Perioperative Anesthetic Management in Pediatric Scoliosis Surgery: A Narrative Review with Focus on Neuromuscular Disorders
Highlights
- Individualized anesthetic planning with total intravenous anesthesia and blood conservation is essential in neuromuscular scoliosis surgery.
- Implementing Enhanced Recovery After Surgery principles facilitates faster recovery and reduces complications in this high-risk population.
- A multidisciplinary, evidence-based approach improves perioperative outcomes.
- Standardized protocols enhance safety and recovery in pediatric neuromuscular scoliosis surgery.
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Scope
2.2. Data Sources and Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection and Yield
2.5. Quality Considerations and Evidence Weighting
- (i)
- Society guidance (SRS, POSNA, anesthesia societies) and multicenter/prospective pediatric cohorts were weighted more heavily than single-center descriptive series;
- (ii)
- Age- and diagnosis-specific applicability (pediatric NMS over adolescent idiopathic scoliosis (AIS)/adult data) was prioritized;
- (iii)
- Recency, sample size, and presence of comparators informed interpretation when recommendations conflicted.
2.6. Data Extraction and Synthesis
2.7. Ethics
3. Preoperative Assessment and Optimization
3.1. Anesthetic Pre-Assessment
3.2. Pulmonary Evaluation
3.3. Respiratory Optimization
3.4. Cardiac Evaluation
3.5. Nutritional Optimization and Aspiration Risk
3.6. Psychological Preparation and Family Communication
3.7. Blood Management Planning
3.8. Venous Thromboembolism (VTE) Risk and Prophylaxis
3.9. Premedication and Anxiolysis
4. Surgical Planning and Deformity Preparation
4.1. Preoperative Imaging and Navigation
4.2. Halo-Gravity Traction (HGT) for Severe, Rigid Curves
4.3. Implications for Anesthesia and Team
5. Anesthetic Techniques and Intraoperative Considerations
5.1. Intraoperative Respiratory and Positioning Management in NMS
5.2. IONM
5.3. Perioperative Monitoring
5.4. Perioperative Temperature Management
5.5. Blood Loss in Scoliosis Surgery
6. Perioperative Complications
6.1. Surgical Site and Neurological Complications
6.2. Gastrointestinal Complications
6.3. Hemorrhagic Complications
6.4. Pulmonary Complications
6.5. Summary and Risk Stratification
7. Postoperative Care and Complication Management
7.1. Pain Management
7.2. Respiratory and Hemodynamic Support
7.3. Nutrition and Monitoring
7.4. Complication Surveillance
7.5. Enhanced Recovery and Family Involvement
- A respiratory pathway with planned postoperative NIV, cough-assist devices, early airway clearance, and nocturnal gas-exchange surveillance (e.g., SMA, advanced DMD).
- A cardiac-first plan in DMD/Becker Muscular Dystrophy (BMD), with continuous ECG monitoring or telemetry and cautious fluid targets.
- Agent selection aligned with disease biology and IONM requirements—notably the absolute avoidance of succinylcholine and volatile anesthetics in DMD/BMD, with TIVA as standard.
- Strict latex-free processes for myelomeningocele and other high-risk groups.
- Individualized VTE prophylaxis, prioritizing mechanical measures and aligning low-molecular-weight heparin use with neuraxial management decisions.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIS | Adolescent Idiopathic Scoliosis |
| BMD | Becker Muscular Dystrophy |
| CP | Cerebral Palsy |
| CT | Computed Tomography |
| CVC | Central Venous Catheter |
| DMD | Duchenne Muscular Dystrophy |
| EBV | Estimated Blood Volume |
| ECG | Electrocardiography |
| ERAS | Enhanced Recovery After Surgery |
| ESPB | Erector Spinae Plane Block |
| FVC | Forced Vital Capacity |
| GMFCS | Gross Motor Function Classification System |
| IONM | Intraoperative Neurophysiological Monitoring |
| IV (PIV) | Peripheral Intravenous (catheter) |
| LMWH | Low-Molecular-Weight Heparin |
| MAP | Mean Arterial Pressure |
| MEP | Motor Evoked Potential |
| MMC | Myelomeningocele (Spina Bifida) |
| NDNMB | Non-Depolarizing Neuromuscular Blocker |
| NIV | Non-Invasive Ventilation |
| NMB | Neuromuscular Blocker |
| NMS | Neuromuscular Scoliosis |
| NSAID | Non-Steroidal Anti-Inflammatory Drug |
| OR | Operating Room |
| PBW | Predicted Body Weight |
| PEEP | Positive End-Expiratory Pressure |
| PICU | Pediatric Intensive Care Unit |
| SMA | Spinal Muscular Atrophy |
| SSEP | Somatosensory Evoked Potential |
| TCI | Target-Controlled Infusion |
| TIVA | Total Intravenous Anesthesia |
| TOF | Train-of-Four |
| TXA | Tranexamic Acid |
| UTI | Urinary Tract Infection |
| VTE | Venous Thromboembolism |
| VT | Tidal Volume |
References
- Wang, L.; Du, Y.; Huang, N.; Yin, N.; Du, J.; Yang, J.; Jiang, L.; Mao, Y. Clinical characteristics and anaesthetic management of severe scoliosis patients with spinal muscular atrophy: Case series. Ann. Med. Surg. 2024, 86, 643–649. [Google Scholar] [CrossRef]
- Murphy, R.F.; Mooney, J.F. Current concepts in neuromuscular scoliosis. Curr. Rev. Musculoskelet. Med. 2019, 12, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Cloake, T.; Gardner, A. The management of scoliosis in children with cerebral palsy: A review. J. Spine Surg. 2016, 2, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.J.; de Gonorazky, H.D.; Cohn, R.D.; Campbell, C. Treating pediatric neuromuscular disorders: The future is now. Am. J. Med. Genet. A 2018, 176, 804–841. [Google Scholar] [CrossRef] [PubMed]
- Rummey, C.; Flynn, J.M.; Corben, L.A.; Delatycki, M.B.; Wilmot, G.; Subramony, S.H.; Bushara, K.; Duquette, A.; Gomez, C.M.; Hoyle, J.C.; et al. Scoliosis in Friedreich’s ataxia: Longitudinal characterization in a large heterogeneous cohort. Ann. Clin. Transl. Neurol. 2021, 8, 1239–1250. [Google Scholar] [CrossRef]
- Hägglund, G.; Pettersson, K.; Czuba, T.; Persson-Bunke, M.; Rodby-Bousquet, E. Incidence of scoliosis in cerebral palsy: A population-based study of 962 young individuals. Acta Orthop. 2018, 89, 443–447. [Google Scholar] [CrossRef]
- Pettersson, K.; Wagner, P.; Rodby-Bousquet, E. Development of a risk score for scoliosis in children with cerebral palsy. Acta Orthop. 2020, 91, 203–208. [Google Scholar] [CrossRef]
- Ciftci, S.; Ulusaloglu, A.C.; Shrader, M.W.; Scavina, M.T.; Mackenzie, W.G.; Heinle, R.; Neal, K.M.; Stall, A.; Howard, J.J. Scoliosis development in spinal muscular atrophy: The influences of genetic severity, functional level, and disease-modifying treatments. J. Pediatr. Orthop. 2024; ahead of print. [Google Scholar] [CrossRef]
- Ruythooren, F.; Moens, P. Spinal muscular atrophy scoliosis in the era of background therapies—A review of the literature. J. Clin. Med. 2024, 13, 3467. [Google Scholar] [CrossRef]
- Archer, J.E.; Gardner, A.; Roper, H.P.; Chikermane, A.A.; Tatman, A.J. Duchenne muscular dystrophy: The management of scoliosis. J. Spine Surg. 2016, 2, 185–194. [Google Scholar] [CrossRef]
- Scoliosis Research Society (SRS). Informational & Position Statements (Quality & Safety Resource Library). Scoliosis Research Society. Available online: https://www.srs.org/Education/Quality-and-Safety/Informational--Position-Statements (accessed on 15 September 2025).
- Fletcher, N.D.; Ghag, R.; Hedequist, D.J.; Imrie, M.N.; Bennett, J.T.; Glotzbecker, M.P.; POSNA QSVI Spine Committee. Perioperative blood pressure management for patients undergoing spinal fusion for pediatric spinal deformity: Current concept review. J. Pediatr. Orthop. Soc. N. Am. 2023, 5, 602. [Google Scholar] [CrossRef]
- Welborn, M.C.; Redding, G.; Evers, P.; Nicol, L.; Bauer, D.F.; Iyer, R.R.; Poon, S.; Hwang, S. Pre-op considerations in neuromuscular scoliosis deformity surgery: Proceedings of the half-day course at the 58th annual meeting of the Scoliosis Research Society. Spine Deform. 2024, 12, 867–876. [Google Scholar] [CrossRef]
- Vitale, M.; Roye, B.; Bloom, Z.; Kunes, J.; Matsumoto, H.; Roye, D.; Farrington, D.; Flynn, J.; Halanski, M.; Hasler, C.; et al. Best practices for the orthopaedic care of children with spinal muscular atrophy: A consensus statement from the European Neuromuscular Centre Standard of Care Orthopaedic Working Group. J. Pediatr. Orthop. Soc. N. Am. 2022, 4, 296. [Google Scholar] [CrossRef]
- Hudec, J.; Prokopová, T.; Kosinová, M.; Gál, R. Anesthesia and perioperative management for surgical correction of neuromuscular scoliosis in children: A narrative review. J. Clin. Med. 2023, 12, 3651. [Google Scholar] [CrossRef] [PubMed]
- Sedra, F.; Shafafy, R.; Sadek, A.-R.; Aftab, S.; Montgomery, A.; Nadarajah, R. Perioperative Optimization of Patients with Neuromuscular Disorders Undergoing Scoliosis Corrective Surgery: A Multidisciplinary Team Approach. Glob. Spine J. 2021, 11, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Khirani, S.; Bersanini, C.; Aubertin, G.; Bachy, M.; Vialle, R.; Fauroux, B. Non-Invasive positive pressure ventilation to facilitate the post-operative respiratory outcome of spine surgery in neuromuscular children. Eur. Spine J. 2014, 23 (Suppl. S4), S406–S411. [Google Scholar] [CrossRef] [PubMed]
- Chatwin, M.; Wakeman, R.H. Mechanical Insufflation–Exsufflation: Considerations for Improving Clinical Practice. J. Clin. Med. 2023, 12, 2626. [Google Scholar] [CrossRef]
- Willis, L.D. 2022 Year in Review: Mechanical Insufflation–Exsufflation. Respir. Care 2023, 68, 275–283. [Google Scholar] [CrossRef]
- Buddhe, S.; Cripe, L.; Friedland-Little, J.; Kertesz, N.; Eghtesady, P.; Finder, J.; Hor, K.; Judge, D.P.; Kinnett, K.; McNally, E.M.; et al. Cardiac Management of the Patient with Duchenne Muscular Dystrophy. Pediatrics 2018, 142 (Suppl. S2), S72–S81. [Google Scholar] [CrossRef]
- OrphanAnesthesia. Duchenne Muscular Dystrophy—Anaesthesia Recommendations. Available online: https://www.orphananesthesia.eu (accessed on 15 September 2024).
- Naume, M.M.; Hoei-Hansen, C.E.; Born, A.P.; Brekke, G.; Høj, A.; Nielsen, M.R.; Borgwardt, L.; Vissing, J.; Dirks, J.; Rye, A.K.S.; et al. A Prospective Study on the Feasibility and Effect of an Optimized Perioperative Care Protocol in Pediatric Neuromuscular Scoliosis Surgery. J. Clin. Med. 2024, 13, 7848. [Google Scholar] [CrossRef]
- Mustafa, M.S.; Shafique, M.A.; Zaidi, S.D.E.Z.; Qamber, A.; Rangwala, B.S.; Ahmed, A.; Zaidi, S.M.F.; Rangwala, H.S.; Uddin, M.M.N.; Ali, M.; et al. Preoperative anxiety management in pediatric patients: A systematic review and meta-analysis of randomized controlled trials on the efficacy of distraction techniques. Front. Pediatr. 2024, 12, 1353508. [Google Scholar] [CrossRef]
- Johnson, D.J.; Johnson, C.C.; Goobie, S.M.; Nami, N.; Wetzler, J.A.; Sponseller, P.D.; Frank, S.M. High-dose Versus Low-dose Tranexamic Acid to Reduce Transfusion Requirements in Pediatric Scoliosis Surgery. J. Pediatr. Orthop. 2017, 37, e552–e557. [Google Scholar] [CrossRef]
- Shrestha, I.K.; Ruan, T.-Y.; Lin, L.; Tan, M.; Na, X.-Q.; Qu, Q.-C.; Chen, J.-C.; Si, Y.-Y.; Tao, J.-P. The efficacy and safety of high-dose tranexamic acid in adolescent idiopathic scoliosis: A meta-analysis. J. Orthop. Surg. Res. 2021, 16, 53. [Google Scholar] [CrossRef]
- Oetgen, M.E.; Litrenta, J. Perioperative blood management in pediatric spine surgery. J. Am. Acad. Orthop. Surg. 2017, 25, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Ahlers, C.G.; Lan, M.; Schoenecker, J.G.; Borst, A.J. Blood Loss and Transfusion in a Pediatric Scoliosis Surgery Cohort in the Antifibrinolytic Era. J. Pediatr. Hematol. Oncol. 2022, 44, e701–e706. [Google Scholar] [CrossRef] [PubMed]
- ICM-VTE Pediatric Delegates. Recommendations from the ICM-VTE: Pediatric. J. Bone Joint Surg. Am. 2022, 104 (Suppl. S1), 238–251. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Zhang, W.; Peng, Y.; Liang, A.; Du, K.; Huang, D. Use of computed tomographic reconstruction to establish the ideal entry point for pedicle screws in idiopathic scoliosis. Eur. Spine J. 2012, 21, 23–30. [Google Scholar] [CrossRef]
- Linden, G.S.; Ghessese, S.; Cook, D.; Hedequist, D.J. Pedicle Screw Placement in Adolescent Idiopathic Scoliosis: A Comparison between Robotics Coupled with Navigation versus the Freehand Technique. Sensors 2022, 22, 5204. [Google Scholar] [CrossRef]
- Baky, F.J.; Milbrandt, T.; Echternacht, S.; Stans, A.A.; Shaughnessy, W.J.; Larson, A.N. Intraoperative Computed Tomography-Guided Navigation for Pediatric Spine Patients Reduced Return to Operating Room for Screw Malposition Compared with Freehand/Fluoroscopic Techniques. Spine Deform. 2019, 7, 577–581. [Google Scholar] [CrossRef]
- LaValva, S.M.; Pahys, J.M.; Garg, S.; Bumpass, D.B.; Sucato, D.J.; Kelly, M.P.; Lenke, L.G.; Gupta, M.C.; Sponseller, P.D.; Boachie-Adjei, O.; et al. Preoperative Halo-Gravity Traction for Severe Pediatric Spinal Deformity: Can It Replace a Vertebral Column Resection? J. Pediatr. Orthop. Soc. N. Am. 2023, 5, e496. [Google Scholar] [CrossRef]
- Nemani, V.M.; Kim, H.J.; Bjerke-Kroll, B.T.; Yagi, M.; Sacramento-Dominguez, C.; Akoto, H.; Papadopoulos, E.C.; Sanchez-Perez-Grueso, F.; Pellise, F.; Nguyen, J.T.; et al. Preoperative halo-gravity traction for severe spinal deformities at an SRS-GOP site in West Africa: Protocols, complications, and results. Spine 2015, 40, 153–161. [Google Scholar] [CrossRef]
- Garabekyan, T.; Hosseinzadeh, P.; Iwinski, H.J.; Muchow, R.D.; Talwalkar, V.R.; Walker, J.; Milbrandt, T.A. The results of preoperative halo-gravity traction in children with severe spinal deformity. J. Pediatr. Orthop. B 2014, 23, 1–5. [Google Scholar] [CrossRef]
- Popescu, M.B.; Ulici, A.; Carp, M.; Haram, O.; Ionescu, N.S. The use and complications of halo-gravity traction in children with scoliosis. Children 2022, 9, 1701. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Y.; Qi, L.; Wu, S.; Li, J.; Wang, Y.; Jiang, B. Does preoperative halo-gravity traction reduce the degree of deformity and improve pulmonary function in severe scoliosis patients with pulmonary insufficiency? A systematic review and meta-analysis. Front. Med. 2021, 8, 767238. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hai, Y.; Han, B.; Zhou, L.; Zhang, Y. Preoperative halo-gravity traction combined with one-stage posterior spinal fusion surgery following for severe rigid scoliosis with pulmonary dysfunction: A cohort study. BMC Surg. 2024, 24, 286. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.L.; Ramo, B.S.; Johnston, C.E. Halo gravity traction for severe pediatric spinal deformity: A clinical concepts review. Spine Deform. 2019, 7, 395–403. [Google Scholar] [CrossRef]
- Antolovich, G.C.; Cooper, M.S.; Johnson, M.B.; Lundine, K.; Yang, Y.; Frayman, K.; Vandeleur, M.; Sutherland, I.; Peachey, D.; Gadish, T.; et al. Perioperative Care of Children with Severe Neurological Impairment and Neuromuscular Scoliosis—A Practical Pathway to Optimize Perioperative Health and Guide Decision Making. J. Clin. Med. 2022, 11, 6769. [Google Scholar] [CrossRef]
- Children’s Mercy Kansas City. Neuromuscular Spinal Fusion Enhanced Recovery After Surgery (ERAS) Pathway: Synopsis. 2025. Available online: https://www.childrensmercy.org/siteassets/media-documents-for-depts-section/documents-for-health-care-providers/block-clinical-practice-guidelines/mobileview/neuromuscular-spinal-fusion-synopsis.pdf (accessed on 16 September 2025).
- Elmeshneb, M.A.; Hassanin, M.A.; Elnady, B.; Sleem, A.; Le, G.T.; Patel, M.S.; Quraishi, N.A. Surgical complications in neuromuscular scoliosis surgery: Systematic review and meta-analysis of the last ten years. Eur. Spine J. 2024, 33, 2666–2676. [Google Scholar] [CrossRef]
- Kwee, M.M.; Ho, Y.H.; Rozen, W.M. The prone position during surgery and its complications: A systematic review and evidence-based guidelines. Int. Surg. 2015, 100, 292–303. [Google Scholar] [CrossRef]
- Afrasinei, M.; Greaney, D. Face monitoring during prone position. Paediatr. Anaesth. 2022, 32, 486–487. [Google Scholar] [CrossRef]
- Shen, Y.; Drum, M.; Roth, S. The prevalence of perioperative visual loss in the United States: A 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth. Analg. 2009, 109, 1534–1545. [Google Scholar] [CrossRef]
- Nickels, T.J.; Manlapaz, M.R.; Farag, E. Perioperative visual loss after spine surgery. World J. Orthop. 2014, 5, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.A.; Roth, S.; Posner, K.L.; Cheney, F.W.; Caplan, R.A.; Newman, N.J.; Domino, K.B. The American Society of Anesthesiologists Postoperative Visual Loss Registry: Analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology 2006, 105, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, L.; Ghosh, S.; Dillon, D.; Palumbo, L.; Woodland, P.; Thalayasingam, P.; Lethbridge, M. Intraoperative neurophysiology monitoring in scoliosis surgery in children. Clin. Neurophysiol. Pract. 2019, 4, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tsirikos, A.I.; Duckworth, A.D.; Henderson, L.E.; Michaelson, C. Multimodal intraoperative spinal cord monitoring during spinal deformity surgery: Efficacy, diagnostic characteristics, and algorithm development. Med. Princ. Pract. 2020, 29, 6–17. [Google Scholar] [CrossRef]
- Zuccaro, M.; Zuccaro, J.; Samdani, A.F.; Pahys, J.M.; Hwang, S.W. Intraoperative neuromonitoring alerts in a pediatric deformity center. Neurosurg. Focus 2017, 43, E8. [Google Scholar] [CrossRef]
- Walker, C.T.; Kim, H.J.; Park, P.; Lenke, L.G.; Weller, M.A.; Smith, J.S.; Nemergut, E.C.; Sciubba, D.M.; Wang, M.Y.; Shaffrey, C.; et al. Neuroanesthesia guidelines for optimizing transcranial motor evoked potential neuromonitoring during deformity and complex spinal surgery: A Delphi consensus study. Spine 2020, 45, 911–920. [Google Scholar] [CrossRef]
- Alkhatip, A.A.A.M.; Mills, K.E.; Hogue, O.; Sallam, A.; Hamza, M.K.; Farag, E.; Yassin, H.M.; Wagih, M.; Ahmed, A.M.I.; Helmy, M.H.; et al. The effects of dexmedetomidine on intraoperative neurophysiologic monitoring modalities during corrective scoliosis surgery in pediatric patients: A systematic review. Paediatr. Anaesth. 2024, 34, 112–120. [Google Scholar] [CrossRef]
- Nakahari, H.; Wilton, N.C.T.; Kojima, T. Anesthesia management of neonates and infants requiring intraoperative neurophysiological monitoring: A concise review. Paediatr. Anaesth. 2023, 33, 526–531. [Google Scholar] [CrossRef]
- Okamura, M.; Saito, W.; Miyagi, M.; Shirasawa, E.; Imura, T.; Nakazawa, T.; Mimura, Y.; Yokozeki, Y.; Kuroda, A.; Kawakubo, A.; et al. Incidence of unintentional intraoperative hypothermia in pediatric scoliosis surgery and associated preoperative risk factors. Spine Surg. Relat. Res. 2021, 5, 154–159. [Google Scholar] [CrossRef]
- Lai, L.L.; See, M.H.; Rampal, S.; Ng, K.S.; Chan, L. Significant factors influencing inadvertent hypothermia in pediatric anesthesia. J. Clin. Monit. Comput. 2019, 33, 1105–1112. [Google Scholar] [CrossRef]
- Vrbica, K.; Hudec, J.; Hrdy, O.; Galko, M.; Horalkova, H.; Demlova, R.; Kubelova, M.; Repko, M.; Gal, R. Effect of prophylactic fibrinogen concentrate in scoliosis surgery (EFISS): A study protocol of two-arm, randomised trial. BMJ Open 2023, 13, e071547. [Google Scholar] [CrossRef]
- Carson, J.L.; Stanworth, S.J.; Guyatt, G.; Valentine, S.; Dennis, J.; Bakhtary, S.; Cohn, C.S.; Dubon, A.; Grossman, B.J.; Gupta, G.K.; et al. Red blood cell transfusion: 2023 AABB international guidelines. JAMA 2023, 330, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.S.; Yunus, S.N.; Ng, C.C.; Chan, C.Y.W.; Chiu, C.K.; Kwan, M.K. Tranexamic acid in pediatric scoliosis surgery: A prospective randomized trial comparing high-dose and low-dose tranexamic acid in adolescent idiopathic scoliosis undergoing posterior spinal fusion surgery. Spine 2021, 46, E1170–E1177. [Google Scholar] [CrossRef] [PubMed]
- Dhawale, A.A.; Shah, S.A.; Sponseller, P.D.; Bastrom, T.; Neiss, G.; Yorgova, P.; Newton, P.O.; Yaszay, B.; Abel, M.F.; Shufflebarger, H.; et al. Are antifibrinolytics helpful in decreasing blood loss and transfusions during spinal fusion surgery in children with cerebral palsy scoliosis? Spine (Phila Pa 1976) 2012, 37, E549–E555. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-H.; Lin, S.-Y.; Wu, M.-H.; Tien, Y.-C.; Jong, Y.-J.; Liang, W.-C.; Lu, Y.-M.; Shih, C.-L.; Lu, C.-C. Intravenous tranexamic acid reduces blood loss and transfusion volume in scoliosis surgery for spinal muscular atrophy: Results of a 20-year retrospective analysis. Int. J. Environ. Res. Public Health 2021, 18, 9959. [Google Scholar] [CrossRef]
- Murphy, R.F.; Mooney, J.F., III. Complications following spine fusion for adolescent idiopathic scoliosis. Curr. Rev. Musculoskelet. Med. 2016, 9, 462–469. [Google Scholar] [CrossRef]
- Rudic, T.N.; Althoff, A.D.; Kamalapathy, P.; Bachmann, K.R. Surgical site infection after primary spinal fusion surgery for adolescent idiopathic scoliosis: An analysis of risk factors from a nationwide insurance database. Spine (Phila Pa 1976) 2023, 48, E101–E106. [Google Scholar] [CrossRef]
- Novy, J.; Carruzzo, A.; Maeder, P.; Bogousslavsky, J. Spinal cord ischemia: Clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch. Neurol. 2006, 63, 1113–1120. [Google Scholar] [CrossRef]
- Rokitansky, C. Handbuch der Pathologischen Anatomie; Braumüller & Seidel: Wien, Austria, 1842; Volume 3. [Google Scholar]
- Bernotavičius, G.; Saniukas, K.; Karmonaitė, I.; Zagorskis, R. Superior mesenteric artery syndrome. Acta Med. Litu. 2016, 23, 155–164. [Google Scholar] [CrossRef]
- Altiok, H.; Lubicky, J.P.; DeWald, C.J.; Herman, J.E. The superior mesenteric artery syndrome in patients with spinal deformity. Spine (Phila Pa 1976) 2005, 30, 2164–2170. [Google Scholar] [CrossRef]
- Shapiro, F.; Zurakowski, D.; Sethna, N.F. Tranexamic acid diminishes intraoperative blood loss and transfusion in spinal fusions for Duchenne muscular dystrophy scoliosis. Spine (Phila Pa 1976) 2007, 32, 2278–2283. [Google Scholar] [CrossRef] [PubMed]
- Al-Iede, M.M.; Al-Zayadneh, E.; Bridge, C.; Alqutawneh, B.; Waters, K. Risk factors for postoperative pulmonary complications in children with severely compromised pulmonary function secondary to severe scoliosis. Pediatr. Pulmonol. 2020, 55, 2782–2790. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohrej, O.A.; Aldakhil, S.S.; Al-Rabiah, M.A.; Al-Rabiah, A.M. Surgical treatment of adolescent idiopathic scoliosis: Complications. Ann. Med. Surg. 2020, 52, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Reames, D.L.; Smith, J.S.; Fu, K.M.G.; Polly, D.W., Jr.; Ames, C.P.; Berven, S.H.; Perra, J.H.; Glassman, S.D.; McCarthy, R.E.; Knapp, R.D., Jr.; et al. Complications in the surgical treatment of 19,360 cases of pediatric scoliosis: A review of the Scoliosis Research Society Morbidity and Mortality database. Spine 2011, 36, 1484–1491. [Google Scholar] [CrossRef]
- Sharma, S.; Wu, C.; Andersen, T.; Wang, Y.; Hansen, E.S.; Bünger, C.E. Prevalence of complications in neuromuscular scoliosis surgery: A literature meta-analysis from the past 15 years. Eur. Spine J. 2013, 22, 1230–1249. [Google Scholar] [CrossRef]
- Yin, S.; Tao, H.; Du, H.; Feng, C.; Yang, Y.; Yang, W.; Duan, C. Postoperative pulmonary complications following posterior spinal instrumentation and fusion for congenital scoliosis. PLoS ONE 2018, 13, e0207657. [Google Scholar] [CrossRef]
- Gabel, B.C.; Schnell, E.C.; Dettori, J.R.; Jeyamohan, S.; Oskouian, R. Pulmonary complications following thoracic spinal surgery: A systematic review. Glob. Spine J. 2016, 6, 296–303. [Google Scholar] [CrossRef]
- Ma, L.; Yu, X.; Zhang, J.; Shen, J.; Zhao, Y.; Li, S.; Huang, Y. Risk factors of postoperative pulmonary complications after primary posterior fusion and hemivertebra resection in congenital scoliosis patients younger than 10 years old: A retrospective study. BMC Musculoskelet. Disord. 2022, 23, 89. [Google Scholar] [CrossRef]
- Monagle, P.; Cuello, C.A.; Augustine, C.; Bonduel, M.; Brandão, L.R.; Capman, T.; Chan, A.K.C.; Hanson, S.; Male, C.; Meerpohl, J.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Treatment of pediatric venous thromboembolism. Blood Adv. 2018, 2, 3292–3316. [Google Scholar] [CrossRef]
- Mulpuri, N.; Sanborn, R.M.; Pradhan, P.; Miller, P.E.; Canizares, M.F.; Shore, B.J. Pediatric orthopaedic venous thromboembo-lism: A systematic review investigating incidence, risk factors, and outcome. JBJS Open Access 2024, 9, e23.00107. [Google Scholar] [CrossRef]
- Seki, H.; Ideno, S.; Ishihara, T.; Watanabe, K.; Matsumoto, M.; Morisaki, H. Postoperative pain management in patients undergoing posterior spinal fusion for adolescent idiopathic scoliosis: A narrative review. Scoliosis Spinal Disord. 2018, 13, 17. [Google Scholar] [CrossRef]
- Chin, K.J.; Dinsmore, M.J.; Lewis, S.; Chan, V. Opioid-sparing multimodal analgesia with bilateral bi-level erector spinae plane blocks in scoliosis surgery: A case report of two patients. Eur. Spine J. 2020, 29, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hong, R.A.; Robbins, C.B.; Gibbons, K.M.; Holman, A.E.; Caird, M.S.; Farley, F.A.; Abbott, M.D.; Burke, M.C. Intrathecal morphine and oral analgesics provide safe and effective pain control after posterior spinal fusion for adolescent idiopathic scoliosis. Spine 2018, 43, E98–E104. [Google Scholar] [CrossRef] [PubMed]
- Domagalska, M.; Ciftsi, B.; Janusz, P.; Reysner, T.; Daroszewski, P.; Kowalski, G.; Wieczorowska-Tobis, K.; Kotwicki, T. Effectiveness of the bilateral and bilevel erector spinae plane block (ESPB) in pediatric idiopathic scoliosis surgery: A randomized, double-blinded, controlled trial. J. Pediatr. Orthop. 2024, 44, e634–e640. [Google Scholar] [CrossRef] [PubMed]
- Akesen, S.; Güler, S.B.; Akesen, B. Bilateral bi-level erector spinae plane blocks in scoliosis surgery: A retrospective comparative study. Acta Orthop. Traumatol. Turc. 2022, 56, 327–332. [Google Scholar] [CrossRef]
- Changoor, S.; Giakas, A.; Sacks, K.; Asma, A.; Lang, R.S.; Yorgova, P.; Rogers, K.; Gabos, P.G.; Shah, S.A. The role of liposomal bupivacaine in multimodal pain management following posterior spinal fusion for adolescent idiopathic scoliosis: Faster and farther with less opioids. Spine (Phila Pa 1976) 2024, 49, E11–E16. [Google Scholar] [CrossRef]
- Hey, G.; Mehkri, Y.; Mehkri, I.; Boatright, S.; Duncan, A.; Patel, K.; Gendreau, J.; Chandra, V. Enhanced recovery after surgery pathways in pediatric spinal surgery: A systematic review and meta-analysis. World Neurosurg. 2024, 190, 329–338. [Google Scholar] [CrossRef]
- Mooney, J.F., III. Perioperative enteric nutritional supplementation in pediatric patients with neuromuscular scoliosis. J. S. Orthop. Assoc. 2000, 9, 202–206. [Google Scholar] [PubMed]
- Chrenko, R. Open-door laminoplasty in cervical myelopathy using titanium miniplate system: Initial clinical experience. Miniinvaz. Chir. Endosk. 2015, 1, 15–21. [Google Scholar]
- Chrenko, R. Unilateral laminotomy and bilateral decompression of degenerative lumbar stenosis—Clinical outcome of 169 operated patients. Miniinvaz. Chir. Endosk. 2015, 4, 13–20. [Google Scholar]
- Gadiya, A.D.; Koch, J.E.J.; Patel, M.S.; Shafafy, M.; Grevitt, M.P.; Quraishi, N.A. Enhanced recovery after surgery (ERAS) in adolescent idiopathic scoliosis (AIS): A meta-analysis and systematic review. Spine Deform. 2021, 9, 893–904. [Google Scholar] [CrossRef]
- Pennington, Z.; Cottrill, E.; Lubelski, D.; Ehresman, J.; Lehner, K.; Groves, M.L.; Sponseller, P.; Sciubba, D.M. Clinical utility of enhanced recovery after surgery pathways in pediatric spinal deformity surgery: Systematic review of the literature. J. Neurosurg. Pediatr. 2021, 27, 225–238. [Google Scholar] [CrossRef]
- Rafeeqi, T.; Pearson, E.G. Enhanced recovery after surgery in children. Transl Gastroenterol Hepatol. 2021, 6, 46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vattipalli, S.; Rath, G.P.; Athiraman, U. Anesthesia for Children with Neuromuscular Diseases. In Fundamentals of Pediatric Neuroanesthesia; Rath, G.P., Ed.; Springer: Singapore, 2021; pp. 579–594. [Google Scholar] [CrossRef]
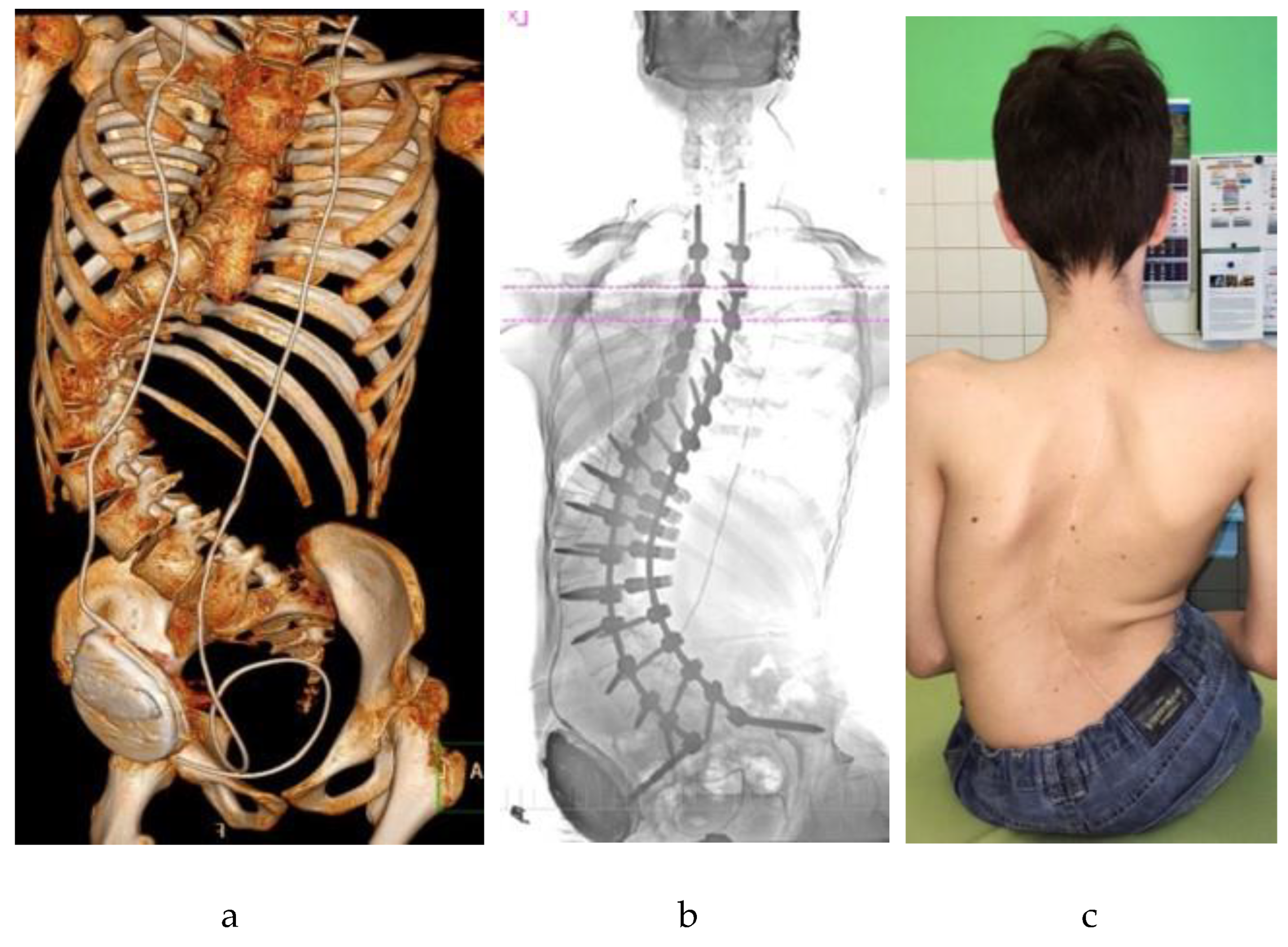

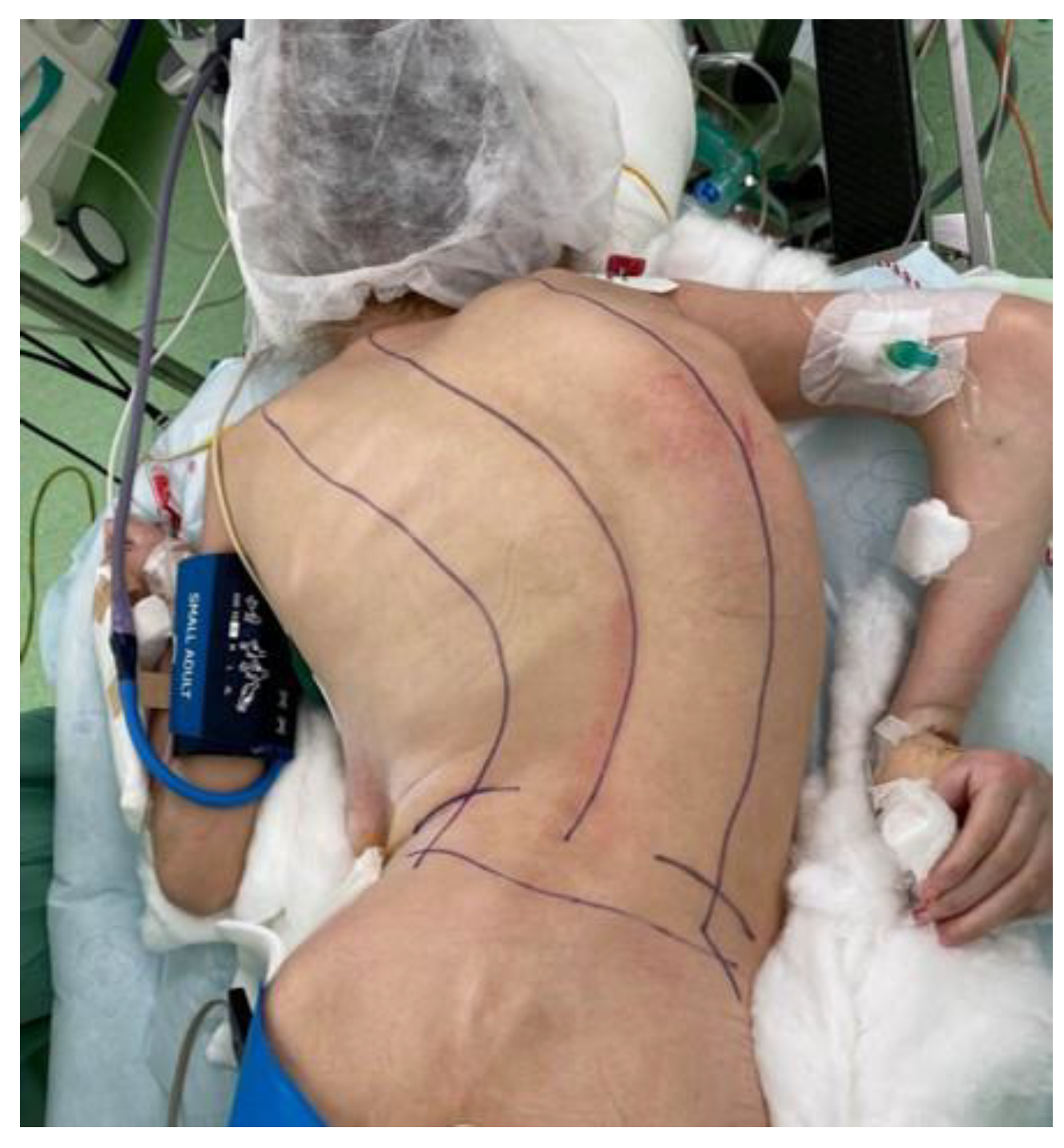
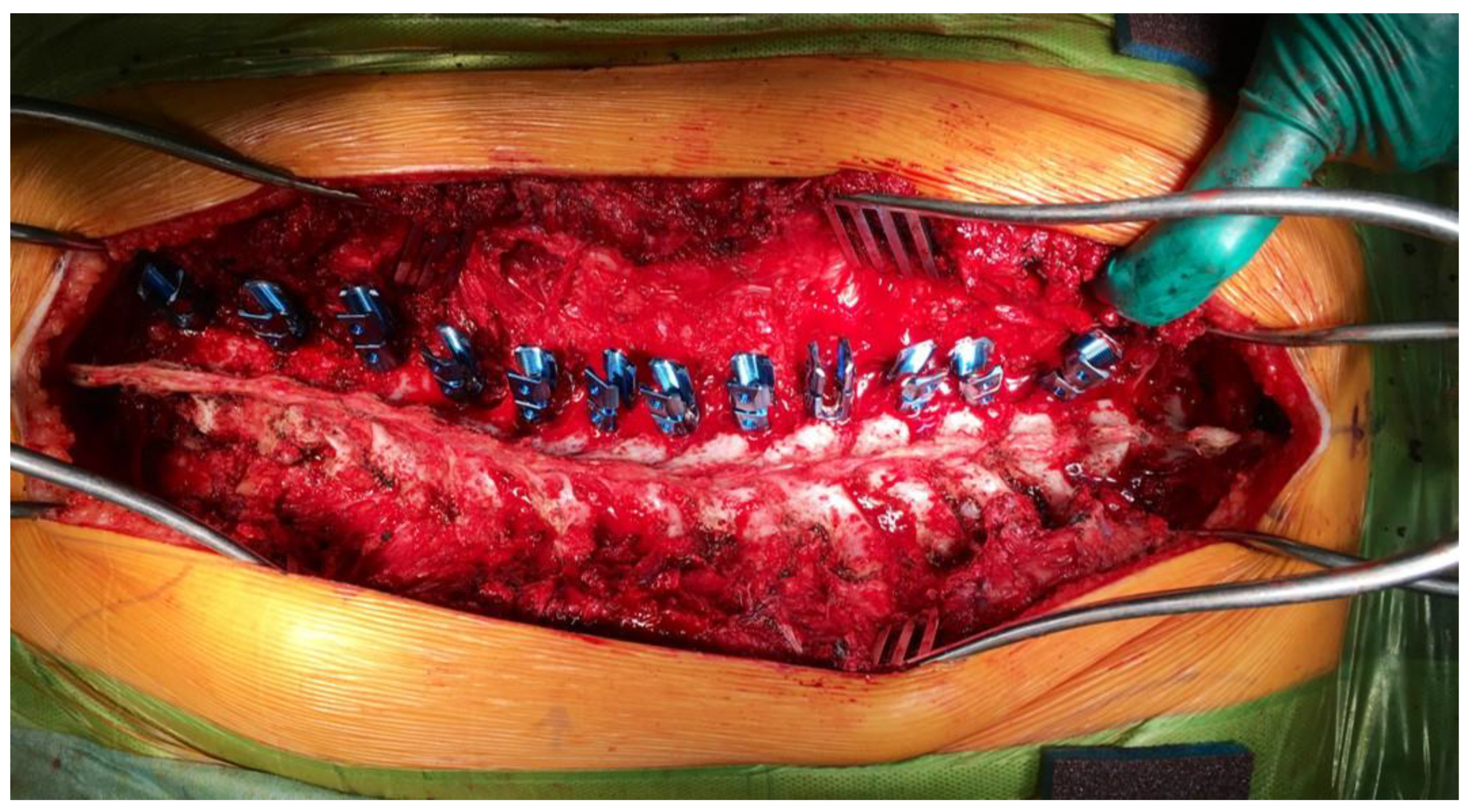
| Domain | DMD/BMD | SMA | CP | MMC |
|---|---|---|---|---|
| Airway & aspiration | Macroglossia, OSA; late aspiration risk. | Bulbar dysfunction; high aspiration risk. | Drooling, GERD; secretions. | GERD common; postsurgical airway changes possible. |
| Ventilation | Restrictive disease; plan postop NIV, cough-assist. | Severe respiratory insufficiency; NIV training, cough-assist. | Variable capacity; atelectasis-prone—gentle strategy. | Prone abdomen may impair venous return. |
| Cardiac | Dilated cardiomyopathy, arrhythmias → echo/ECG. | No primary cardiomyopathy; respiratory load fatigue. | Occasional congenital defects; PH if chronic hypoventilation. | Structural defects possible; preload-sensitive in prone. |
| Anesthetic agents | Absolute: no succinylcholine, no volatile → TIVA. | Prefer TIVA with IONM; volatile only if necessary. | Either approach; watch antiepileptic interactions. | Latex-free OR; agents per IONM/hemodynamics. |
| Neuromuscular blockers | Sux contraindicated; ↑ sensitivity to NDNMB; use TOF, consider sugammadex. | ↑ sensitivity to NDNMB; minimal dosing; TOF; sugammadex. | Spasticity may confound TOF; individualize dosing. | Prior neurosurgery; anatomic variation—use TOF. |
| IONM strategy | TIVA mandatory, MEP/SSEP preserved. | Prefer TIVA with IONM; minimize volatiles. | Either; with IONM prefer TIVA. | Either; with IONM prefer TIVA. |
| Perioperative therapy interactions | Chronic steroids → consider stress-dose; cardiac meds. | Nusinersen/risdiplam do not change anesthesia plan; prioritize respiratory support. | Continue antiepileptics; check baclofen pump. | Latex allergy risk very high → strict latex-free setup. |
| Neurophysiologist/IONM Specialist | Anesthesiologist | Surgeon |
|---|---|---|
| Repeat IONM test. | Ensure no interfering anesthetic agents are being administered. | Assess any surgical intervention immediately preceding the IONM alert. |
| Verify electrode placement and impedance; optimize stimulation parameters and IONM settings. | Maintain mean arterial pressure (MAP) ≥ 70 mmHg or 10–20% above preoperative baseline. | Consider temporarily halting the procedure; observe for recovery of IONM signals. |
| Eliminate artifacts and electrical noise. | Reassess head and limb positioning, particularly in cases of unilateral signal loss. | If necessary, ensure intraoperative imaging is available for further assessment. |
| Evaluate potential waveform changes and onset timing. | Adjust hematocrit (target Hct > 30%), correct pH and pCO2, maintain normothermia and normoglycemia. | Assess signal recovery post-intervention. Be prepared for a wake-up test or to modify the surgical strategy. |
| Type of Complication | Typical Incidence | Main Risk Factors | Preventive/Mitigating Strategies |
|---|---|---|---|
| Neurological injury | AIS: ~0.3–1% [68,69]; NMS: higher than AIS (heterogeneous cohorts) [70] | Severe curve > 90°, rapid correction, hypotension | Maintain MAP ≥ 65 mmHg; combined SSEP/MEP IONM; avoid overly rapid correction |
| Surgical site infection (SSI) | ~2.7% within 90 days after posterior fusion for AIS [61] (higher ranges reported across pediatric spinal fusion) | NMS, obesity, male sex, prolonged surgery | Antibiotic prophylaxis; normothermia; meticulous wound care |
| Hemorrhage > 50% EBV | Incidence varies by etiology and fusion length; highest in DMD, followed by MMC, SMA, and CP [66] | Long, rigid fusion; NMS; high Cobb angle; prolonged operative time | TXA protocol; cell salvage; viscoelastic-guided hemostasis (see Section 5.4) |
| Pulmonary complications | ~18% overall in pediatric scoliosis surgery; higher in non-idiopathic vs. AIS; higher after anterior approach [67,71,72,73] | NMS, Cobb > 90°, low FVC, ↑HCO3− | Preoperative polysomnography; prophylactic NIV; lung-protective ventilation; early extubation |
| Superior mesenteric artery syndrome (SMAS) | 0.5–2.4% after scoliosis correction [64,65] | Low BMI/asthenic habitus; rapid correction; prior spinal surgery | Nutritional optimization; gradual correction; early feeding protocol |
| Venous thromboembolism (VTE) | <1% in pediatric orthopedics; pharmacologic thromboprophylaxis is not routine—individualize by risk [74,75] | Prolonged immobility, CVC, obesity, trauma | Mechanical prophylaxis; selective LMWH per risk (see Section 3: Preoperative Preparation) |
| ERAS Domain | Core Components | Specific Adaptations for Neuromuscular Scoliosis (NMS) |
|---|---|---|
| Preoperative optimization | Multidisciplinary evaluation (anesthesiology, neurology, pulmonology, cardiology, nutrition, physiotherapy); patient and family education; anemia management; antifibrinolytic planning | Prehabilitation—Cough training; airway clearance; PEG feeding if malnourished; latex avoidance; individualized anesthesia plan. |
| Anesthetic management | IONM-compatible TIVA (propofol/remifentanil); lung-protective ventilation; temperature and fluid management; antifibrinolytic therapy (TXA); PONV prophylaxis; intubation strategy compatible with IONM (short-acting NMB for intubation only, then avoid) | Anesthesia (DMD/BMD)—TIVA mandatory; strict avoidance of succinylcholine and volatile agents; real-time IONM coordination. |
| Intraoperative care | Normothermia; goal-directed fluid therapy; viscoelastic-guided hemostasis; restrictive transfusion thresholds. | Higher vigilance for coagulopathy; maintain age-appropriate MAP targets (near baseline; avoid hypotension); minimize mechanical stress during deformity correction |
| Postoperative pain control | Scheduled acetaminophen ± NSAIDs (if not contraindicated); adjuncts (gabapentinoids, low-dose ketamine or dexmedetomidine); regional techniques (ESPB/parasagittal blocks, wound infiltration); early mobilization. | Prefer regional/wound infiltration over neuraxial if neuro exam or respiratory risk; avoid routine epidural; consider liposomal bupivacaine (limited evidence); no basal opioid (PCA demand-only); continuous oximetry ± capnography, planned nocturnal NIV; monitor for respiratory depression. |
| Respiratory and cardiovascular support | Incentive spirometry; chest physiotherapy; early ambulation; hemodynamic stability monitoring | Planned extubation with immediate NIV (BiPAP); nocturnal gas-exchange monitoring; telemetry in cardiac involvement |
| Nutrition and early recovery | Early enteral feeding; avoidance of prolonged fasting; prevention of ileus and nausea | PEG or nasogastric feeding for undernourished or dysphagic children; nutritional supplementation |
| Family involvement | Teach-back education on airway equipment, pain plan, wound/infection red flags (incl. UTI), and a clear escalation protocol. | Extended caregiver training and participation in daily respiratory care; coordination with home-ventilation teams |
| Preoperative Preparation | Intraoperative Management | Postoperative Management |
|---|---|---|
| • Determine scoliosis type/etiology and age at onset. | • TIVA with TCI (propofol/remifentanil) and continuous depth monitoring (DMD/BMD: no volatile). | • Early extubation in OR when feasible (plan NIV/cough-assist if SMA/advanced DMD). |
| • Focused history: pulmonary function (cough effectiveness, obstruction); palpitations/syncope (esp. DMD/BMD). | • IV induction of anesthesia. | • Multimodal analgesia; antiemetic prophylaxis as indicated. |
| • Targeted exam: airway & cervical mobility, neurologic status, pulmonary, cardiac assessment (echo/ECG if DMD/BMD). | • Non-depolarizing NMB for intubation; avoid succinylcholine (DMD/BMD). | • Ensure adequate ventilation/oxygenation; lung-protective strategy if restrictive mechanics. |
| • Labs and type & screen/cross-match; plan blood products by anticipated loss. | • Prepare for difficult airway (bulbar SMA/advanced CP; adjuncts/backup). | • Anticipate postoperative mechanical ventilation based on—Preop: etiology, Cobb angle, spirometry (FEV1%, FVC%), comorbidities—Intraop: levels fused, instability/course—Modifiable: transfusion burden, hypothermia |
| • Optimization: nutrition, hydration, infection screen; ERAS briefing (latex-free plan if MMC). | • Advanced monitoring: invasive arterial pressure; consider central venous access if vasoactives likely; hourly urine output; core temperature. | • ICU/step-down as indicated; early physiotherapy and cough-assist/NIV where appropriate. |
| • Vascular access plan (US-guided if difficult); anesthesia/analgesia plan aligned with IONM if used. | • Secure reliable IV access (≥2 large-bore peripherals; ultrasound guidance as needed). | • Catheter care (PIV/CVC/urinary) with early removal when safe; early enteral nutrition. |
| • Prone positioning with chest/abdominal support; protect pressure points/eyes (fragile skin; MMC: strict latex-free OR). | • VTE prevention: mechanical for all; pharmacologic selectively per risk and neuraxial timing. | |
| • Blood-loss management: cell salvage; minimize allogeneic transfusion; antifibrinolytics; viscoelastic testing to guide therapy. | • Discharge education (analgesics schedule, wound care, red flags, respiratory device use/alarms). |
| Phase | Core ERAS Elements (Apply to All) | NMS-Specific Adaptations (High-Impact Nuances) |
|---|---|---|
| Preoperative | Patient/parent education; premedication; fasting; nutritional assessment. | Respiratory stratification with planned NIV and cough-assist (SMA/advanced DMD); cardiac-first planning in DMD/BMD (continuous ECG monitoring as indicated, cautious fluids); agent selection briefing (no succinylcholine/volatile in DMD/BMD → TIVA); latex-free pathway for MMC; continue antiepileptics (CP), check baclofen pump; aspiration mitigation in bulbar phenotypes. |
| Intraoperative | Goal-directed fluids; opioid-sparing anesthesia; temperature monitoring/normothermia; antibiotic prophylaxis; meticulous hemostasis; IONM-compatible technique. | TIVA with IONM (minimize volatiles; DMD/BMD: TIVA mandatory); lung-protective ventilation (VT ~6–7 mL·kg−1 PBW, individualized PEEP, gentle recruitment); cautious NDNMB titration with TOF (prefer sugammadex in DMD/SMA); latex-free OR for MMC; enhanced padding/pressure-injury prevention. |
| Postoperative | ICU/step-down as indicated; fluid optimization; appropriate catheter care; multimodal analgesia; early nutrition; structured discharge education. | Risk-stratified PICU with planned NIV/cough-assist (SMA/advanced DMD); telemetry where cardiomyopathy/arrhythmia risk (DMD/BMD); mechanical VTE prophylaxis for all, pharmacologic selectively and timed vs. neuraxial; UTI surveillance (MMC). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedomová, B.; Liščák, B.; Urbanová, S.; Pavlík, Š.; Riedel, R.; Dostálová, V. Perioperative Anesthetic Management in Pediatric Scoliosis Surgery: A Narrative Review with Focus on Neuromuscular Disorders. Children 2025, 12, 1481. https://doi.org/10.3390/children12111481
Nedomová B, Liščák B, Urbanová S, Pavlík Š, Riedel R, Dostálová V. Perioperative Anesthetic Management in Pediatric Scoliosis Surgery: A Narrative Review with Focus on Neuromuscular Disorders. Children. 2025; 12(11):1481. https://doi.org/10.3390/children12111481
Chicago/Turabian StyleNedomová, Barbora, Boris Liščák, Soňa Urbanová, Štefan Pavlík, Rudolf Riedel, and Vlasta Dostálová. 2025. "Perioperative Anesthetic Management in Pediatric Scoliosis Surgery: A Narrative Review with Focus on Neuromuscular Disorders" Children 12, no. 11: 1481. https://doi.org/10.3390/children12111481
APA StyleNedomová, B., Liščák, B., Urbanová, S., Pavlík, Š., Riedel, R., & Dostálová, V. (2025). Perioperative Anesthetic Management in Pediatric Scoliosis Surgery: A Narrative Review with Focus on Neuromuscular Disorders. Children, 12(11), 1481. https://doi.org/10.3390/children12111481









