Effect of the Different Dietary Supplements on the Average Surface Roughness and Color Stability of Direct Restorative Materials Used in Pediatric Dentistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Immersion Time
2.3. Measurement of Color Parameters
2.4. Measurements of Average Surface Roughness (Ra)
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kale, Y.J.; Nalwade, A.V.; Dahake, P.T.; Dadpe, M.V.; Kendre, S.B. Effect of different pediatric drug formulations on color stability of composite, zirconia-reinforced glass ionomer cement, and glass ionomer cement. J. Indian. Soc. Pedod. Prev. Dent. 2019, 37, 151–156. [Google Scholar]
- Almutairi, M.; Moussa, I.; Alsaeri, N.; Alqahtani, A.; Alsulaiman, S.; Alhajri, M. The effects of different pediatric drugs and brushing on the color stability of esthetic restorative materials used in pediatric dentistry: An in vitro study. Children 2022, 9, 1026. [Google Scholar] [CrossRef] [PubMed]
- Čulina, M.Z.; Rajić, V.B.; Šalinović, I.; Klarić, E.; Marković, L.; Ivanišević, A. Influence of pH Cycling on Erosive Wear and Color Stability of High-Viscosity Glass Ionomer Cements. Materials 2022, 15, 923. [Google Scholar] [CrossRef] [PubMed]
- Sharafeddin, F.; Bahrani, S. Effect of hydroxyapatite on surface roughness of zirconomer, and conventional and resin-modified glass ionomers. Front. Dent. 2020, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Adsul, P.S.; Dhawan, P.; Tuli, A.; Khanduri, N.; Singh, A. Evaluation and comparison of physical properties of Cention N with other restorative materials in artificial saliva: An in vitro study. Int. J. Clin. Pediatr. Dent. 2022, 15, 350–355. [Google Scholar]
- Manisha, S.; Shetty, S.S.; Mehta, V.; Sa, R.; Meto, A. A comprehensive evaluation of zirconia-reinforced glass ionomer cement’s effectiveness in dental caries: A systematic review and network meta-analysis. Dent. J. 2023, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Panetta, A.; Lopes, P.; Novaes, T.F.; Rio, R.; Fernandes, G.V.O.; Mello-Moura, A.C.V. Evaluating Glass Ionomer Cement Longevity in the Primary and Permanent Teeth—An Umbrella Review. J. Funct. Biomater. 2024, 15, 48. [Google Scholar] [CrossRef]
- Asafarlal, S. Comparative evaluation of microleakage, surface roughness and hardness of three glass ionomer cements—Zirconomer, Fujii IX Extra GC and Ketac Molar: An in vitro study. J. Dent. 2017, 7, 427. [Google Scholar] [CrossRef]
- Nanavati, K.; Katge, F.; Chimata, V.K.; Pradhan, D.; Kamble, A.; Patil, D. Comparative evaluation of shear bond strength of bioactive restorative material, zirconia reinforced glass ionomer cement and conventional glass ionomer cement to the dentinal surface of primary molars: An in vitro study. J. Dent. 2021, 4, 260. [Google Scholar]
- Safy, R.K.; Elmohsen, H.A. Assessment of the fracture resistance of novel zirconia reinforced glass ionomer in comparison to nano hybrid resin composite restorations. Egypt. Dent. J. 2019, 65, 3735–3744. [Google Scholar] [CrossRef]
- Faghihi, T.; Heidarzadeh, Z.; Jafari, K.; Farhoudi, I.; Hekmatfar, S. An experimental study on the effect of four pediatric drug types on color stability in different tooth-colored restorative materials. Dent. Res. J. 2021, 18, 75. [Google Scholar]
- Sardana, A.; Kumar, M.; Taneja, S. Comparative evaluation of microleakage and hardness of newer posterior restorative materials. J. Oral Biol. Craniofacial Res. 2022, 12, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Pai, D.; Anirudhmaadhava, P.A.; Ginjupalli, K. In Vitro Evaluation of Mechanical Properties of Cention N and Its Comparison with Resin Modified Glass Ionomer Cement (RMGIC) Restorative Material as Used in Primary Teeth. Sci. World J. 2024, 2024, 9420336. [Google Scholar] [CrossRef] [PubMed]
- Sujith, R.; Yadav, T.G.; Pitalia, D.; Babaji, P.; Apoorva, K.; Sharma, A. Comparative evaluation of mechanical and microleakage properties of Cention-N, composite, and glass ionomer cement restorative materials. J. Contemp. Dent. Pract. 2020, 21, 691–695. [Google Scholar] [PubMed]
- Costa, M.P.; Jacomine, J.C.; Mosquim, V.; Santin, D.C.; Zabeu, G.S.; Agulhari, M.A.S.; Mondelli, R.F.L.; Honório, H.M.; Wang, L. Analysis of color stability and degree of conversion of different types of resin composites. Braz. Oral Res. 2024, 38, e003. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA 101: Dietary Supplements. Available online: https://www.fda.gov/consumers/consumer-updates/fda-101-dietary-supplements (accessed on 17 December 2023).
- Arora, I.; White, S.; Mathews, R. Global dietary and herbal supplement use during COVID-19—A scoping review. Nutrients 2023, 15, 771. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, M.; Bedi, O.; Gupta, M.; Kumar, S.; Jaiswal, G.; Rahi, V.; Yedke, N.G.; Bijalwan, A.; Sharma, S.; et al. Role of vitamins and minerals as immunity boosters in COVID-19. Inflammopharmacology 2021, 29, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Quispe, C.; Martorell, M.; Docea, A.O.; Salehi, B.; Calina, D.; Reiner, Ž.; Sharifi-Rad, J. Dietary supplements, vitamins and minerals as potential interventions against viruses: Perspectives for COVID-19. Int. J. Vitam. Nutr. Res. 2022, 92, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Gurdogan Guler, E.B.; Bayrak, G.D.; Unsal, M.; Selvi Kuvvetli, S. Effect of pediatric multivitamin syrups and effervescent tablets on the surface microhardness and roughness of restorative materials: An in vitro study. J. Dent. Sci. 2021, 16, 311–317. [Google Scholar] [CrossRef]
- Sezer Efe, Y.; Gül Tamer, F.; Tekcan, P.; Bayat, M. The effect of mothers’ fears of Covid-19 on their attitudes towards feeding their children and using food supplements. Women Health 2023, 63, 454–463. [Google Scholar] [CrossRef]
- Peker, O.; Bolgul, B. Evaluation of surface roughness and color changes of restorative materials used with different polishing procedures in pediatric dentistry. J. Clin. Pediatr. Dent. 2023, 47, 72–79. [Google Scholar] [PubMed]
- Nalwade Apeksha, V.; Kale Yogesh, J.; Dadpe Mahesh, V.; Dahake Prasanna, T.; Kendre Shrikant, B. Effect of paediatric liquid medications on surface roughness of dental restorative materials. J. Adv. Med. Dent. Sci. Res. 2019, 7, 123–127. [Google Scholar]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Dogan, M.S.; Yıldız, Ş. Effects of Different Anti-Epileptic Drug Groups and Brushing on the Color Stability of Restorative Materials Used in Pedodontics: An In Vitro Evaluation. Children 2024, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Tüzüner, T.; Turgut, S.; Baygin, O.; Yilmaz, N.; Tuna, E.B.; Ozen, B. Effects of different pediatric drugs on the color stability of various restorative materials applicable in pediatric dentistry. BioMed Res. Int. 2017, 2017, 9684193. [Google Scholar] [CrossRef]
- Kathiria, H.; Panda, A.; Virda, M.; Budakoti, V.; Dave, P.; Malge, R. Effect of pediatric drugs on color stability of various esthetic restorations in pediatric dentistry. IJPCDR 2021, 8, 35–37. [Google Scholar] [CrossRef]
- Valera, B.; Bhatt, R.; Patel, M.; Patel, C.; Makwani, D.; Goyal, S. Effect of different pediatric medications on various tooth colored restorative materials used in pediatric dentistry: A comparative study. Int. J. Health Sci. 2022, 6, 578–591. [Google Scholar] [CrossRef]
- Singh, A.; Grover, C.; Raina, D.; Pandey, A.; Sri Chaitanya Krishna, A. Color stability of pediatric restorative material over pediatric drug formulation. Cureus 2023, 15, e42953. [Google Scholar] [CrossRef] [PubMed]
- Çevik, N.; Hazar Bodrumlu, E. The effect of fluoride varnish application on colour change due to paediatric drug usage in polyacid-modified composite resin: An in vitro study. Eur. Arch. Paediatr. Dent. 2024. [Google Scholar] [CrossRef]
- Candan, M.; Ünal, M. The effect of various inhaled asthma medications on the color stability of paediatric dental restorative materials. BMC Oral Health 2024, 24, 384. [Google Scholar] [CrossRef]
- Hekmatfar, S.; Fahim, Z.; Davan, M.; Jafari, K. The effect of pediatric drugs on color stability of bulk-fill and conventional composite resins. Gen. Dent. 2024, 72, 72–77. [Google Scholar]
- Yildirim, S.; Uslu, Y.S. Effects of different pediatric drugs and toothbrushing on color change of restorative materials used in pediatric dentistry. Niger. J. Clin. Pract. 2020, 23, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Çınar, B.; Eren, D.; Akın, Ş. Effect of low pH dietary supplements on discoloration of resin composites. Niger. J. Clin. Pract. 2023, 26, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, N.; Baygin, O.; Tüzüner, T.; Turgut, S.N.; Erbek, Ş.M. Evaluation of the effect of pediatric drugs and an oral rinse on primary teeth discoloration. Dent. Med. Probl. 2022, 59, 225–231. [Google Scholar] [CrossRef]
- Paravina, R.D.; Pérez, M.M.; Ghinea, R. Acceptability and perceptibility thresholds in dentistry: A comprehensive review of clinical and research applications. J. Esthet. Restor. Dent. 2019, 31, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Paravina, R.D. Critical appraisal. Color in dentistry: Match me, match me not. J. Esthet. Restor. Dent. 2009, 21, 133–139. [Google Scholar] [CrossRef] [PubMed]
- ISO/TR 28642; Dentistry—Guidance on Color Measurement. ISO: Geneva, Switzerland, 2016.
- Kumari, C.M.; Bhat, K.M.; Bansal, R. Evaluation of surface roughness of different restorative composites after polishing using atomic force microscopy. J. Conserv. Dent. 2016, 19, 56–62. [Google Scholar] [CrossRef]
- Borilova Linhartova, P.; Izakovicova Holla, L. Drugs and dosage forms as risk factors for dental caries. Ceska Slov. Farm. 2017, 66, 103–106. [Google Scholar]
- Neves, B.G.; Farah, A.; Lucas, E.; de Sousa, V.P.; Maia, L.C. Are paediatric medicines risk factors for dental caries and dental erosion? Community Dent. Health 2010, 27, 46–51. [Google Scholar]
- Thomas, M.S.; Vivekananda Pai, A.R.; Yadav, A. Medication-related dental erosion: A review. Compend. Contin. Educ. Dent. 2015, 36, 662–666. [Google Scholar]
- Neha, N.; Sandeep, A.H.; Bhandari, S.; Solete, P.; Choudhari, S. Comparative analysis of the surface roughness of class V composite restorations using a conventional polishing system and pre-contoured cervical matrices: An in vitro study. Cureus 2023, 15, e45901. [Google Scholar]
- Hamza, B.; Eliades, T.; Attin, T.; Schwendener, S.; Karygianni, L. Initial bacterial adherence and biofilm formation on novel restorative materials used in paediatric dentistry. Dent. Mater. 2024, 40, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Karatas, O.; Delikan, E.; Erturk Avunduk, A.T. Comparative evaluation of probiotic solutions on surface roughness and microhardness of different restorative materials and enamel. J. Clin. Pediatr. Dent. 2024, 48, 107–119. [Google Scholar] [PubMed]
- Prabhakar, A.; Kalimireddy, P.; Yavagal, C.; Sugandhan, S. Assessment of the clinical performance of zirconia infused glass ionomer cement: An in vivo study. Int. J. Oral Heal. Sci. 2015, 5, 74–79. [Google Scholar]
- Gladys, S.; Van Meerbeek, B.; Braem, M.; Lambrechts, P.; Vanherle, G. Comparative physico-mechanical characterization of new hybrid restorative materials with conventional glass-ionomer and resin composite restorative materials. J. Dent. Res. 1997, 76, 883–894. [Google Scholar] [CrossRef]
- Nica, I.; Stoleriu, S.; Iovan, A.; Tărăboanță, I.; Pancu, G.; Tofan, N.; Brânzan, R.; Andrian, S. Conventional and resin-modified glass ionomer cement surface characteristics after acidic challenges. Biomedicines 2022, 10, 1755. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, H.; Avula, J.S.; Kakarla, P.; Bandi, S.; Mallela, G.M.; Vallabhaneni, K. Color stability of esthetic restorative materials used in pediatric dentistry: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2016, 34, 233–237. [Google Scholar]
- Çapan, B.Ş.; Birant, S. Effect of pediatric drugs on the color stability of dental restorative materials currently used in pediatric dentistry. J. Aust. Ceram. Soc. 2024, 60, 601–608. [Google Scholar] [CrossRef]
- Fontes, S.T.; Fernández, M.R.; de Moura, C.M.; Meireles, S.S. Color stability of a nanofill composite: Effect of different immersion media. J. Appl. Oral. Sci. 2009, 17, 388–391. [Google Scholar] [CrossRef]
- Bezgin, T.; Özer, L.; Tulga Öz, F.; Özkan, P. Effect of toothbrushing on color changes of esthetic restorative materials. J. Esthet. Restor. Dent. 2015, 27, 65–73. [Google Scholar] [CrossRef]
- Bagheri, R.; Burrow, M.F.; Tyas, M. Influence of food-simulating solutions and surface finish on susceptibility to staining of aesthetic restorative materials. J. Dent. 2005, 33, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Nasoohi, N.; Hoorizad, M.; Torabzadeh Tari, N. Effect of tea and coffee on color change of two types composite resins: Nanofilled and Micro hybrid. J. Res. Dent. Sci. 2011, 7, 18–22. [Google Scholar]
- Tunc, E.S.; Bayrak, S.; Guler, A.U.; Tuloglu, N. The effects of children’s drinks on the color stability of various restorative materials. J. Clin. Pediatr. Dent. 2009, 34, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Özdaş, D.Ö.; Kazak, M.; Çilingir, A.; Subaşı, M.G.; Tiryaki, M.; Günal, Ş. Color stability of composites after short-term oral simulation: An in vitro study. Open Dent. J. 2016, 10, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, N.B.; Arikan, V.; Akbay Oba, A. Effect of mouthwashes on the discolouration of restorative materials commonly used in paediatric dentistry. Eur. Arch. Paediatr. Dent. 2018, 19, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.V.; Nagy, P.M.; Chass, G.A.; Fejerdy, P.; Nicholson, J.W.; Csizmadia, I.G.; Dobó-Nagy, C. Qualitative assessment of microstructure and Hertzian indentation failure in biocompatible glass ionomer cements. J. Mater. Sci. Mater. Med. 2012, 23, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Hotwani, K.; Thosar, N.; Baliga, S. Comparative in vitro assessment of color stability of hybrid esthetic restorative materials against various children’s beverages. J. Conserv. Dent. 2014, 17, 70–74. [Google Scholar] [CrossRef]
- Mutlu-Sagesen, L.; Ergün, G.; Ozkan, Y.; Semiz, M. Color stability of a dental composite after immersion in various media. Dent. Mater. J. 2005, 24, 382–390. [Google Scholar] [CrossRef]

| Material | Classification | Composition | Manufacturer |
|---|---|---|---|
| Fuji IX GP | Conventional glass ionomer cement | Powder: aluminum–calcium–lanthanum fluorosilicate glass, acrylic acid, maleic acid; Liquid: poly(alkenoic acid) tartaric acid, water | 3M ESPE, St. Paul, MN, USA |
| Fuji II LC | Resin-modified glass ionomer cement | Powder: fluoroaluminosilicate glass, HEMA, urethane dimethacrylate, water, photoinitiator (camphorquinone); Liquid: poly(acrylic acid) | GC, Tokyo, Japan |
| Zirconomer Improved | Zirconia-reinforced glass ionomer cement | Zirconium oxide (nano-sized zirconia filler particles ranging from 96.5% to 98.5%), glass powder, tartaric acid, polyacrylic acid, and deionized water | Shofu Inc., Ashford, UK |
| Dyract®XTRA | Polyacid-modified composite resin (compomer) | UDMA, TCB resin, TEGDMA, trimethacrylate resin, camphorquinone, ethyl-4-dimethylaminobenzoate, BHT, UV stabilizer, strontium-alumino-sodiumfluorophosphor-silicate glass, highly dispersed silicon dioxide, strontium fluoride, iron oxide and titanium dioxide pigments (mean filler size: 0.8, filler volume 47%) | Dentsply, Konstanz, Germany |
| Equia Forte HT Fill | Bulk-fill glass hybrid restorative system | Powder: fluoroaluminosilicate glass, polyacrylic acid, iron oxide; Liquid: polybasic carboxylic acid, water | GC, Tokyo, Japan |
| Charisma Smart | Conventional composite resin | Bis-EMA, HEDMA, TEGDMA, barium aluminium fluoride glass (0.02–2 μm), pyrogenic silicon dioxide (0.02–0.07 μm) | Kulzer, Hanau, Germany |
| Cention N | “Alkasite” (composite resin with reactive glass fillers) | Powder: barium aluminum silicate glass, ytterbium trifluoride, isofiller, calcium barium aluminum fluorosilicate glass, calcium fluoro silicate glass; Liquid: urethane dimethacrylate, tricyclodecandimethanol dimethacrylate, tetramethyl-xylylene, diurethane dimethacrylate, polyethylene glycol 400, dimethacrylate, ivocerin, hydroperoxide | Ivoclar Vivadent, Schaan, Lichtenstein |
| Product | Composition | Manufacturer |
|---|---|---|
| Sambucol kids | Glucose syrup, black elderberry juice concentrate, L-ascorbic acid (vitamin C). Acidity regulator: citric acid. Preservative: potassium sorbate | PharmaCare, Karlsruhe, Germany |
| Resverol | Resveratrol, quercetin, vitamin C | Armin, İzmir, Türkiye |
| Imunol | Echinacea extract, 1.3–1.6 beta glucan, zinc, propolis, vitamin C | Orzax, İstanbul, Türkiye |
| Umca | Pelargonium sidoide liquid extract, maltodextrin, xylitol, glycerol, citric acid anhydrous, potassium sorbate, xanthan gum, pure water | ISO Arzneimittel GmbH&Co., Karlsruhe, Germany |
| Microfer | Iron (lipophere ‘microencapsulated iron source’) | Orzax, İstanbul, Türkiye |
| Material | Specimen Preparation | Polymerization Procedures |
|---|---|---|
| Fuji IX GP | A standard powder/liquid ratio of 1 level scoop of powder and 1 drop of liquid (3.6 g/1.0 g) was placed on the pad. The powder was divided into two parts with the help of a plastic spatula. The first portion was mixed with all the liquid for 10 s. The remaining powder was added, and the whole sample was mixed for 15–20 s. | Self-curing material, setting time of 2 min 20 s. |
| Fuji II LC | The capsule was shaken to loosen the powder inside the capsule, and its piston was pushed until it was aligned with the main body to activate the capsule. The capsule was placed in an amalgamator and mixed for 10 s. The mixed capsule was loaded into the GC capsule applicator, and the application was made. | Light-curing for 20 s using a visible light-curing device (LED/Halogen > 700 mW/cm2). |
| Zirconomer Improved | Two parts powder and one drop (standard powder/liquid ratio: 3.6 g/1.0 g) of liquid were dispersed onto the mixing pad. The dispensed powder was divided into 2 equal portions; the first half was added to the distributed liquid and mixed with a plastic spatula for 5–10 s. Then, the remaining half was added and mixed for a total of 30 s until it reached a thick paste consistency. | Self-curing material, setting time of 3 min (from the end of mixing) |
| Dyract®XTRA | The compule tip was inserted into the notched opening of the gun. The material was placed by applying constant pressure to the gun. | Curing to 10 s (depth of at least 2 mm) using a lamp with an output over 500 mW/cm2 |
| Equia Forte HT Fill | The capsule was shaken to loosen the powder. Then, the plunger was pressed and held firmly for 2 s. It was mixed in the amalgamator for 10 s. The capsule was immediately inserted into the applicator and applied within 10 s. | Setting time of 2 min 30 s (from the start of mixing) |
| Charisma Smart | The ready-made material in the syringe was applied directly. | Curing time of 20 sn (for maximum layer thickness 2 mm) for blue-light-curing units (wavelength peak at 450–480 nm; light output of 1550–600 mW/cm2 |
| Cention N | One measuring spoon of powder and one drop of liquid were used as the mixing ratio. The powder and liquid were taken onto a mixing pad, and the powder was divided into two equal-sized pieces with a plastic spatula. The first part was mixed first, and then the remaining powder was added. It was mixed again for 45–60 s until a homogeneous consistency was obtained. | * Self-curing material, with light-curing option Setting time of 5 min (from the start of mixing) The restoration can be optionally light-cured after placement (for ≥500 mW/cm2 light intensity, exposure time 40 sn; for >1000 mW/cm2 light intensity, exposure times 20 sn). |
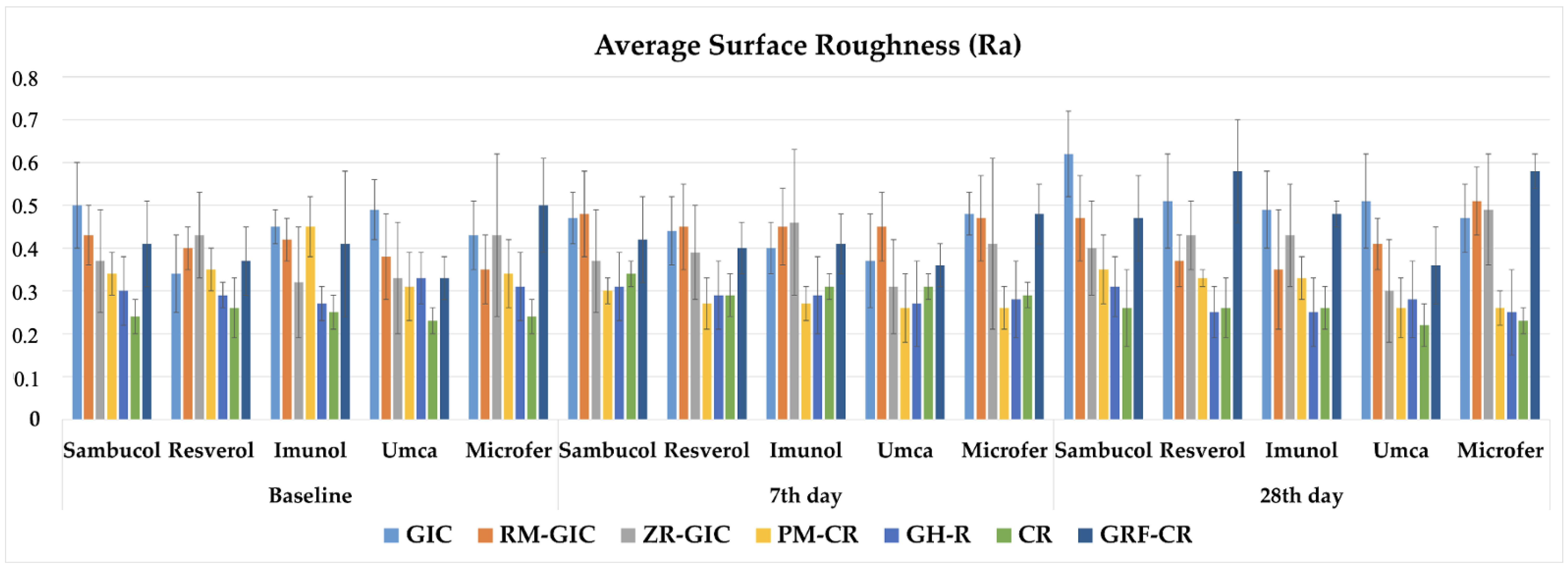
| Material | Baseline | 7th Day | 28th Day | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sambucol | Resverol | Imunol | Umca | Microfer | Sambucol | Resverol | Imunol | Umca | Microfer | Sambucol | Resverol | Imunol | Umca | Microfer | |
| GIC | 0.5 (±0.10) A a 2 | 0.34 (±0.09) BC b 3 | 0.45 (±0.04) A a 12 | 0.49 (±0.07) A a 1 | 0.43 (±0.08) A a 1 | 0.47 (±0.06) A ab 2 | 0.44 (±0.08) A abc 2 | 0.40 (±0.06) A bc 2 | 0.37 (±0.11) B c 2 | 0.48 (±0.05) A a 1 | 0.62 (±0.10) A a 1 | 0.51 (±0.11) A b 1 | 0.49 (±0.09) A b 1 | 0.51 (±0.11) A b 1 | 0.47 (±0.08) A b 1 |
| RM-GIC | 0.43 (±0.07) AB a 1 | 0.40 (±0.05) AB ab 12 | 0.42 (±0.05) A a 1 | 0.38 (±0.10) B ab 2 | 0.35 (±0.08) B b 2 | 0.48 (±0.10) A a 1 | 0.45 (±0.10) A a 1 | 0.45 (±0.09) A a 1 | 0.45 (±0.08) A a 1 | 0.47 (±0.10) A a 1 | 0.47 (±0.10) B ab 1 | 0.37 (±0.06) BC c 2 | 0.35 (±0.14) BC c 2 | 0.41 (±0.06) B bc 12 | 0.51 (±0.08) AB a 1 |
| ZR-GIC | 0.37 (±0.12) BC ab 1 | 0.43 (±0.10) A a 1 | 0.32 (±0.13) B b 2 | 0.33 (±0.13) B b 1 | 0.43 (±0.19) A a 12 | 0.37 (±0.12) BC ab 1 | 0.39 (±0.11) A bc 1 | 0.46 (±0.17) A c 1 | 0.31 (±0.11) BC a 1 | 0.41 (±0.20) A bc 2 | 0.40 (±0.11) BC b 1 | 0.43 (±0.08) B bc 1 | 0.43 (±0.12) AB bc 1 | 0.30 (±0.12) CD a 1 | 0.49 (±0.13) A c 1 |
| PM-CR | 0.34 (±0.05) C b 1 | 0.35 (±0.05) ABC b 1 | 0.45 (±0.07) A a 1 | 0.31 (±0.08) B b 1 | 0.34 (±0.08) B b 1 | 0.30 (±0.03) C a 1 | 0.27 (±0.06) B a 2 | 0.27 (±0.04) B a 2 | 0.26 (±0.08) C a 1 | 0.26 (±0.05) B a 2 | 0.35 (±0.08) CD ac 1 | 0.33 (±0.02) CD b 12 | 0.33 (±0.05) CD ab 2 | 0.26 (±0.07) DE a 1 | 0.26 (±0.04) C bc 2 |
| GH-R | 0.30 (±0.08) CD a 1 | 0.29 (±0.03) CD a 1 | 0.27 (±0.04) B a 1 | 0.33 (±0.06) B a 1 | 0.31 (±0.08) BC a 1 | 0.31 (±0.08) C a 1 | 0.29 (±0.08) B a 1 | 0.29 (±0.09) B a 1 | 0.27 (±0.10) C a 1 | 0.28 (±0.09) B a 1 | 0.31 (±0.07) DE a 1 | 0.25 (±0.06) E a 1 | 0.25 (±0.08) E a 1 | 0.28 (±0.09) DE a 1 | 0.25 (±0.10) C a 1 |
| CR | 0.24 (±0.04) D a 2 | 0.26 (±0.07) D a 1 | 0.25 (±0.04) B a 2 | 0.23 (±0.03) C a 2 | 0.24 (±0.04) C a 1 | 0.34 (±0.03) C a 1 | 0.29 (±0.05) B a 1 | 0.31 (±0.03) B a 1 | 0.31 (±0.03) BC a 1 | 0.29 (±0.03) B a 1 | 0.26 (±0.09) E a 2 | 0.26 (±0.07) DE a 1 | 0.26 (±0.05) DE a 12 | 0.22 (±0.05) E a 2 | 0.23 (±0.03) C a 1 |
| GRF-CR | 0.41 (±0.10) B c 1 | 0.37 (±0.08) AB bc 2 | 0.41 (±0.17) A c 1 | 0.33 (±0.05) B b 1 | 0.50 (±0.11) A a 2 | 0.42 (±0.10) AB bc 1 | 0.40 (±0.06) A b 2 | 0.41 (±0.07) A abc 1 | 0.36 (±0.05) B b 1 | 0.48 (±0.07) A c 2 | 0.47 (±0.10) B b 1 | 0.58 (±0.12) A a 1 | 0.48 (±0.03) A b 1 | 0.36 (±0.09) BC c 1 | 0.58 (±0.04) B a 1 |
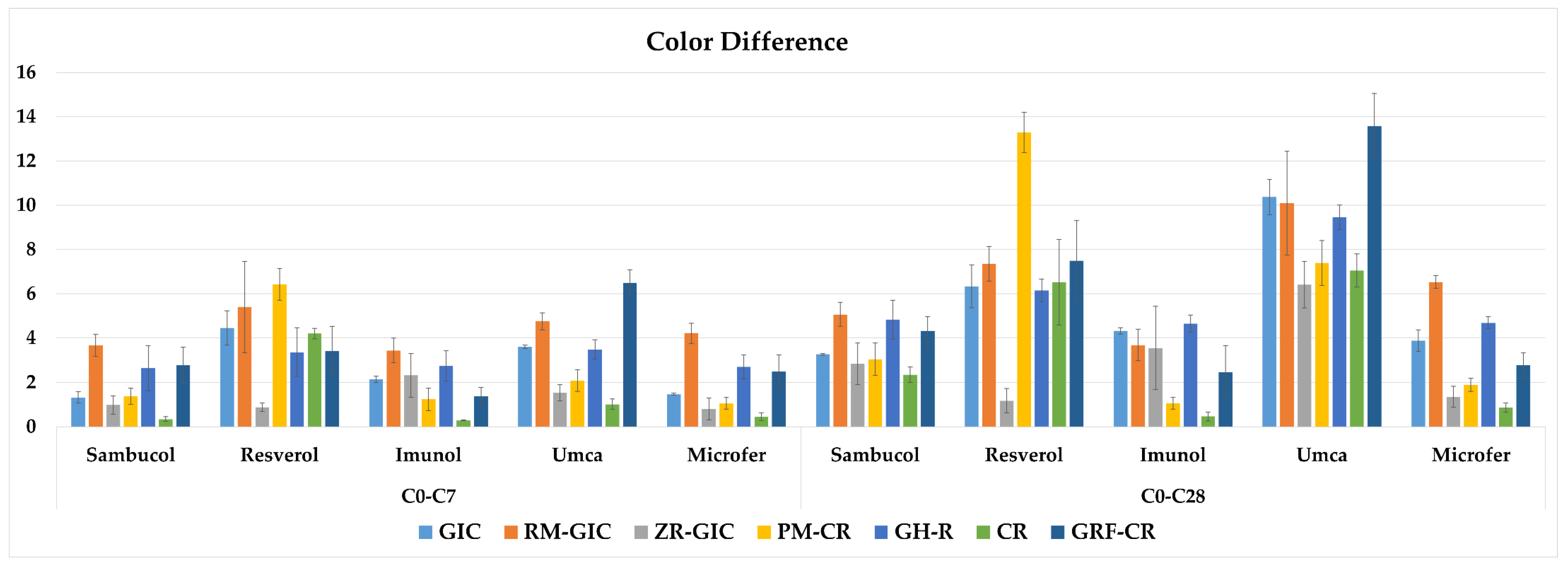
| Material | C0–C7 | C0–C28 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sambucol | Resverol | Imunol | Umca | Microfer | Sambucol | Resverol | Imunol | Umca | Microfer | |
| GIC | 1.32 (±0.25) C d 2 | 4.45 (±0.76) C b 2 | 2.15 (±0.14) B c 2 | 3.61 (±0.08) C a 2 | 1.46 (±0.05) C d 2 | 3.27 (±0.04) B d 1 | 6.33 (±0.96) C b 1 | 4.32 (±0.14) AB c 1 | 10.37 (±0.80) C a 1 | 3.88 (±0.48) B cd 1 |
| RM-GIC | 3.67 (±0.49) A cd 2 | 5.39 (±2.06) B a 2 | 3.44 (±0.55) A d 1 | 4.75 (±0.39) B b 2 | 4.21 (±0.45) A bc 2 | 5.06 (±0.55) A d 1 | 7.35 (±0.78) B b 1 | 3.68 (±0.70) B c 1 | 10.09 (±2.34) BC a 1 | 6.53 (±0.28) A b 1 |
| ZR-GIC | 0.98 (±0.42) C bc 2 | 0.88 (±0.19) E c 1 | 2.32 (±0.99) BC a 2 | 1.53 (±0.37) DE b 2 | 0.80 (±0.49) DE c 1 | 2.85 (±0.94) BC b 1 | 1.17 (±0.55) D c 1 | 3.55 (±1.88) B b 1 | 6.41 (±1.05) E a 1 | 1.35 (±0.47) CD c 1 |
| PM-CR | 1.38 (±0.37) C c 2 | 6.42 (±0.72) A b 2 | 1.24 (±0.51) D c 1 | 2.08 (±0.49) D a 2 | 1.05 (±0.26) CD c 2 | 3.05 (±0.74) BC c 1 | 13.29 (±0.91) A a 1 | 1.06 (±0.26) C e 1 | 7.39 (±1.01) D b 1 | 1.89 (±0.30) C d 1 |
| GH-R | 2.65 (±1.02) B b 2 | 3.36 (±1.09) D a 2 | 2.74 (±0.69) C b 2 | 3.49 (±0.43) C a 2 | 2.70 (±0.55) B b 2 | 4.83 (±0.88) A c 1 | 6.15 (±0.50) C b 1 | 4.65 (±0.39) A c 1 | 9.45 (±0.55) B a 1 | 4.68 (±0.27) B c 1 |
| CR | 0.34 (±0.11) D c 2 | 4.2 (±0.24) C a 2 | 0.28 (±0.02) E c 1 | 1.01 (±0.24) E b 2 | 0.44 (±0.18) E bc 1 | 2.35 (±0.35) C c 1 | 6.52 (±1.94) C a 1 | 0.46 (±0.21) C b 1 | 7.05 (±0.75) DE a 1 | 0.86 (±0.21) D b 1 |
| GRF-CR | 2.79 (±0.79) B d 2 | 3.43 (±1.08) D b 2 | 1.37 (±0.4) D c 2 | 6.48 (±0.6) A a 2 | 2.5 (±0.74) B d 1 | 4.31 (±0.64) A d 1 | 7.49 (±1.81) B b 1 | 2.46 (±1.21) D c 1 | 13.57 (±1.48) A a 1 | 2.79 (±0.54) E c 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aktaş, N.; Akın, Y.; Bal, C.; Bani, M.; Bankoğlu Güngör, M. Effect of the Different Dietary Supplements on the Average Surface Roughness and Color Stability of Direct Restorative Materials Used in Pediatric Dentistry. Children 2024, 11, 645. https://doi.org/10.3390/children11060645
Aktaş N, Akın Y, Bal C, Bani M, Bankoğlu Güngör M. Effect of the Different Dietary Supplements on the Average Surface Roughness and Color Stability of Direct Restorative Materials Used in Pediatric Dentistry. Children. 2024; 11(6):645. https://doi.org/10.3390/children11060645
Chicago/Turabian StyleAktaş, Nagehan, Yasemin Akın, Cenkhan Bal, Mehmet Bani, and Merve Bankoğlu Güngör. 2024. "Effect of the Different Dietary Supplements on the Average Surface Roughness and Color Stability of Direct Restorative Materials Used in Pediatric Dentistry" Children 11, no. 6: 645. https://doi.org/10.3390/children11060645
APA StyleAktaş, N., Akın, Y., Bal, C., Bani, M., & Bankoğlu Güngör, M. (2024). Effect of the Different Dietary Supplements on the Average Surface Roughness and Color Stability of Direct Restorative Materials Used in Pediatric Dentistry. Children, 11(6), 645. https://doi.org/10.3390/children11060645






