Complete Transposition of the Great Arteries in the Pediatric Field: A Multimodality Imaging Approach
Abstract
1. Introduction
2. Fetal Echocardiogram in Complete Transposition of the Great Arteries
3. Transthoracic Echocardiography in Complete Transposition of the Great Arteries
3.1. Preoperative Evaluation
3.2. Postoperative Evaluation
3.3. Stress Echocardiography in Complete Transposition of the Great Arteries
4. Cardiovascular Magnetic Resonance (CMR) in Complete Transposition of the Great Arteries
4.1. Fetal CMR
4.2. CMR in Neonatal and Pediatric Life
4.3. Preoperative
4.4. Postoperative
5. Cardiac Computed Tomography (CCT) in Complete Transposition of the Great Arteries
5.1. Preoperative Imaging
5.2. Postoperative Imaging
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASD | atrial septal defect |
| ASO | arterial switch operation |
| AtrSO | atrial switch operation |
| AV | aortic valve |
| b-SSFP | balanced steady-state free precession |
| CA | coronary arteries |
| CAD | coronary artery disease |
| CCT | cardiac computed tomography |
| CTGA | complete TGA |
| CCTGA | congenital corrected TGA/L-TGA |
| CFR | coronary flow reserve |
| CHD | congenital heart disease |
| CX | circumflex artery |
| DORV | double outlet right ventricle |
| FFSE | fast spin echo sequences |
| GAA | gothic aortic arch |
| GBCA | gadolinium-based contrast agents |
| HASTE | (half-Fourier acquisition single shot) |
| LAD | left anterior descending artery |
| LCA | left coronary artery |
| LCx | left circumflex |
| LGE | late gadolinium enhancement |
| LV | left ventricle |
| LVOT | left ventricular outflow tract |
| LVOTO | left ventricular outflow obstruction |
| MRI | magnetic resonance imaging |
| PA | pulmonary artery |
| PDA | patent ductus arteriosus |
| PFO | patent foramen ovale |
| PS | pulmonary stenosis |
| RA | right atrium |
| RCA | right coronary artery |
| RV | right ventricle |
| RVOT | right ventricular outflow tract |
| SAX | short axis |
| SVC | superior vena cava |
| TGA | transposition great arteries |
| TTE | transthoracic echocardiography |
| US | ultrasound |
| VSD | ventricular septal defect |
| WSS | wall shear stress |
| PS | pulmonary stenosis |
| PV | pulmonary valve |
| PVs | pulmonary veins |
| 2D | two dimension |
| 4CH | four chamber |
References
- Samánek, M.; Slavík, Z.; Zborilová, B.; Hrobonová, V.; Vorísková, M.; Skovránek, J. Prevalence, Treatment, and Outcome of Heart Disease in Live-Born Children: A Prospective Analysis of 91,823 Live-Born Children. Pediatr. Cardiol. 1989, 10, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sarris, G.E.; Balmer, C.; Bonou, P.; Comas, J.V.; da Cruz, E.; Chiara, L.D.; Di Donato, R.M.; Fragata, J.; Jokinen, T.E.; Kirvassilis, G.; et al. Clinical Guidelines for the Management of Patients with Transposition of the Great Arteries with Intact Ventricular Septum. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2017, 51, e1–e32. [Google Scholar] [CrossRef] [PubMed]
- Wernovsky, G. Transposition of the Great Arteries and Common Variants. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2016, 17, S337–S343. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the Management of Adult Congenital Heart Disease: The Task Force for the Management of Adult Congenital Heart Disease of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Adult Congenital Heart Disease (ISACHD). Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Warnes, C.A. Transposition of the Great Arteries. Circulation 2006, 114, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Salerno, N.; Panuccio, G.; Sabatino, J.; Leo, I.; Torella, M.; Sorrentino, S.; De Rosa, S.; Torella, D. Cellular and Molecular Mechanisms Underlying Tricuspid Valve Development and Disease. J. Clin. Med. 2023, 12, 3454. [Google Scholar] [CrossRef] [PubMed]
- Van Praagh, R.; Van Praagh, S. Isolated Ventricular Inversion. A Consideration of the Morphogenesis, Definition and Diagnosis of Nontransposed and Transposed Great Arteries. Am. J. Cardiol. 1966, 17, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, M.; Al Adnani, M.; Sebire, N.J. Neonatal Death Due to Transposition in Association with Premature Closure of the Oval Foramen. Cardiol. Young 2006, 16, 586–589. [Google Scholar] [CrossRef]
- Senning, A. Surgical Correction of Transposition of the Great Vessels. Surgery 1959, 45, 966–980. [Google Scholar]
- Mustard, W.T. Successful two-stage correction of transposition of the great vessels. Surgery 1964, 55, 469–472. [Google Scholar]
- Yacoub, M.H.; Radley-Smith, R.; Hilton, C.J. Anatomical Correction of Complete Transposition of the Great Arteries and Ventricular Septal Defect in Infancy. Br. Med. J. 1976, 1, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Jatene, A.D.; Fontes, V.F.; Paulista, P.P.; de Souza, L.C.; Neger, F.; Galantier, M.; Souza, J.E. Successful Anatomic Correction of Transposition of the Great Vessels. A Preliminary Report. Arq. Bras. Cardiol. 1975, 28, 461–464. [Google Scholar] [PubMed]
- Rastelli, G.C.; Wallace, R.B.; Ongley, P.A. Complete Repair of Transposition of the Great Arteries with Pulmonary Stenosis. A Review and Report of a Case Corrected by Using a New Surgical Technique. Circulation 1969, 39, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Nikaidoh, H. Aortic Translocation and Biventricular Outflow Tract Reconstruction. A New Surgical Repair for Transposition of the Great Arteries Associated with Ventricular Septal Defect and Pulmonary Stenosis. J. Thorac. Cardiovasc. Surg. 1984, 88, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, C. L-Transposition of the Great Arteries. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2016, 17, S344–S346. [Google Scholar] [CrossRef] [PubMed]
- Warnes, C.A. Congenitally Corrected Transposition: The Uncorrected Misnomer. J. Am. Coll. Cardiol. 1996, 27, 1244–1245. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.P.; Bernard, Y.D.; Mellen, B.G.; Celermajer, D.; Baumgartner, H.; Cetta, F.; Connolly, H.M.; Davidson, W.R.; Dellborg, M.; Foster, E.; et al. Long-Term Outcome in Congenitally Corrected Transposition of the Great Arteries: A Multi-Institutional Study. J. Am. Coll. Cardiol. 2000, 36, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Kutty, S.; Danford, D.A.; Diller, G.-P.; Tutarel, O. Contemporary Management and Outcomes in Congenitally Corrected Transposition of the Great Arteries. Heart Br. Card. Soc. 2018, 104, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, D.Z.; Nadas, A.S. Clinical Profile of Patients with Congenital Corrected Transposition of the Great Arteries. A Study of 60 Cases. N. Engl. J. Med. 1970, 282, 1053–1059. [Google Scholar] [CrossRef]
- Marek, J.; Tomek, V.; Skovránek, J.; Povysilová, V.; Samánek, M. Prenatal Ultrasound Screening of Congenital Heart Disease in an Unselected National Population: A 21-Year Experience. Heart Br. Card. Soc. 2011, 97, 124–130. [Google Scholar] [CrossRef]
- van Velzen, C.L.; Haak, M.C.; Reijnders, G.; Rijlaarsdam, M.E.B.; Bax, C.J.; Pajkrt, E.; Hruda, J.; Galindo-Garre, F.; Bilardo, C.M.; de Groot, C.J.M.; et al. Prenatal Detection of Transposition of the Great Arteries Reduces Mortality and Morbidity. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2015, 45, 320–325. [Google Scholar] [CrossRef] [PubMed]
- American Institute of Ultrasound in Medicine. AIUM Practice Guideline for the Performance of Obstetric Ultrasound Examinations. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2010, 29, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Alfirevic, Z.; Berghella, V.; Bilardo, C.; Hernandez-Andrade, E.; Johnsen, S.L.; Kalache, K.; Leung, K.-Y.; Malinger, G.; Munoz, H.; et al. Practice Guidelines for Performance of the Routine Mid-Trimester Fetal Ultrasound Scan. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2011, 37, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Moon-Grady, A.J.; Donofrio, M.T.; Gelehrter, S.; Hornberger, L.; Kreeger, J.; Lee, W.; Michelfelder, E.; Morris, S.A.; Peyvandi, S.; Pinto, N.M.; et al. Guidelines and Recommendations for Performance of the Fetal Echocardiogram: An Update from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2023, 36, 679–723. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.; Allan, L.; Chaoui, R.; Copel, J.; DeVore, G.; Hecher, K.; Lee, W.; Munoz, H.; Paladini, D.; Tutschek, B.; et al. ISUOG Practice Guidelines (Updated): Sonographic Screening Examination of the Fetal Heart. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2013, 41, 348–359. [Google Scholar] [CrossRef]
- Huggon, I.C.; Ghi, T.; Cook, A.C.; Zosmer, N.; Allan, L.D.; Nicolaides, K.H. Fetal Cardiac Abnormalities Identified Prior to 14 Weeks’ Gestation. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2002, 20, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Coltri, A.; Butera, G.; Fermont, L.; Le Bidois, J.; Kachaner, J.; Sidi, D. Detection of Transposition of the Great Arteries in Fetuses Reduces Neonatal Morbidity and Mortality. Circulation 1999, 99, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.S.; Axt-Fliedner, R.; Chaoui, R.; Copel, J.A.; Cuneo, B.F.; Goff, D.; Gordin Kopylov, L.; Hecher, K.; Lee, W.; Moon-Grady, A.J.; et al. ISUOG Practice Guidelines (Updated): Fetal Cardiac Screening. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2023, 61, 788–803. [Google Scholar] [CrossRef]
- Allen, H.D.; Driscoll, D.J.; Shaddy, R.E.; Feltes, T.F. Moss & Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; ISBN 978-1-4511-7127-3. [Google Scholar]
- Kaji, T.; Hayabuchi, Y.; Maeda, K.; Irahara, S.N.M. Prenatal assessment of coronary artery anatomy using color Doppler in cases of D-transposition of the great arteries: Case reports. J. Obstet. Gynaecol. Res. 2017, 43, 397–402. [Google Scholar] [CrossRef]
- Haligheri, G.; Patel, C.R.; Komarlu, R. Prenatal Delineation of Coronary Anatomy in Dextro-Transposition of Great Arteries. J. Cardiovasc. Echogr. 2021, 31, 171–174. [Google Scholar]
- Wilson, A.D.; Rao, P.S.; Aeschlimann, S. Normal Fetal Foramen Flap and Transatrial Doppler Velocity Pattern. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 1990, 3, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Słodki, M.; Axt-Fliedner, R.; Zych-Krekora, K.; Wolter, A.; Kawecki, A.; Enzensberger, C.; Gulczyńska, E.; Respondek-Liberska, M. International Prenatal Cardiology Collaboration Group New Method to Predict Need for Rashkind Procedure in Fetuses with Dextro-Transposition of the Great Arteries. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2018, 51, 531–536. [Google Scholar] [CrossRef]
- Cohen, M.S.; Eidem, B.W.; Cetta, F.; Fogel, M.A.; Frommelt, P.C.; Ganame, J.; Han, B.K.; Kimball, T.R.; Johnson, R.K.; Mertens, L.; et al. Multimodality Imaging Guidelines of Patients with Transposition of the Great Arteries: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2016, 29, 571–621. [Google Scholar] [CrossRef] [PubMed]
- Graziano, J.N.; Heidelberger, K.P.; Ensing, G.J.; Gomez, C.A.; Ludomirsky, A. The Influence of a Restrictive Atrial Septal Defect on Pulmonary Vascular Morphology in Patients with Hypoplastic Left Heart Syndrome. Pediatr. Cardiol. 2002, 23, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Hoque, T.; Richmond, M.; Vincent, J.A.; Bacha, E.; Torres, A. Current Outcomes of Hypoplastic Left Heart Syndrome with Restrictive Atrial Septum: A Single-Center Experience. Pediatr. Cardiol. 2013, 34, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, N.; Avesani, M.; Sabatino, J.; Ibrahim, A.; Josen, M.; Paredes, J.; Di Salvo, G. Blood Speckle Imaging: A New Echocardiographic Approach to Study Fluid Dynamics in Congenital Heart Disease. Int. J. Cardiol. Congenit. Heart Dis. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Cohen, M.S.; Mertens, L.L. Educational Series in Congenital Heart Disease: Echocardiographic Assessment of Transposition of the Great Arteries and Congenitally Corrected Transposition of the Great Arteries. Echo Res. Pract. 2019, 6, R107–R119. [Google Scholar] [CrossRef] [PubMed]
- Baraona, F.; Valente, A.M.; Porayette, P.; Pluchinotta, F.R.; Sanders, S.P. Coronary Arteries in Childhood Heart Disease: Implications for Management of Young Adults. J. Clin. Exp. Cardiolog. 2012, (Suppl. S8), S006. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Rajiah, P. Single coronary artery with a pre-pulmonic dual left anterior descending artery and a retro-aortic left circumflex artery. Cardiol. Young 2016, 26, 1241–1245. [Google Scholar] [CrossRef]
- Mahle, W.T.; Gonzalez, J.H.; Kreeger, J.; Marx, G.; Duldani, G.; Silverman, N.H. Echocardiography of Transposition of the Great Arteries. Cardiol. Young 2012, 22, 664–670. [Google Scholar] [CrossRef]
- Martins, P.; Castela, E. Transposition of the great arteries. Orphanet J. Rare Dis. 2008, 3, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Salvo, G.; Al Bulbul, Z.; Issa, Z.; Fadel, B.; Al-Sehly, A.; Pergola, V.; Al Halees, Z.; Al Fayyadh, M. Left Ventricular Mechanics after Arterial Switch Operation: A Speckle-Tracking Echocardiography Study. J. Cardiovasc. Med. 2016, 17, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Bragantini, G.; Bartolacelli, Y.; Balducci, A.; Ciuca, C.; Gesuete, V.; Palleri, D.; Egidy Assenza, G.; Mariucci, E.; Angeli, E.; Gargiulo, G.D.; et al. Left Ventricle Function after Arterial Switch Procedure for D-Transposition of the Great Arteries: Long Term Evaluation by Speckle-Tracking Analysis. Int. J. Cardiol. Congenit. Heart Dis. 2022, 8, 100374. [Google Scholar] [CrossRef]
- Ro, S.S.; Wan, Q.; Pasumarti, N.; Keelan, J.; Shah, A.; Krishnamurthy, G.; Choudhury, T.A.; Anderson, B.R.; LaPar, D.; Bacha, E.; et al. Post-Operative Troponin Levels and Left Ventricular Function in Patients with d-Transposition of the Great Arteries Following the Arterial Switch Operation. Int. J. Cardiovasc. Imaging 2023, 39, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Buendía-Fuentes, F.; Lozano-Edo, S.; Jover-Pastor, P.; Sánchez-Martínez, J.C.; Martínez-Sole, J.; Rodríguez-Serrano, M.; Aguero, J.; Arnau-Vives, M.A.; Osa-Sáez, A.; Martínez-Dolz, L.V.; et al. Left Atrial Strain in Adults after the Arterial Switch Operation for Transposition of the Great Arteries. Echocardiography 2024, 41, e15750. [Google Scholar] [CrossRef]
- van der Palen, R.L.F.; van der Bom, T.; Dekker, A.; Tsonaka, R.; van Geloven, N.; Kuipers, I.M.; Konings, T.C.; Rammeloo, L.A.J.; Ten Harkel, A.D.J.; Jongbloed, M.R.M.; et al. Progression of Aortic Root Dilatation and Aortic Valve Regurgitation after the Arterial Switch Operation. Heart Br. Card. Soc. 2019, 105, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Cifra, B.; Dragulescu, A.; Border, W.L.; Mertens, L. Stress echocardiography in paediatric cardiology. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Ermis, P. Stress Echocardiography: An Overview for Use in Pediatric and Congenital Cardiology. Congenit. Heart Dis. 2017, 12, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Luijnenburg, S.E. Prevalence and Predictors of Myocardial Ischemia in Patients with Transposition of the Great Arteries 25 Years after Arterial Switch Operation. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 554–562. [Google Scholar] [CrossRef]
- Kumar, V.; Ward, C.; Justo, R.; Anderson, B. The Gore Septal Occluder (GSO) for Multiple Indications in Children—An Intention to Treat Analysis. Heart Lung Circ. 2021, 30, 1578–1581. [Google Scholar] [CrossRef]
- Silvilairat, S. Speckle-Tracking Echocardiography in Pediatric Patients with Transposition of the Great Arteries: A Comparative Study with Magnetic Resonance Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 355–363. [Google Scholar]
- Valsangiacomo Buechel, E.R.; Grosse-Wortmann, L.; Fratz, S.; Eichhorn, J.; Sarikouch, S.; Greil, G.F.; Beerbaum, P.; Bucciarelli- Ducci, C.; Bonello, B.; Sieverding, L.; et al. Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: An expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, S.; Leo, I.; Lisignoli, V.; Boyle, S.; Bucciarelli-Ducci, C.; Secinaro, A.; Montanaro, C. Cardiovascular Magnetic Resonance from Fetal to Adult Life-Indications and Challenges: A State-of-the-Art Review. Children 2023, 10, 763. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azarine, A.; Garçon, P.; Stansal, A.; Canepa, N.; Angelopoulos, G.; Silvera, S.; Sidi, D.; Marteau, V.; Zins, M. Four-dimensional Flow MRI: Principles and Cardiovascular Applications. RadioGraphics 2019, 39, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Fratz, S.; Chung, T.; Greil, G.F.; Samyn, M.M.; Taylor, A.M.; Buechel, E.R.V.; Yoo, S.-J.; Powell, A.J. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J. Cardiovasc. Magn. Reson. 2013, 15, 51. [Google Scholar] [CrossRef]
- Jalili, M.H.; Yu, T.; Hassani, C.; Prosper, A.E.; Finn, J.P.; Bedayat, A. Contrast-enhanced MR Angiography without Gadoliniumbased Contrast Material: Clinical Applications Using Ferumoxytol. Radiol. Cardiothorac. Imaging 2022, 4, e210323. [Google Scholar] [CrossRef]
- Nazarian, S.; Beinart, R.; Halperin, H.R. Magnetic resonance imaging and implantable devices. Circ. Arrhythmia Electrophysiol. 2013, 6, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Do, C.; DeAguero, J.; Brearley, A.; Trejo, X.; Howard, T.; Escobar, G.P.; Wagner, B. Gadolinium-Based Contrast Agent Use, TheirnSafety, and Practice Evolution. Kidney360 2020, 1, 561–568. [Google Scholar] [CrossRef]
- Sun, L.; Lee, F.-T.; van Amerom, J.F.P.; Freud, L.; Jaeggi, E.; Macgowan, C.K.; Seed, M. Update on fetal cardiovascular magnetic resonance and utility in congenital heart disease. J. Congenit. Heart Dis. 2021, 5, 4. [Google Scholar] [CrossRef]
- Pozza, A.; Reffo, E.; Castaldi, B.; Cattapan, I.; Avesani, M.; Biffanti, R.; Cavaliere, A.; Cerutti, A.; Di Salvo, G. Utility of Fetal Cardiac Resonance Imaging in Prenatal Clinical Practice: Current State of the Art. Diagnostics 2023, 13, 3523. [Google Scholar] [CrossRef]
- Lloyd, D.F.A.; van Amerom, J.F.P.; Pushparajah, K.; Simpson, J.M.; Zidere, V.; Miller, O.; Sharland, G.; Allsop, J.; Fox, M.; Lohezic, M.; et al. An exploration of the potential utility of fetal cardiovascular MRI as an adjunct to fetal echocardiography. Prenat Diagn. 2016, 36, 916–925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Porayette, P.; van Amerom, J.F.; Yoo, S.-J.; Jaeggi, E.; Macgowan, C.K.; Seed, M. MRI shows limited mixing between systemic and pulmonary circulations in foetal transposition of the great arteries: A potential cause of in utero pulmonary vascular disease. Cardiol. Young 2015, 25, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Panayiotou, H.R.; Mills, L.K.; Broadbent, D.A.; Shelley, D.; Scheffczik, J.; Olaru, A.M.; Jin, N.; Greenwood, J.P.; Michael, H.; Plein, S.; et al. Comprehensive Neonatal Cardiac, Feed and Wrap, Non-contrast, Non-sedated, Free-breathing Compressed Sensing 4D Flow MRI Assessment. J. Magn. Reason. Imaging 2023, 57, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Canan, A.; Ashwath, R.; Agarwal, P.P.; François, C.; Rajiah, P. Multimodality Imaging of Transposition of the Great Arteries. Radiographics 2021, 41, 338–360. [Google Scholar] [CrossRef] [PubMed]
- Beek, F.J.A.; Beekman, R.P.; Dillon, E.H.; Mali, W.P.T.M.; Meiners, L.C.; Kramer, P.P.G.; Meyboom, E.J. MRI of the pulmonary artery after arterial switch operation for transposition of the great arteries. Pediatr. Radiol. 1993, 23, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Gutberlet, M.; Boeckel, T.; Hosten, N.; Vogel, M.; Kühne, T.; Oellinger, H.; Ehrenstein, T.; Venz, S.; Hetzer, R.; Bein, G.; et al. Arterial switch procedure for D-transposition of the great arteries: Quantitative midterm evaluation of hemodynamic changes with cine MR imaging and phase-shift velocity mapping-initial experience. Radiology 2000, 214, 467–475. [Google Scholar] [CrossRef]
- McConnell, M.V.; Ganz, P.; Selwyn, A.P.; Li, W.; Edelman, R.R.; Manning, W.J. Identification of anomalous coronary arteries and their anatomic course by magnetic resonance coronary angiography. Circulation 1995, 92, 3158–3162. [Google Scholar] [CrossRef]
- Stagnaro, N.; Moscatelli, S.; Cheli, M.; Bondanza, S.; Marasini, M.; Trocchio, G. Dobutamine Stress Cardiac MRI in Pediatric Patients with Suspected Coronary Artery Disease. Pediatr. Cardiol. 2023, 44, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, S.; Bianco, F.; Cimini, A.; Panebianco, M.; Leo, I.; Bucciarelli-Ducci, C.; Perrone, M.A. The Use of Stress Cardiovascular Imaging in Pediatric Population. Children 2023, 10, 218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sampson, C.; Kilner, P.J.; Hirsch, R.; Rees, R.S.; Somerville, J.; Underwood, S.R. Venoatrial pathways after the Mustard operation for transposition of the great arteries: Anatomic and functional MR imaging. Radiology 1994, 193, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Groenink, M.; Mulder, B.; van der Wall, E. Value of magnetic resonance imaging in functional assessment of baffle obstruction after the Mustard procedure. J. Cardiovasc. Magn. Reson. 1999, 1, 49–51. [Google Scholar] [CrossRef]
- Holmqvist, C.; Oskarsson, G.; Stahlberg, F.; Thilen, U.; Bjorkhem, G.; Laurin, S. Functional evaluation of extracardiac ventriculopulmonary conduits and of the right ventricle with magnetic resonance imaging and velocity mapping. Am. J. Cardiol. 1999, 83, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Ntsinjana, H.N.; Capelli, C.; Biglino, G.; Cook, A.C.; Tann, O.; Derrick, G.; Taylor, A.M.; Schievano, S. 3D Morphometric Analysis of the Arterial Switch Operation Using In Vivo MRI Data. Clin. Anat. 2014, 27, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.R.; Villemain, O.; Marini, D.; Dragulescu, A.; Yoo, S.-J.; Barron, D.J. Utility of a bespoke 3-dimensional printed model in complex transposition. JTCVS Tech. 2021, 7, 199–202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sachdeva, R.; Valente, A.M.; Armstrong, A.K.; Cook, S.C.; Han, B.K.; Lopez, L.; Lui, G.K.; Pickard, S.S.; Powell, A.J.; Bhave, N.M.; et al. ACC/AHA/ASE/HRS/ISACHD/SCAI/SCCT/SCMR/SOPE 2020 Appropriate Use Criteria for Multimodality Imaging During the Follow-Up Care of Patients with Congenital Heart Disease: A Report of the American College of Cardiology Solution Set Oversight Committee and Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Pediatric Echocardiography. J. Am. Coll. Cardiol. 2020, 75, 657–703. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.H.; Tann, O. Computed Tomography in Paediatric Heart Disease. Br. J. Radiol. 2018, 91, 20180201. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bhatia, M. Role of CT in the Pre- and Postoperative Assessment of Conotruncal Anomalies. Radiol. Cardiothorac. Imaging 2022, 4, e210089. [Google Scholar] [CrossRef] [PubMed]
- Han, B.K.; Rigsby, C.K.; Hlavacek, A.; Leipsic, J.; Nicol, E.D.; Siegel, M.J.; Bardo, D.; Abbara, S.; Ghoshhajra, B.; Lesser, J.R.; et al. Computed Tomography Imaging in Patients with Congenital Heart Disease Part I: Rationale and Utility. An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT): Endorsed by the Society of Pediatric Radiology (SPR) and the North American Society of Cardiac Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2015, 9, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Taylor, A.M.; Owens, C.M. Paediatric Cardiac Computed Tomography: A Review of Imaging Techniques and Radiation Dose Consideration. Eur. Radiol. 2011, 21, 518–529. [Google Scholar] [CrossRef]
- Francone, M.; Gimelli, A.; Budde, R.P.J.; Caro-Dominguez, P.; Einstein, A.J.; Gutberlet, M.; Maurovich-Horvat, P.; Miller, O.; Nagy, E.; Natale, L.; et al. Radiation Safety for Cardiovascular Computed Tomography Imaging in Paediatric Cardiology: A Joint Expert Consensus Document of the EACVI, ESCR, AEPC, and ESPR. Eur. Hear. J.-Cardiovasc. Imaging 2022, 23, e279–e289. [Google Scholar] [CrossRef]
- Pickard, S.S.; Armstrong, A.K.; Balasubramanian, S.; Buddhe, S.; Crum, K.; Kong, G.; Lang, S.M.; Lee, M.V.; Lopez, L.; Natarajan, S.S.; et al. Appropriateness of Cardiovascular Computed Tomography and Magnetic Resonance Imaging in Patients with Conotruncal Defects. J. Cardiovasc. Comput. Tomogr. 2023, 17, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.K.; Sayyouh, M.M.; Bardo, D.M.E.; Ghadimi Mahani, M.; Lu, J.C.; Dorfman, A.L.; Agarwal, P.P. Interpretation and Reporting of Coronary Arteries in Transposition of the Great Arteries: Cross-Sectional Imaging Perspective. J. Thorac. Imaging 2018, 33, W14–W21. [Google Scholar] [CrossRef] [PubMed]
- Lembcke, A.; Koch, C.; Dohmen, P.M.; Rutsch, W.; Abbara, S.; Krug, L.D.; Muehler, M.R.; Rogalla, P. Electrocardiographic-Gated Multislice Computed Tomography for Visualization of Cardiac Morphology in Congenitally Corrected Transposition of the Great Arteries. J. Comput. Assist. Tomogr. 2005, 29, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Pergola, V.; Avesani, M.; Reffo, E.; Da Pozzo, S.; Cavaliere, A.; Padalino, M.; Vida, V.; Motta, R.; Di Salvo, G. Unveiling the gothic aortic arch and cardiac mechanics: Insights from young patients after arterial switch operation for d-transposition of the great arteries. Monaldi Arch. Chest Dis. 2023, 94, 2712. [Google Scholar] [CrossRef] [PubMed]
- Zucker, E.J. Computed Tomography in Tetralogy of Fallot: Pre- and Postoperative Imaging Evaluation. Pediatr. Radiol. 2022, 52, 2485–2497. [Google Scholar] [CrossRef] [PubMed]
- Tsai-Goodman, B.; Geva, T.; Odegard, K.C.; Sena, L.M.; Powell, A.J. Clinical Role, Accuracy, and Technical Aspects of Cardiovascular Magnetic Resonance Imaging in Infants. Am. J. Cardiol. 2004, 94, 69–74. [Google Scholar] [CrossRef]
- Ranganath, P.; Singh, S.; Abbara, S.; Agarwal, P.P.; Rajiah, P. Computed Tomography in Adult Congenital Heart Disease. Radiol. Clin. N. Am. 2019, 57, 85–111. [Google Scholar] [CrossRef]

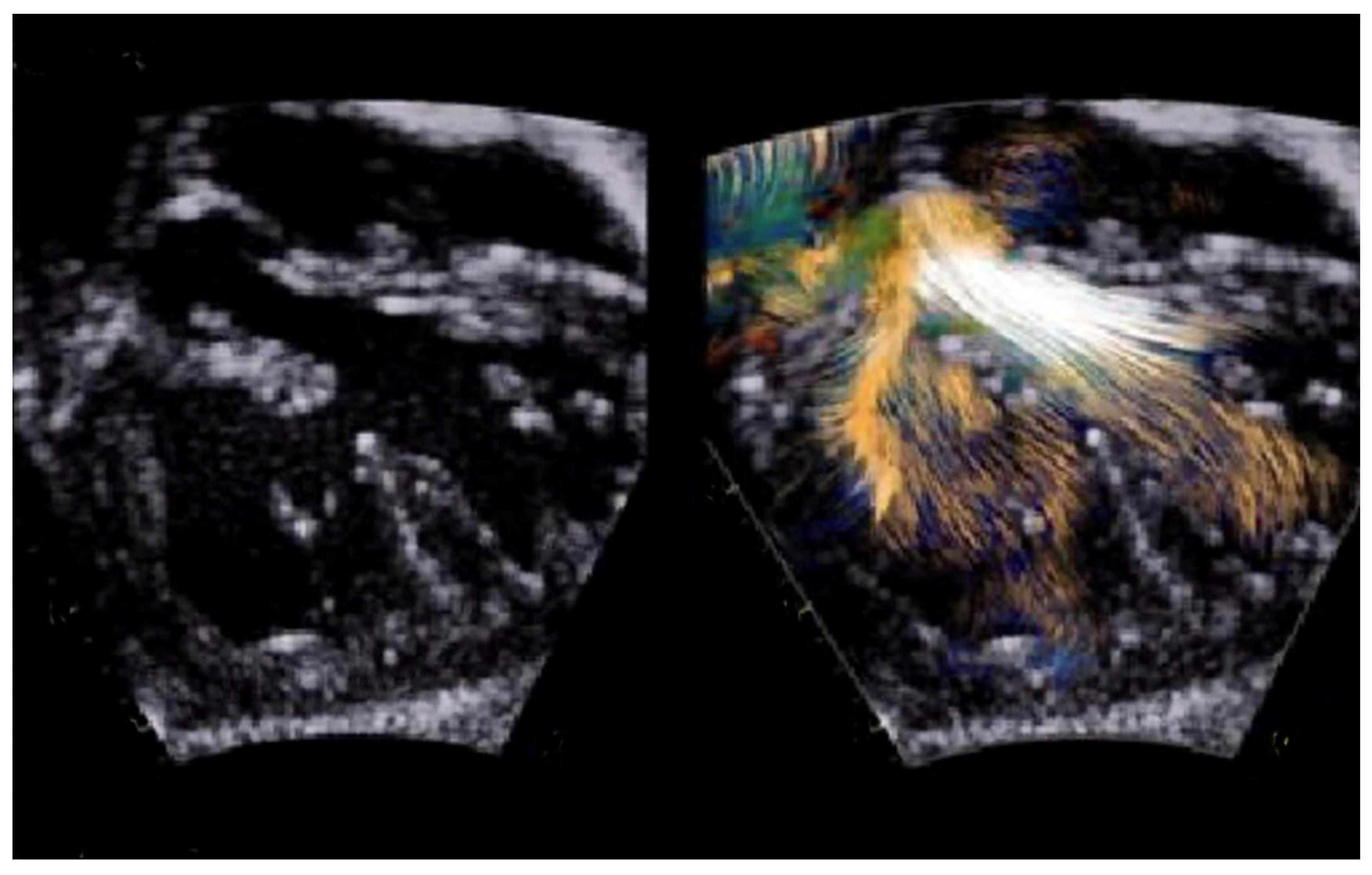

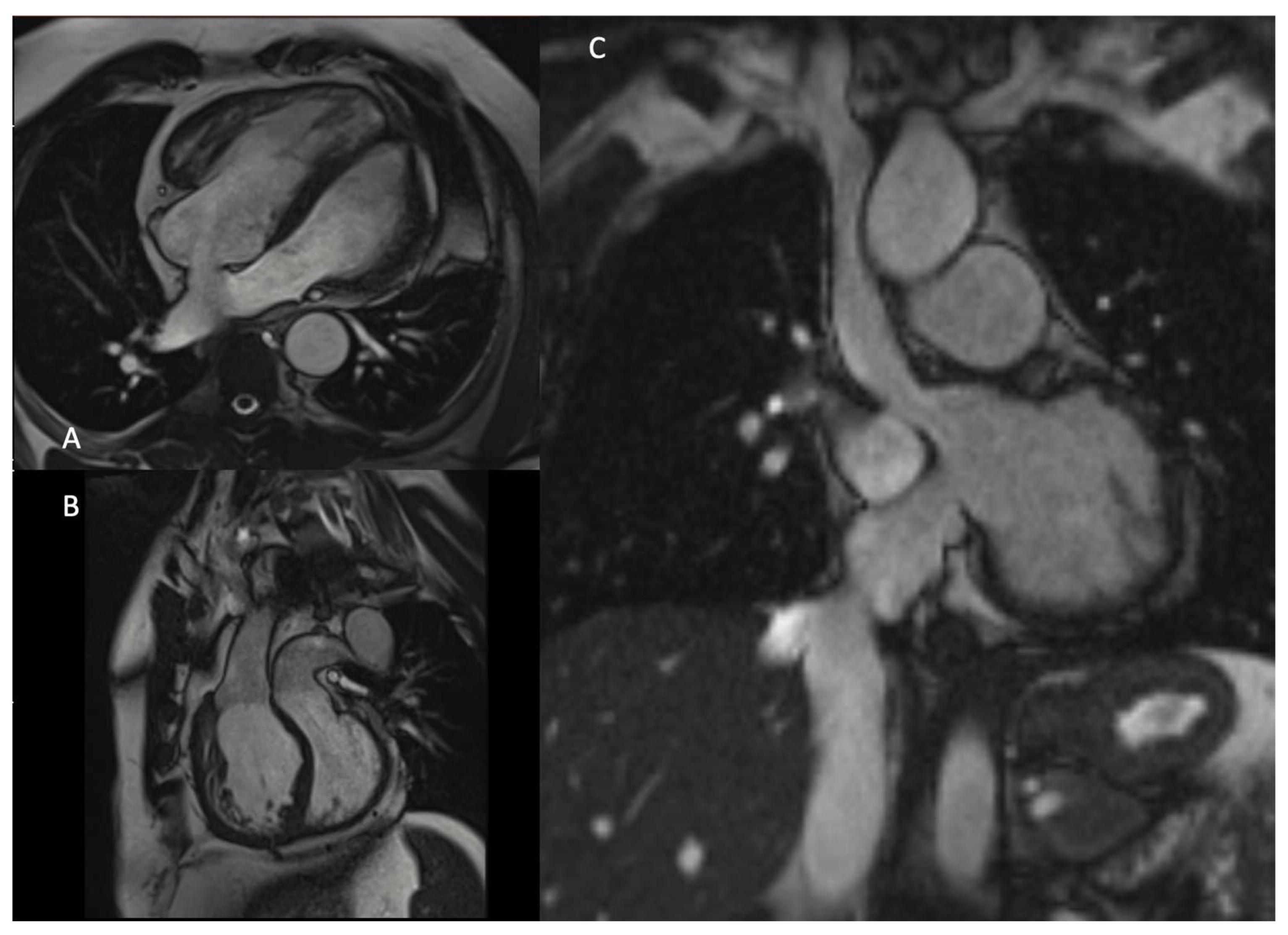
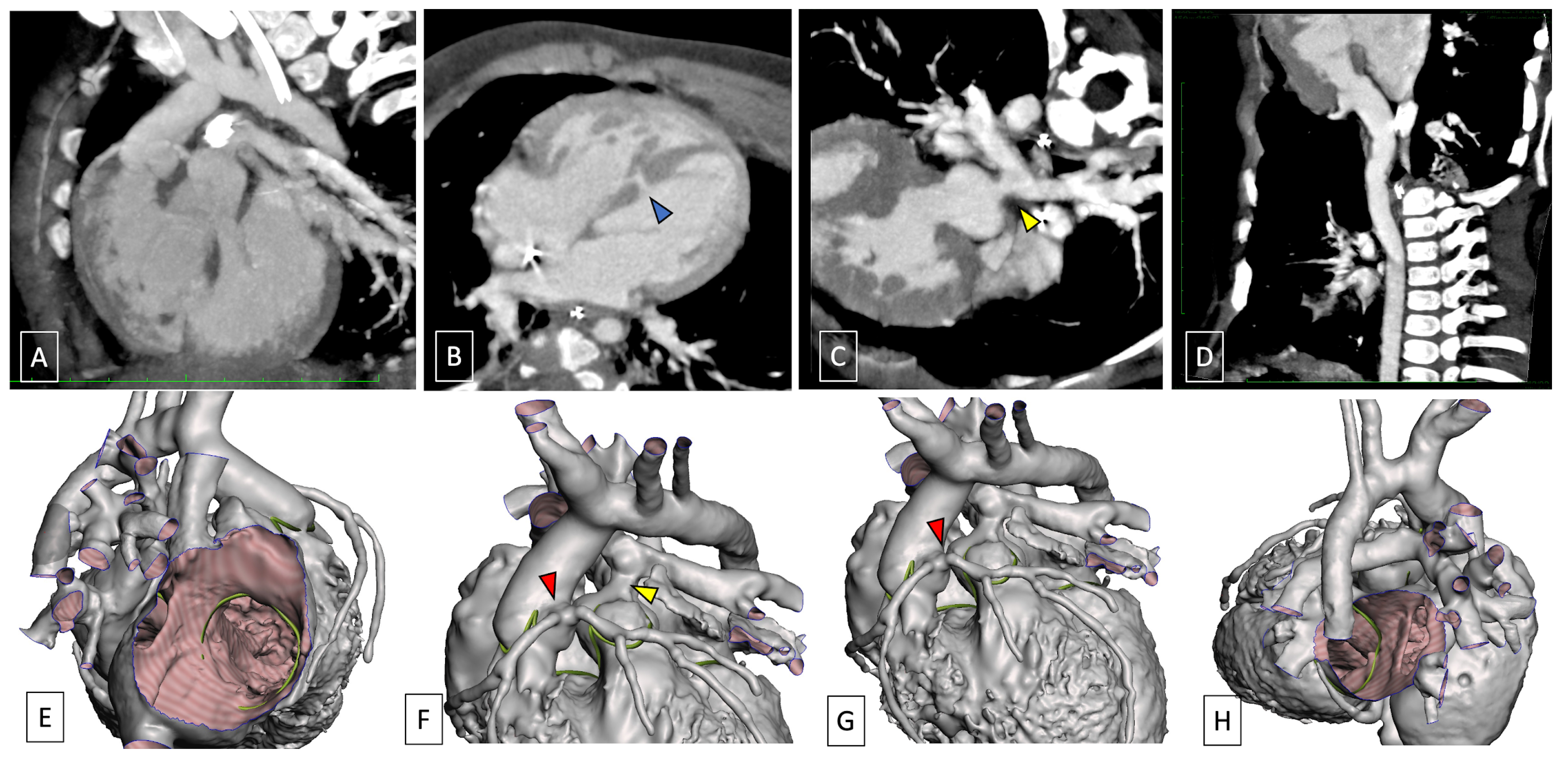
| Prenatal | Preoperative | Postoperative | |
|---|---|---|---|
| Echocardiography |
|
|
|
| Cardiovascular Magnetic Resonance |
|
|
|
| Cardiac Computed Tomography |
|
|
|
| Preoperative Assessment | |
|---|---|
| Spatial relationship between the aorta and the pulmonary artery | Subxiphoid frontal Subxiphoid sagittal Parasternal long axis |
| Presence and size of the atrial septal defect | Subxiphoid frontal |
| Presence, location, number, and size of VSD | Subxiphoid sagittal Apical five chamber Parasternal short axis |
| AV valve morphology, function, and abnormalities | Subxiphoid sagittal Apical four and five chamber |
| Outflow tract obstruction | Subxiphoid sagittal Apical five chamber Parasternal long axis |
| Coronary artery anatomy and anomalies | High parasternal short axis Apical four chamber Subxiphoid |
| Aortic arch anatomy and sideness and PDA | Suprasternal sagital |
| Postoperative Assessment | |
| Residual ASD | Subxiphoid frontal |
| Residual VSD | Subxiphoid frontal Subxiphoid sagittal Parasternal short axis |
| Ventricular function, size, regional wall motion, AV valve function | Apical four chamber Parasternal long axis Parasternal short axis |
| Outflow tract obstruction, neoaortic root dilation, semilunar valve regurgitation/stenosis, supravalvar stenosis | Subxiphoid frontal Subxiphoid sagittal Apical five chamber Parasternal long axis Parasternal short axis |
| Branch pulmonary arteries stenosis | High parasternal plane |
| Residual arch obstruction or residual PDA | Suprasternal sagittal Subxiphoid sagittal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moscatelli, S.; Avesani, M.; Borrelli, N.; Sabatino, J.; Pergola, V.; Leo, I.; Montanaro, C.; Contini, F.V.; Gaudieri, G.; Ielapi, J.; et al. Complete Transposition of the Great Arteries in the Pediatric Field: A Multimodality Imaging Approach. Children 2024, 11, 626. https://doi.org/10.3390/children11060626
Moscatelli S, Avesani M, Borrelli N, Sabatino J, Pergola V, Leo I, Montanaro C, Contini FV, Gaudieri G, Ielapi J, et al. Complete Transposition of the Great Arteries in the Pediatric Field: A Multimodality Imaging Approach. Children. 2024; 11(6):626. https://doi.org/10.3390/children11060626
Chicago/Turabian StyleMoscatelli, Sara, Martina Avesani, Nunzia Borrelli, Jolanda Sabatino, Valeria Pergola, Isabella Leo, Claudia Montanaro, Francesca Valeria Contini, Gabriella Gaudieri, Jessica Ielapi, and et al. 2024. "Complete Transposition of the Great Arteries in the Pediatric Field: A Multimodality Imaging Approach" Children 11, no. 6: 626. https://doi.org/10.3390/children11060626
APA StyleMoscatelli, S., Avesani, M., Borrelli, N., Sabatino, J., Pergola, V., Leo, I., Montanaro, C., Contini, F. V., Gaudieri, G., Ielapi, J., Motta, R., Perrone, M. A., & Di Salvo, G. (2024). Complete Transposition of the Great Arteries in the Pediatric Field: A Multimodality Imaging Approach. Children, 11(6), 626. https://doi.org/10.3390/children11060626










