Interplay Between VCAM-1 and PGE2 Levels and Autism Spectrum Disorder Severity in Children—A Preliminary Single-Center Analysis
Highlights
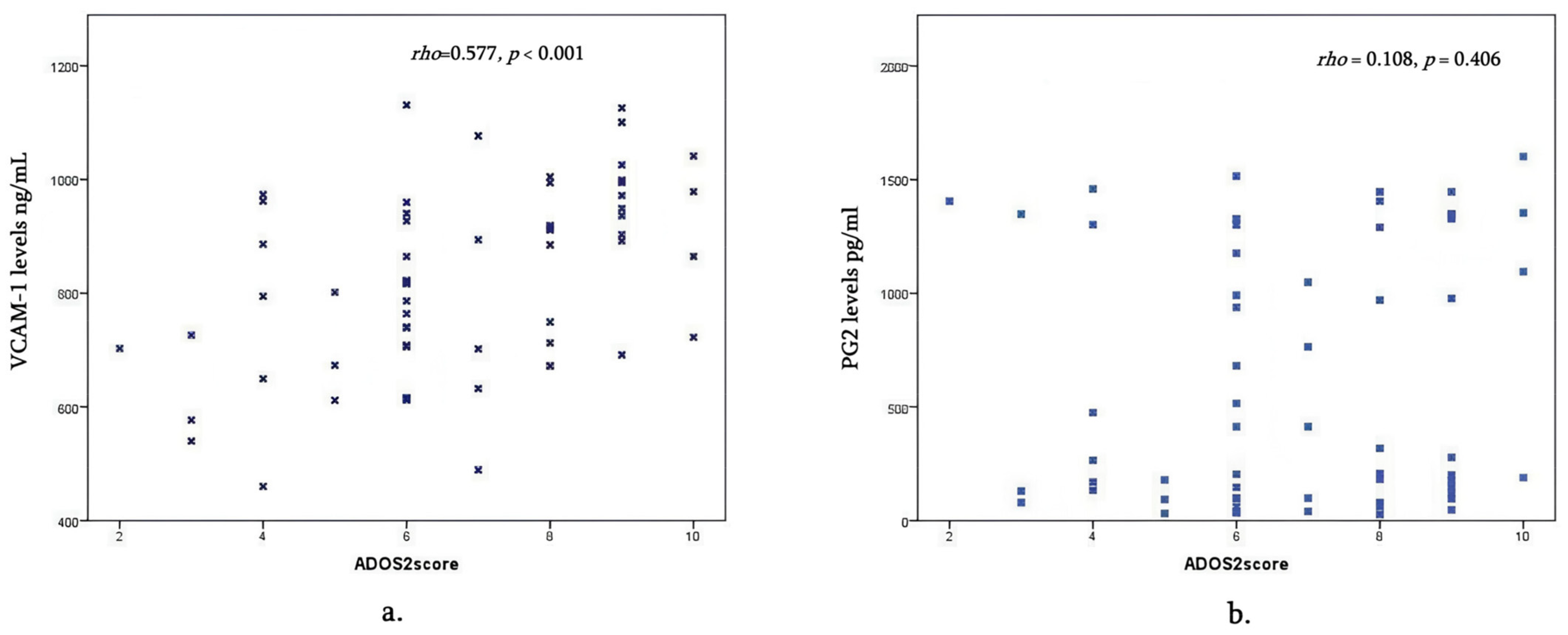
- VCAM-1 is higher in the moderate and severe ASD behavior group than in mild cases and declines with age; PGE2 did not differ between severity and age groups.
- Elevated VCAM-1 is linked to greater behavioral severity in ASD.
- VCAM-1 could be potential biomarker of ASD severity.
- Autism Spectrum Disorders (ASDs) may involve neuroimmune dysregulation.
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Investigations
2.3. Statistics
2.4. Ethics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism Spectrum Disorder |
| VCAM-1 | Vascular Adhesion Molecule |
| PGE 2 | Prostaglandin 2 |
| ADOS 2 | Autism Diagnostic Observation Schedule-2 |
| TNF-α | Tumor Necrosis Factor-α |
| IL | Interleukin |
| JNK | c-Jun N-terminal kinases |
| IL1RAPL1 | Interleukin-1 (IL-1) Receptor Accessory Protein-like 1 |
| NK | Natural Killer |
| CNS | Central Nervous System |
| BBB | Blood Brain Barrier |
| PECAM-1 | Platelet Endothelial Adhesion Molecule-1 |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| sVCAM-1 | Serum Vascular Adhesion Molecule-1 |
| sPECAM-1 | Soluble Platelet Endothelial Adhesion Molecule-1 |
| COX | Cyclooxygenase |
| COX-1 | Cyclooxygenase-1 |
| SCID | Structured Clinical Interview for DSMIV |
| ADI-R | Autism Diagnostic Interview-Revised |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; text revision; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Shaw, K.A.; Williams, S.; Patrick, M.E.; Valencia-Prado, M.; Durkin, M.S.; Howerton, E.M.; Ladd-Acosta, C.M.; Pas, E.T.; Bakian, A.V.; Bartholomew, P.; et al. Prevalence and Early Identification of Autism Spectrum Disorder Among Children Aged 4 and 8 Years—Autism and Developmental Disabilities Monitoring Network, 16 Sites, United States, 2022. Morb. Mortal. Wkly. Rep. 2025, 74, 1–22. Available online: https://www.cdc.gov/mmwr/volumes/74/ss/ss7402a1.htm (accessed on 28 September 2025). [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; text revision; American Psychiatric Publishing: Arlington, VA, USA, 2000. [Google Scholar]
- Blank, M.S.; Turner, J.B.; Fisher, P.W.; Guthrie, E.B.; Whitaker, A.H. The Need for a Clinically Useful Schema of Social Communication. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 1198–1200. [Google Scholar] [CrossRef] [PubMed]
- Onore, C.E.; Nordahl, C.W.; Young, G.S.; Van de Water, J.A.; Rogers, S.J.; Ashwood, P. Levels of soluble platelet endothelial cell adhesion molecule-1 and P-Selectin are decreased in children with autism spectrum disorder. Biol. Psychiatry 2012, 72, 1020–1025. [Google Scholar] [CrossRef]
- Atladottir, H.; Thorsen, P.; Ostergaard, L.; Schendel, D.E.; Lemcke, S.; Abdallah, M.; Parner, E.T. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2010, 40, 1423–1430. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef]
- Vergas, D.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Enstrom, A.M.; Van de Water, J.A.; Ashwood, P. Autoimmunity in autism. Curr. Opin. Investig. Drugs 2009, 10, 463–473. [Google Scholar]
- Chez, M.; Dowling, T.; Patel, P.; Khanna, P.; Kominsky, M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr. Neurol. 2007, 36, 361–365. [Google Scholar] [CrossRef]
- Singh, V.; Warren, R.; Odell, J.D.; Cole, P. Changes of soluble interleukin-2, interleukin -2 receptor, T8 antigen and interleukin-1 in the serum of autistic children. Clin. Immunol. Immunopathol. 1991, 61, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Carrie, A.; Jun, L.; Bienvenu, T.; Vinet, M.C.; McDonell, N.; Couvert, P.; Zemni, R.; Cardona, A.; Van Buggenhout, G.; Frints, S.; et al. A new member of the IL-1 receptor family highly expressed in hippocampus and involved in X-linked mental retardation. Nat. Genet. 1999, 23, 25–31. [Google Scholar] [CrossRef]
- Vojdani, A.; Campbell, E.; Anyanwu, E.; Kashanian, A.; Bock, K.; Vojdani, E. Antibodies to neuron-specific antigens in children with autism: Possible cross-reaction with encephalitogenic proteins from milk, Chlamydia pneumoniae and Streptococcus group A. J. Neuroimmunol. 2002, 129, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Singh, V. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J. Neuroimmunol. 1996, 66, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zou, H.; Sheikh, A.; Malik, M.; Dobkin, C.; Brown, W.T.; Li, X. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J. Neuroinflamm. 2011, 8, 52. [Google Scholar] [CrossRef]
- Xu, N.; Li, X.; Zhong, Y. Inflammatory cytokines: Potential biomarkers of immunologic dysfunction in Autism Spectrum Disorders. Mediat. Inflamm. 2015, 1, 531518. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Kameno, Y.; Iwata, K.; Matsuzaki, H.; Miyachi, T.; Tsuchiya, K.J.; Matsumoto, K.; Iwata, Y.; Suzuki, K.; Nakamura, K.; Maekawa, M.; et al. Serum levels of soluble platelet endothelial cell adhesion molecule-1 and vascular cell adhesion molecule-1 are decreased in subjects with autism spectrum disorder. Mol. Autism 2013, 7, 19. [Google Scholar] [CrossRef]
- Suzuki, K.; Matsuzaki, H.; Iwata, K.; Kameno, Y.; Shimmura, C.; Kawai, S.; Yoshihara, Y.; Wakuda, T.; Takebayashi, K.; Takagai, S.; et al. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS ONE 2011, 6, e20470. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Matsumoto, K.; Yagi, A.; Inada, N.; Kuroda, M.; Inokuchi, E.; Koyama, T.; Kamio, Y.; Tsujii, M.; Sakai, S.; et al. Reliability and validity of Autism Diagnostic Interview Revised, Japanese Version. J. Autism Dev. Disord. 2013, 43, 643–662. [Google Scholar] [CrossRef]
- Akaneya, Y. The remarkable mechanism of prostaglandin E2 on synaptic plasticity. Gene Regul. Syst. Biol. 2008, 1, 83–89. [Google Scholar] [CrossRef]
- Famitafreshi, H.; Karimian, M. Prostaglandins as the agents that modulate the course of Brain disorders. Degener. Neurol. Neuromuscul. Dis. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Brigandi, S.; Shao, H.; Qian, S.; Shen, Y.; Wu, B.-L.; Kang, J.X. Autistic children exhibit decreased levels of essentials fatty acids on red blood cells. Int. J. Mol. Sci. 2015, 16, 10061–10076. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Wu, C.; Wang, J.; Sun, M. Autism Spectrum Disorder: Neurodevelopmental Risk Factors, Biological Mechanism, and Precision Therapy. Int. J. Mol. Sci. 2023, 24, 1819. [Google Scholar] [CrossRef]
- Robinson-Agramonte, M.d.L.A.; García, E.N.; Guerra, J.F.; Hurtado, Y.V.; Antonucci, N.; Semprún-Hernández, N.; Schultz, S.; Siniscalco, D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int. J. Mol. Sci. 2022, 23, 3033. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Liu, S.; Luo, W.; Jiang, Y.; Gao, J. Association of Peripheral Blood Levels of Cytokines with Autism Spectrum Disorder: A Meta-Analysis. Front. Psychiatry 2021, 12, 670200. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.S.; Rajabi, P.; Sargolzaei, J.; Alaghmand, A. Correlation of biochemical markers and inflammatory cytokines in autism spectrum disorder (ASD). BMC Pediatr. 2024, 24, 696. [Google Scholar] [CrossRef] [PubMed]
- Andrýs, C.; Pozler, O.; Krejsek, J.; Derner, V.; Drahosová, M.; Kopecký, O. Serum soluble adhesion molecules (sICAM-1, sVCAM-1 and sE-selectin) in healthy school aged children and adults. Acta Med. 2000, 43, 103–106. [Google Scholar] [CrossRef]
- Bryn, V.; Aass, H.; Skjeldal, O.; Isaksen, J.; Saugstad, O.D.; Ormstad, H. Cytokine profile in autism spectrum disorders in children. J. Mol. Neurosci. 2017, 61, 1–7. [Google Scholar] [CrossRef]
- Napolioni, V.; Ober-Reynolds, B.; Szelinger, S.; Corneveaux, J.J.; Pawlowski, T.; Ober-Reynolds, S.; Kirwan, J.; Persico, A.M.; Melmed, R.D.; Craig, D.W.; et al. Plasma cytokine profiling in sibling pairs discordant for autism spectrum disorder. J. Neuroinflamm. 2013, 10, 38. [Google Scholar] [CrossRef]
- Ashwood, K.L.; Gillan, N.; Horder, J.; Hayward, H.; Woodhouse, E.; McEwen, F.S.; Findon, J.; Eklund, H.; Spain, D.; Wilson, C.E.; et al. Predicting the diagnosis of autism ain adults using the Autism-Spectrum Quotient (AQ) questionnaire. Psychol. Med. 2016, 46, 2595–2604. [Google Scholar] [CrossRef]
- Abdallah, M.; Larsen, N.; Mortensen, E.; Atladottir, H.; Nørgaard-Pedersen, B.; Bonefeld-Jørgensen, E.C.; Grove, J.; Hougaard, D.M. Neonatal levels of cytokines and risk of autism spectrum disorders: Anexploratory register-based historical birth cohort study utilizing the Danish Newborn Screening Biobank. J. Neuroimmunol. 2012, 252, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Fernandez, A.; de la Torre-Aguilar, M.J.; Gil-Campos, M.; Flores-Rojas, K.; Cruz-Rico, M.D.; Martin-Borreguero, P.; Perez-Navero, J.L. Children with Autism Spectrum Disorder with Regression Exhibit a Different Profile in Plasma Cytokines and Adhesion Molecules Compared to Children Without Such Regression. Front. Pediatr. 2018, 6, 264. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Iwata, Y.; Nakamura, K.; Tsujii, M.; Tsuchya, K.J.; Sekine, Y.; Suzuki, K.; Minabe, Y.; Takei, N.; Iyo, M.; et al. Reduced serum levels of brain-derived neurotrophic factor in adult male patients with autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 1529–1531. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Hornig, M.; Bresnahan, M.; Stoltenberg, C.; Magnus, P.; Surén, P.; Mjaaland, S.; Reichborn-Kjennerud, T.; Susser, E.; Lipkin, W.I. Maternal mid-gestational and child cord blood immune signatures are strongly associated with offspring risk of ASD. Mol. Psychiatry 2011, 27, 1527–1542. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 2011, 15, 1607–1638. [Google Scholar] [CrossRef]
- Madore, C.; Leyrolle, O.; Lacabanne, C.; Benmamar-Badel, A.; Joffre, C.; Nadjar, A.; Layé, S. Neuroinflammation in Autism: Plausible Role of Maternal Inflammation, Dietary Omega 3, and Microbiota. Neural Plast. 2016, 2016, 3597209. [Google Scholar] [CrossRef]
- El-Ansary, A.; Al-Ayadhi, L. Lipid mediators in plasma of autism spectrum disorders. Lipids Health Disord. 2012, 11, 160. [Google Scholar] [CrossRef]
- Than, U.T.T.; Nguyen, L.T.; Nguyen, P.H.; Nguyen, X.H.; Trinh, D.P.; Hoang, D.H.; Nguyen, P.A.T.; Dang, V.D. Inflammatory mediators drive neuroinflammation in autism spectrum disorder and cerebral palsy. Sci. Rep. 2023, 13, 22587. [Google Scholar] [CrossRef]
- El-Ansary, A.; Alhakbany, M.; Aldbass, A.; Qasem, H.; Al-Mazidi, S.; Bhat, R.S.; Al-Ayadhi, L. Alpha-Synuclein, cyclooxygenase-2 and prostaglandins-EP2 receptors as neuroinflammatory biomarkers of autism spectrum disorders: Use of combined ROC curves to increase their diagnostic value. Lipids Health Disord. 2021, 20, 155. [Google Scholar] [CrossRef]
- Wong, C.T.; Bestard-Lorigados, I.; Crawford, D.A. Autism-related behaviors in the cyclooxygenase-2-deficient mouse model. Genes, brain, and behavior. Genes Brain Behav. 2019, 18, e12506. [Google Scholar] [CrossRef]
- Kamal, D.; Abidin, S.; Saudi, W.S.W.; Kumar, J. Roles of Prostaglandins and Cyclooxygenases in Autism Spectrum Disorder: A comprehensive Review. Curr. Behav. Neurosci. Rep. 2025, 12, 2. [Google Scholar] [CrossRef]
- Yui, K.; Imataka, G.; Shiohama, T. Lipid Peroxidation via Regulating the Metabolism of Docosahexaenoic Acid and Arachidonic Acid in Autistic Behavioral Symptoms. Curr. Issues Mol. Biol. 2023, 45, 9149–9164. [Google Scholar] [CrossRef]
- Qasem, H.; Al-Ayadhi, L.; Bjørklund, G.; Chirumbolo, S.; El-Ansary, A. Impaired lipid metabolism markers to assess the risk of neuroinflammation in autism spectrum disorder. Metab. Brain Dis. 2018, 33, 1141–1153. [Google Scholar] [CrossRef]


| Age Group (Years) | VCAM-1 (Mean ± SE) | PGE2 (Mean ± SE) |
|---|---|---|
| 2–3 | 956.2 ± 38.9 | 683.2 ± 226.1 |
| 3–4 | 873.0 ± 57.6 | 577.5 ± 174.7 |
| 4–5 | 834.7 ± 42.2 | 609.3 ± 154.7 |
| 5–6 | 763.1 ± 29.9 | 643.3 ± 106.4 |
| Group | n | Mean VCAM-1 | SE | 95% CI (Lower–Upper) | SD | Skewness |
|---|---|---|---|---|---|---|
| Group 1 | 13 | 615.08 | 51.50 | 502.88–727.29 | 185.68 | 0.88 |
| Group 2 | 22 | 789.05 | 33.63 | 719.12–858.99 | 157.74 | 0.37 |
| Group 3 | 26 | 912.41 | 26.45 | 857.94–966.89 | 134.87 | −0.50 |
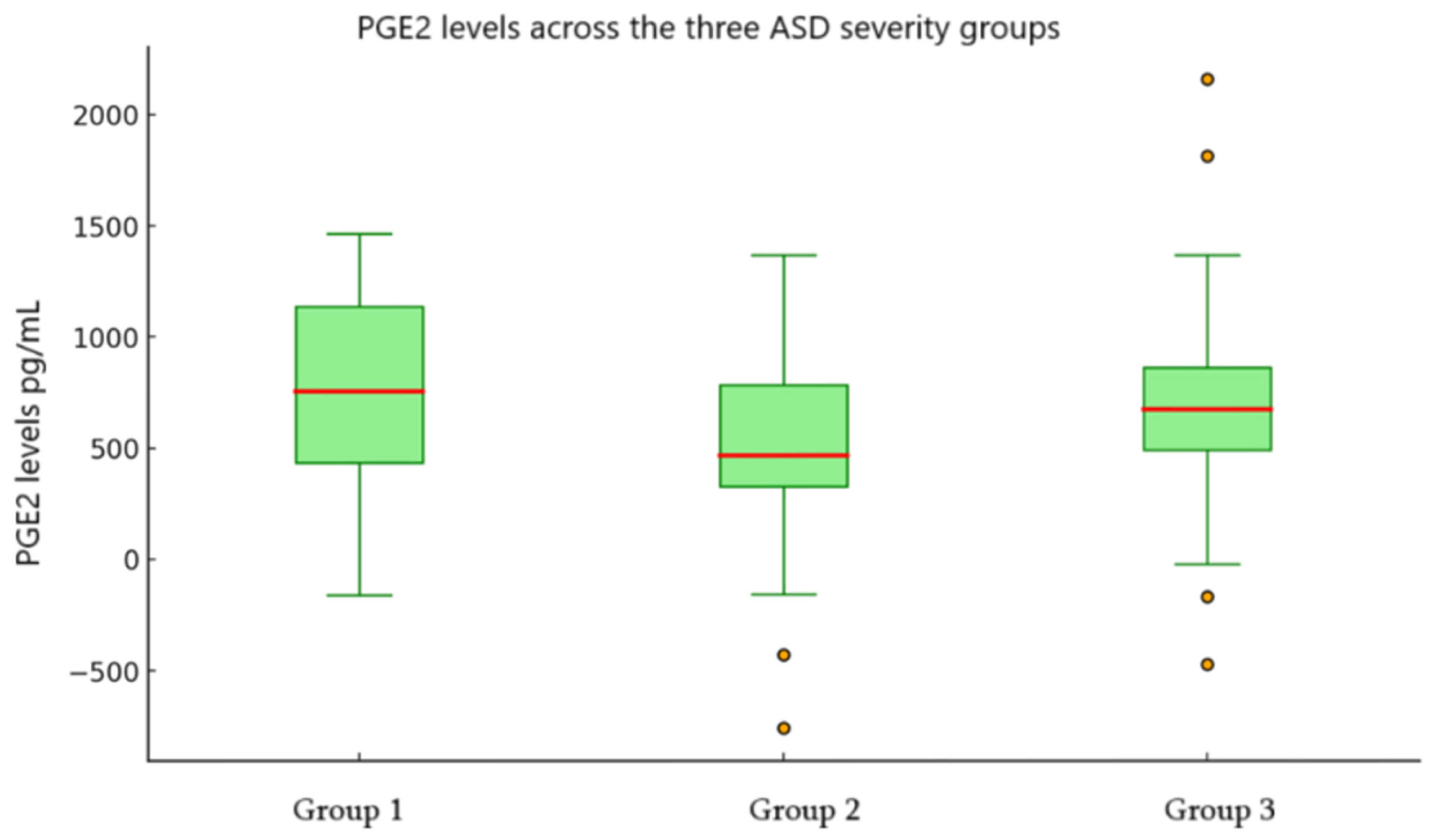
| Group | Mean PGE2 | SE | 95% CI (Lower–Upper) | SD | Skewness |
|---|---|---|---|---|---|
| Group 1 | 543.90 | 163.56 | 187.53–900.27 | 589.73 | 0.85 |
| Group 2 | 601.48 | 110.53 | 371.63–831.34 | 518.42 | 0.38 |
| Group 3 | 681.62 | 117.68 | 439.25–924.00 | 600.07 | 0.26 |
| Comparison | n | U Value | Z Score | Mean Rank | p-Value |
|---|---|---|---|---|---|
| Group 1 vs. Group 2 | 35 | 61.00 | −2.80 | 11.69 vs. 21.73 | 0.005 |
| Group 1 vs. Group 3 | 39 | 36.00 | −3.96 | 9.77 vs. 25.12 | <0.001 |
| Group 2 vs. Group 3 | 48 | 155.50 | −2.70 | 18.57 vs. 29.52 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natroshvili, I.; Gakharia, T.; Bakhtadze, S.; Natsvlishvili, T.; Khachapuridze, N. Interplay Between VCAM-1 and PGE2 Levels and Autism Spectrum Disorder Severity in Children—A Preliminary Single-Center Analysis. Children 2025, 12, 1488. https://doi.org/10.3390/children12111488
Natroshvili I, Gakharia T, Bakhtadze S, Natsvlishvili T, Khachapuridze N. Interplay Between VCAM-1 and PGE2 Levels and Autism Spectrum Disorder Severity in Children—A Preliminary Single-Center Analysis. Children. 2025; 12(11):1488. https://doi.org/10.3390/children12111488
Chicago/Turabian StyleNatroshvili, Irakli, Tatia Gakharia, Sophia Bakhtadze, Tamar Natsvlishvili, and Nana Khachapuridze. 2025. "Interplay Between VCAM-1 and PGE2 Levels and Autism Spectrum Disorder Severity in Children—A Preliminary Single-Center Analysis" Children 12, no. 11: 1488. https://doi.org/10.3390/children12111488
APA StyleNatroshvili, I., Gakharia, T., Bakhtadze, S., Natsvlishvili, T., & Khachapuridze, N. (2025). Interplay Between VCAM-1 and PGE2 Levels and Autism Spectrum Disorder Severity in Children—A Preliminary Single-Center Analysis. Children, 12(11), 1488. https://doi.org/10.3390/children12111488






