAG, Renzi S. Significant Response to Denosumab Yet with Severe Rebound Hypercalcemia in a 9-Year-Old Boy with Aneurysmal Bone Cyst: A Case Report. Children. 2025; 12(11):1524. https://doi.org/10.3390/children12111524
Chicago/Turabian StyleAllain, Laurence, Sarah Elbaz, Sayanthen Sathyakumar, William Le Gallou, Christina Coleman, Hallie Coltin, Dardye Eugène, Abha Gupta, Sebastiano A. G. Lava, and Samuele Renzi. 2025. "Significant Response to Denosumab Yet with Severe Rebound Hypercalcemia in a 9-Year-Old Boy with Aneurysmal Bone Cyst: A Case Report" Children 12, no. 11: 1524. https://doi.org/10.3390/children12111524
APA StyleAllain, L., Elbaz, S., Sathyakumar, S., Le Gallou, W., Coleman, C., Coltin, H., Eugène, D., Gupta, A., Lava, S. A. G., & Renzi, S. (2025). Significant Response to Denosumab Yet with Severe Rebound Hypercalcemia in a 9-Year-Old Boy with Aneurysmal Bone Cyst: A Case Report. Children, 12(11), 1524. https://doi.org/10.3390/children12111524







