The Role of Spirometry and MMEF in Pediatric Asthma Monitoring and Prediction of Exacerbations
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Participants
2.2. Methods
- For patients aged 10 years or older: an adequate onset of expiration starting from point 0 [expiratory volume ≤ 5% of FVC or 1500 mL, whichever is greater], a rapid rise to peak expiratory flow with a sharp peak, complete expiration (expiratory plateau ≤ 0.025 L in the last second of expiration and an expiratory time ≥ 6 s), and smooth expiration on the flow-volume curve, free from artefacts (such as cough in the first second of forced expiration, non-maximal effort throughout expiration, premature glottis closure, premature termination of expiration, obstruction or air loss around the mouthpiece).
- For children under 10 years: exhalation over 3 s and absence of coughing or premature glottis closure in the first 0.75 s of exhalation.
- –
- The level of asthma control according to the GINA guideline, based on symptoms, the number of exacerbations, and lung function: controlled (C), partially controlled (PC), or uncontrolled (UC) asthma.
- –
- The long-term treatment regimen: inhaled corticosteroids, alone or combined with long-acting beta2 adrenergic or long-acting muscarinic antagonist, or leukotriene modifier.
2.3. Study Protocol
2.4. Statistics
3. Results
3.1. Quality of Spirometry in Children by Age Groups
3.2. The Pulmonary Function Tests in Asthma Patients
3.3. Spirometric Parameters in Children with Asthma According to the Control Level
3.4. Long-Term Treatment
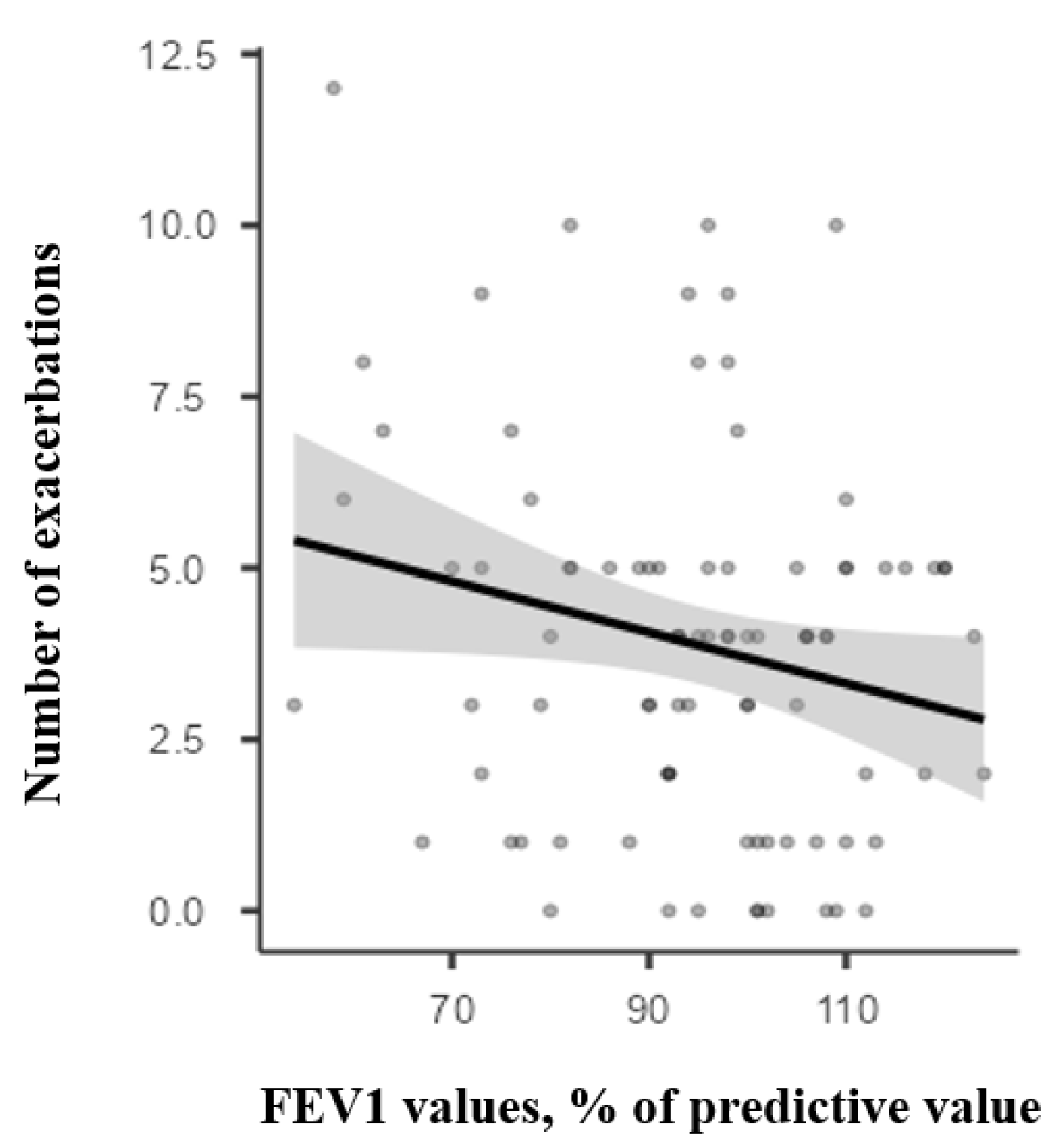
3.5. Predictive Value of Spirometry Parameters for Asthma Exacerbation
3.6. Changes in Long-Term Treatment
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACT | Asthma Control Test |
| ARTP | Association for Respiratory Technology and Physiology |
| ATS | American Thoracic Society |
| BTS | British Thoracic Society |
| C | Controlled (asthma group) |
| ERS | European Respiratory Society |
| FEF25-75 | Forced Expiratory Flow between 25 and 75% of FVC |
| FEF50 | Forced Expiratory Flow at 50% of FVC |
| FEV1 | Forced Expired Volume in 1 s |
| FeNO | Fractional Exhaled Nitric Oxide |
| FVC | Forced Vital Capacity |
| ICS | inhaled corticosteroids |
| LABA | Long-acting β2-Agonists |
| LAMA | Long-Acting Muscarinic Antagonists |
| LTRA | Leukotriene Receptor Antagonist |
| MEF25 | Mean Expiratory Flow rate at 25% of vital capacity |
| MEF50 | Mean Expiratory Flow rate at 50% of vital capacity |
| MMEF | Maximal Mid-Expiratory Flow |
| PC | Partially Controlled asthma group |
| SAD | Small Airway Dysfunction |
| SD | Standard Deviation |
| UC | Uncontrolled asthma group |
References
- Global Initiative for Asthma Global Strategy for Asthma Management and Prevention. 2024. Available online: https://www.ginasthma.org (accessed on 26 January 2025).
- Stanojevic, S.; Filipow, N.; Ratjen, F. Paediatric Reproducibility Limits for the Forced Expiratory Volume in 1 s. Thorax 2020, 75, 891–896. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Lopes Vieira, J.F.; Miskovic, A.; Abel, F. Interpretation of Pulmonary Function Tests in Children. BJA Educ. 2023, 23, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Almeshari, M.A.; Alobaidi, N.Y.; Stockley, J.A.; Stockley, R.A.; Nagakumar, P.; Sutton, B.P.; Sapey, E. Physiological Small Airways Dysfunction and the Bronchodilator Response in Adults with Asthma and Its Risk Factors: A Retrospective Analysis. J. Asthma Allergy 2025, 18, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fu, Z.; Deng, H.; Xie, Q.; Wu, W. Identification and Treatment of Persistent Small Airway Dysfunction in Paediatric Patients with Asthma: A Retrospective Cohort Study. BMC Pulm. Med. 2024, 24, 94. [Google Scholar] [CrossRef]
- Yi, L.; Zhao, Y.; Guo, Z.; Li, Q.; Zhang, G.; Tian, X.; Xu, X.; Luo, Z. The Role of Small Airway Function Parameters in Preschool Asthmatic Children. BMC Pulm. Med. 2023, 23, 219. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Adibi, A.; Zafari, Z.; FitzGerald, J.M.; Aaron, S.D.; Johnson, K.M.; Sadatsafavi, M. Cost-Effectiveness of Implementing Objective Diagnostic Verification of Asthma in the United States. J. Allergy Clin. Immunol. 2020, 145, 1367–1377.e4. [Google Scholar] [CrossRef]
- Zar, H.J.; Ferkol, T.W. The Global Burden of Respiratory Disease-Impact on Child Health. Pediatr. Pulmonol. 2014, 49, 430–434. [Google Scholar] [CrossRef]
- Azaldegi, G.; Korta, J.; Sardón, O.; Corcuera, P.; Pérez-Yarza, E.G. Disfunción de La Pequeña Vía Aérea En Niños Con Asma Controlada. Arch. Bronconeumol. 2019, 55, 208–213. [Google Scholar] [CrossRef]
- Kjellberg, S.; Olin, A.-C.; Schiöler, L.; Robinson, P.D. Detailed Characterization and Impact of Small Airway Dysfunction in School-Age Asthma. J. Asthma Off. J. Assoc. Care Asthma 2024, 61, 1412–1421. [Google Scholar] [CrossRef]
- Cottini, M.; Lombardi, C.; Berti, A.; Comberiati, P. Small-Airway Dysfunction in Paediatric Asthma. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 128–134. [Google Scholar] [CrossRef]
- Meulmeester, F.L.; Mailhot-Larouche, S.; Celis-Preciado, C.; Lemaire-Paquette, S.; Ramakrishnan, S.; Wechsler, M.E.; Brusselle, G.; Corren, J.; Hardy, J.; Diver, S.E.; et al. Inflammatory and Clinical Risk Factors for Asthma Attacks (ORACLE2): A Patient-Level Meta-Analysis of Control Groups of 22 Randomised Trials. Lancet Respir. Med. 2025, 13, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, M.; Bumbacea, D. Prevalence of Asthma Symptoms, Diagnosis and Treatment Use in Romania. Eur. Respir. J. 2013. Available online: https://www.academia.edu/29115074/Prevalence_of_asthma_symptoms_diagnosis_and_treatment_use_in_Romania (accessed on 26 January 2025).
- Chereches-Panta, P.; Sorin, C.; Dumitrescu, D.; Marshall, M.; Mirestean, I.; Muresan, M.; Iacob, D.; Farcau, M.; Ichim, G.E.; Nanulescu, M.V. Epidemiological Survey 6 Years Apart: Increased Prevalence of Asthma and Other Allergic Diseases in Schoolchildren Aged 13–14 Years in Cluj-Napoca, Romania (Based on Isaac Questionnaire). Maedica 2011, 6, 10–16. [Google Scholar] [PubMed]
- Marica, I.; Cherecheş-Panţa, P. The Importance of Spirometry in Bronchial Asthma Management and the Predictive Value of FEV1 and MMEF Parameters for Future Exacerbations. Appl. Med. Inform. 2024, 46, S32. [Google Scholar]
- Sylvester, K.P.; Clayton, N.; Cliff, I.; Hepple, M.; Kendrick, A.; Kirkby, J.; Miller, M.; Moore, A.; Rafferty, G.F.; O’Reilly, L.; et al. ARTP Statement on Pulmonary Function Testing 2020. BMJ Open Respir. Res. 2020, 7, e000575. [Google Scholar] [CrossRef]
- The Global Asthma Report 2022. Int. J. Tuberc. Lung Dis. 2022, 26, 1–104. [CrossRef]
- Lundberg, B.; Melén, E.; Thunqvist, P.; Norman, M.; Hallberg, J. Agreement between Spirometry and Impulse Oscillometry for Lung Function Assessment in 6-Year-Old Children Born Extremely Preterm and at Term. Pediatr. Pulmonol. 2020, 55, 2745–2753. [Google Scholar] [CrossRef]
- Domínguez-Martín, C.; Cano, A.; Díez-Monge, N.; Alonso-Rubio, A.M.; Pérez-García, I.; Arroyo-Romo, M.T.; Casares-Alonso, I.; Barbero-Rodríguez, A.M.; Grande-Alvarez, R.; Martínez-Rivera, M.T.; et al. Spirometry and Respiratory Oscillometry: Feasibility and Concordance in Schoolchildren with Asthma. Pediatr. Pulmonol. 2023, 58, 1896–1903. [Google Scholar] [CrossRef]
- Gunawardana, S.; Tuazon, M.; Wheatley, L.; Cook, J.; Harris, C.; Greenough, A. Airwave Oscillometry and Spirometry in Children with Asthma or Wheeze. J. Asthma Off. J. Assoc. Care Asthma 2023, 60, 1153–1161. [Google Scholar] [CrossRef]
- Beydon, N.; Abou Taam, R.; Delclaux, C.; Du Boisbaudry, C.; Gauthier, R.; Ioan, I.; Le Bourgeois, M.; Giroux-Metges, M.-A.; Matecki, S.; Groupe Efr pédiatrique français. Pulmonary function test: The testing of children. Rev. Mal. Respir. 2024, 41, 488–497. [Google Scholar] [CrossRef]
- Gheorghiu, R.M.; Stan, I.V. Reflections of a Paediatric Pulmonologist: Strategies for Optimising Lung Function Tests in Preschool Children. Breathe 2025, 21, 240178. [Google Scholar] [CrossRef] [PubMed]
- Almeshari, M.A.; Alobaidi, N.Y.; Edgar, R.G.; Stockley, J.; Sapey, E. Physiological Tests of Small Airways Function in Diagnosing Asthma: A Systematic Review. BMJ Open Respir. Res. 2020, 7, e000770. [Google Scholar] [CrossRef] [PubMed]
- Braido, F.; Brusselle, G.; Guastalla, D.; Ingrassia, E.; Nicolini, G.; Price, D.; Roche, N.; Soriano, J.B.; Worth, H.; LIAISON Study Group. Determinants and Impact of Suboptimal Asthma Control in Europe: The INTERNATIONAL CROSS-SECTIONAL AND LONGITUDINAL ASSESSMENT ON ASTHMA CONTROL (LIAISON) Study. Respir. Res. 2016, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Richardson, M.; Hallmark, B.; Billheimer, D.; Van den Berge, M.; Fabbri, L.M.; Van der Molen, T.; Nicolini, G.; Papi, A.; Rabe, K.F.; et al. The Role of Small Airway Dysfunction in Asthma Control and Exacerbations: A Longitudinal, Observational Analysis Using Data from the ATLANTIS Study. Lancet Respir. Med. 2022, 10, 661–668. [Google Scholar] [CrossRef]
- Postma, D.S.; Brightling, C.; Baldi, S.; Van den Berge, M.; Fabbri, L.M.; Gagnatelli, A.; Papi, A.; Van der Molen, T.; Rabe, K.F.; Siddiqui, S.; et al. Exploring the Relevance and Extent of Small Airways Dysfunction in Asthma (ATLANTIS): Baseline Data from a Prospective Cohort Study. Lancet Respir. Med. 2019, 7, 402–416. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Weiner, D.J.; Pretto, J.J.; Brazzale, D.J.; Boros, P.W. Measurement of FEF25-75% and FEF75% Does Not Contribute to Clinical Decision Making. Eur. Respir. J. 2014, 43, 1051–1058. [Google Scholar] [CrossRef]
- Xiao, S.; Ou, J.; Qiu, W.; Ye, C.; Li, N.; Chen, S.; Lai, Y.; Deng, Z.; Wu, F.; Shen, Y. Risk of All-Cause Mortality in US Adults with Preserved Ratio Impaired Spirometry: An Observational Study. Int. J. Chronic Obstr. Pulm. Dis. 2025, 20, 287–302. [Google Scholar] [CrossRef]
- Quezada, W.; Kwak, E.S.; Reibman, J.; Rogers, L.; Mastronarde, J.; Teague, W.G.; Wei, C.; Holbrook, J.T.; DiMango, E. Predictors of Asthma Exacerbation among Patients with Poorly Controlled Asthma despite Inhaled Corticosteroid Treatment. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2016, 116, 112–117. [Google Scholar] [CrossRef]
- Jones, K.G.; Bell, J.; Fehrenbach, C.; Pearce, L.; Grimley, D.; McCarthy, T.P. Understanding Patient Perceptions of Asthma: Results of the Asthma Control and Expectations (ACE) Survey. Int. J. Clin. Pract. 2002, 56, 89–93. [Google Scholar] [CrossRef]
- Francisco, B.; Ner, Z.; Ge, B.; Hewett, J.; König, P. Sensitivity of Different Spirometric Tests for Detecting Airway Obstruction in Childhood Asthma. J. Asthma Off. J. Assoc. Care Asthma 2015, 52, 505–511. [Google Scholar] [CrossRef]
- Rechkina, O.; Opimakh, S.; Kravtsova, O.; Kaydashev, I. Small Airways Response to Bronchodilators as the Marker of the Uncontrolled Asthma in Children. Wiadomosci Lek. 2024, 77, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Lazova, S.; Priftis, S.; Petrova, G.; Naseva, E.; Velikova, T. MMEF25-75 May Predict Significant BDR and Future Risk of Exacerbations in Asthmatic Children with Normal Baseline FEV1. Int. J. Physiol. Pathophysiol. Pharmacol. 2022, 14, 33–47. [Google Scholar] [PubMed]
- Siroux, V.; Boudier, A.; Dolgopoloff, M.; Chanoine, S.; Bousquet, J.; Gormand, F.; Just, J.; Le Moual, N.; Nadif, R.; Pison, C.; et al. Forced Midexpiratory Flow between 25% and 75% of Forced Vital Capacity Is Associated with Long-Term Persistence of Asthma and Poor Asthma Outcomes. J. Allergy Clin. Immunol. 2016, 137, 1709–1716.e6. [Google Scholar] [CrossRef] [PubMed]
- McFadden, E.R.; Linden, D.A. A Reduction in Maximum Mid-Expiratory Flow Rate. A Spirographic Manifestation of Small Airway Disease. Am. J. Med. 1972, 52, 725–737. [Google Scholar] [CrossRef]
- Domínguez-Martín, C.; Cano, A.; Díez-Monge, N.; Investigadoras SADICA. Clinical Performance of Spirometry and Respiratory Oscillometry for Prediction of Severe Exacerbations in Schoolchildren with Asthma. An. Pediatr. 2023, 98, 427–435. [Google Scholar] [CrossRef]
- Peláez, G.; Giubergia, V.; Lucero, B.; Aguerre, V.; Castaños, C.; Figueroa, J.M. Childhood Severe Asthma: Relationship among Asthma Control Scores, FeNO, Spirometry and Impulse Oscillometry. BMC Pulm. Med. 2024, 24, 270. [Google Scholar] [CrossRef]
- Couillard, S.; Laugerud, A.; Jabeen, M.; Ramakrishnan, S.; Melhorn, J.; Hinks, T.; Pavord, I. Derivation of a Prototype Asthma Attack Risk Scale Centred on Blood Eosinophils and Exhaled Nitric Oxide. Thorax 2022, 77, 199–202. [Google Scholar] [CrossRef]
- Louis, R.; Satia, I.; Ojanguren, I.; Schleich, F.; Bonini, M.; Tonia, T.; Rigau, D.; Brinke, A.T.; Buhl, R.; Loukides, S.; et al. European Respiratory Society Guidelines for the Diagnosis of Asthma in Adults. Eur. Respir. J. 2022, 60, 2101585. [Google Scholar] [CrossRef]

| Patients Aged 5–10 Years (N = 208) | Patients Aged 11–18 Years (N = 208) | p | |
|---|---|---|---|
| Correct expiration onset, no (%) | 150 (72.1%) | 137 (66.9%) | 0.168 |
| Presence of expiratory peak, no (%) | 160 (76.9%) | 190 (91.3%) | <0.05 |
| Age-appropriate exhalation time *, no (%) | 67 (32.2%) | 89 (42.8%) | 0.025 |
| Presence of secondary inspiration, no (%) | 12 (5.8%) | 16 (7.7%) | 0.433 |
| Patients Aged 5–10 Years | Patients Aged 11–18 Years | |
|---|---|---|
| Number of cases | 157 | 119 |
| Sex, n (%) | ||
| - Female | 61 (38.9) | 56 (47.1) |
| - Male | 96 (61.2) | 63 (52.9) |
| Age, years, mean (±SD) | 7.76 (±1.8) | 14.4 (±2.1) |
| Controlled asthma, n (%) | 43 (27.4) | 37 (31.1) |
| Partially controlled, n (%) | 78 (49.6) | 47 (39.5) |
| Uncontrolled, n (%) | 36 (22.9) | 35 (29.4) |
| Controlled asthma | ||
| - FEV1 *, mean (±SD) | 99.5 (±11.0) | 99.5 (±11.8) |
| - FEV1 < 80% of pred, n (%) | 2 (4.6) | 1 (2.7) |
| - FEV1/FVC, mean (±SD) | 103 (±7.2) | 103 (±7.68) |
| - MMEF *, mean (±SD) | 101 (±16.6) | 100 (±20.7) |
| - MMEF < 80% of pred, n (%) | 4 (9.3) | 6 (16.2) |
| - MMEF < 65% of pred, n (%) | 2 (4.7) | 1 (2.7) |
| Partially controlled | ||
| - FEV1 *, mean (± SD) | 93.8 (±15.7) | 93 (±14.8) |
| - FEV1 < 80% of pred, n (%) | 18 (23) | 8 (17.0) |
| - FEV1/FVC, mean (±SD) | 99.3 (±9.49) | 98.4 (±8.9) |
| - MMEF *, mean (±SD) | 90.5 (±27.4) | 89.3 (±23.4) |
| - MMEF < 80% of pred, n (%) | 32 (41) | 11 (23.4) |
| - MMEF < 65% of pred, n (%) | 10 (12.8) | 8 (17.0) |
| Uncontrolled | ||
| - FEV1 *, mean (± SD) | 82.9 (±17.6) | 78.8 (±12.5) |
| - FEV1 < 80% of pred, n (%) | 17 (47.2) | 14 (40) |
| - FEV1/FVC, mean (±SD) | 91.4 (±13.3) | 88.7 (±8.1) |
| - MMEF *, mean (±SD) | 66.1 (±21.8) | 61.3 (±16.2) |
| - MMEF < 80% of pred, n (%) | 30 (83.3) | 31 (88.6) |
| - MMEF < 65% of pred, n (%) | 11 (30.6) | 18 (51.4) |
| C (n = 80) | PC (n = 125) | UC (n = 71) | p | |
|---|---|---|---|---|
| FEV1 *, mean (±SD) | 99.3 (±11.2) | 93.5 (±15.3) | 80.9 (±15.4) | 0.0102 |
| MMEF *, mean (±SD) | 101 (±18.5) | 91.5 (±27.3) | 63.7 (±19.3) | 0.0001 |
| C (n = 80) | PC (n = 125) | UC (n = 71) | |
|---|---|---|---|
| No maintenance treatment, n (%) | 49 (61.3) | 24 (19.2) | 0 |
| Monotherapy (low-dose ICS or LTRA), n (%) | 30 (37.5) | 87 (69.6) | 5 (7.0) |
| Medium-dose ICS or combination (low-dose ICS + LTRA/LABA), n (%) | 1 (1.3) | 11 (8.8) | 26 (36.6) |
| High-dose ICS (±LABA and/or LTRA) or medium-dose ICS + LABA, n (%) | 0 | 3 (2.4) | 40 (56.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chereches-Panta, P.; Marica, I.; Sas, V.; Bouari-Coblișan, A.P.; Man, S.C. The Role of Spirometry and MMEF in Pediatric Asthma Monitoring and Prediction of Exacerbations. Children 2025, 12, 1398. https://doi.org/10.3390/children12101398
Chereches-Panta P, Marica I, Sas V, Bouari-Coblișan AP, Man SC. The Role of Spirometry and MMEF in Pediatric Asthma Monitoring and Prediction of Exacerbations. Children. 2025; 12(10):1398. https://doi.org/10.3390/children12101398
Chicago/Turabian StyleChereches-Panta, Paraschiva, Ioana Marica, Valentina Sas, Alina Petronela Bouari-Coblișan, and Sorin Claudiu Man. 2025. "The Role of Spirometry and MMEF in Pediatric Asthma Monitoring and Prediction of Exacerbations" Children 12, no. 10: 1398. https://doi.org/10.3390/children12101398
APA StyleChereches-Panta, P., Marica, I., Sas, V., Bouari-Coblișan, A. P., & Man, S. C. (2025). The Role of Spirometry and MMEF in Pediatric Asthma Monitoring and Prediction of Exacerbations. Children, 12(10), 1398. https://doi.org/10.3390/children12101398






