Serum Biomarker Analysis in Pediatric ADHD: Implications of Homocysteine, Vitamin B12, Vitamin D, Ferritin, and Iron Levels
Abstract
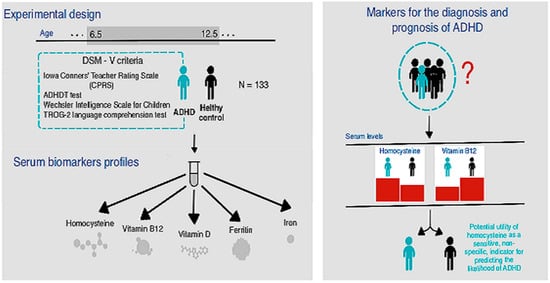
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Polanczyk, G.; Silva de Lima, M.; Horta, B.L.; Biederman, J.; Rohde, L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Couture, J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann. Pharmacother. 2014, 48, 209–225. [Google Scholar] [CrossRef]
- Thapar, A.; Cooper, M. Attention deficit hyperactivity disorder. Lancet 2015, 387, 10024. [Google Scholar]
- Heijer, A.E.D.; Groen, Y.; Tucha, L.; Fuermaier, A.B.M.; Koerts, J.; Lange, K.W.; Thome, J.; Tucha, O. Sweat it out? The effects of physical exercise on cognition and behavior in children and adults with ADHD: A systematic literature review. J. Neural Transm. 2017, 124, 3–26. [Google Scholar] [CrossRef]
- Kooij, S.J.; Bejerot, S.; Blackwell, A.; Caci, H.; Casas-Brugué, M.; Carpentier, P.J.; Edvinsson, D.; Fayyad, J.; Foeken, K.; Fitzgerald, M.; et al. European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry 2010, 10, 67. [Google Scholar] [CrossRef]
- Lukovac, T.; Hil, O.A.; Popović, M.; Savić, T.; Pavlović, A.M.; Pavlović, D. Serum levels of glucose, thyroid stimulating hormone, and free thyroxine in boys diagnosed with attention deficit hyperactivity disorder: A cross-sectional pilot study. BMC Neurol. 2024, 24, 76. [Google Scholar] [CrossRef]
- Caylak, E. Biochemical and genetic analyses of childhood attention deficit/hyperactivity disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2012, 159, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, G.; Piña, R.; Contreras, D.; Godoy, F.; Rubio, D.; Rozas, C.; Zeise, M.; Vidal, R.; Escobar, J.; Morales, B. Attention Deficit-Hyperactivity Disorder (ADHD): From Abnormal Behavior to Impairment in Synaptic Plasticity. Biology 2023, 12, 1241. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Dhaliwal, A.J.; Roy, M. The usefulness of Conners’ Rating Scales-Revised in screening for attention deficit hyperactivity disorder in children with intellectual disabilities and borderline intelligence. J. Intellect. Disabil. Res. 2008, 52, 950–965. [Google Scholar] [CrossRef]
- Cainelli, E.; Bisiacchi, P. Neurodevelopmental Disorders: Past, Present, and Future. Children 2022, 10, 31. [Google Scholar] [CrossRef]
- Alvarez-Arellano, L.; González-García, N.; Salazar-García, M.; Corona, J.C. Antioxidants as a potential target against inflammation and oxidative stress in attention-deficit/hyperactivity disorder. Antioxidants 2020, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Clemow, D.B. Misuse of methylphenidate. Non-Med. Illicit. Use Psychoact. Drugs 2017, 34, 99–124. [Google Scholar]
- Verlaet, A.A.J.; Maasakkers, C.M.; Hermans, N.; Savelkoul, H.F.J. Rationale for dietary antioxidant treatment of ADHD. Nutrients 2018, 10, 405. [Google Scholar] [CrossRef]
- Toomey, S.L.; Sox, C.M.; Rusinak, D.; Finkelstein, J.A. Why do children with ADHD discontinue their medication? Clin. Pediatr. 2012, 51, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.A.; Nigg, J.T.; Sullivan, E.L. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol. Biochem. Behav. 2019, 182, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Roehr, B. American Psychiatric Association explains DSM-5. BMJ Br. Med. J. 2013, 346, f3591. [Google Scholar] [CrossRef]
- Pinto, S.; Correia-De-Sá, T.; Sampaio-Maia, B.; Vasconcelos, C.; Moreira, P.; Ferreira-Gomes, J. Eating patterns and dietary interventions in ADHD: A narrative review. Nutrients 2022, 14, 4332. [Google Scholar] [CrossRef]
- Corona, J.C. Natural compounds for the management of Parkinson’s disease and attention-deficit/hyperactivity disorder. BioMed Res. Int. 2018, 2018, 4067597. [Google Scholar] [CrossRef]
- Miller, A.L. The methionine-homocysteine cycle and its effects on cognitive diseases. (Homocysteine & Cognitive). Altern. Med. Rev. 2003, 8, 7–20. [Google Scholar]
- Ioniță-Radu, F.; Nicolau, I.-N.; Petrache, O.-G.; Groșeanu, M.-L.; Bojincă, V.-C.; Negru, M.-M.; Bucurică, S.; Anghel, D. Correlation between Trabecular Bone Score and Homocysteine Level in Rheumatoid Arthritis Patients on Anti-TNF Inhibitors. Life 2024, 14, 463. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.J. Serum homocysteine levels are correlated with behavioral and psychological symptoms of Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2014, 10, 1887–1896. [Google Scholar] [PubMed]
- Kałużna-Czaplińska, J.; Michalska, M.; Rynkowski, J. Homocysteine level in urine of autistic and healthy children. Acta Biochim. Pol. 2011, 58, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Stanger, O.; Fowler, B.; Piertzik, K.; Huemer, M.; Haschke-Becher, E.; Semmler, A.; Lorenzl, S.; Linnebank, M. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: Review and treatment recommendations. Expert Rev. Neurother. 2009, 9, 1393–1412. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Shea, T.B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003, 26, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T. Folate, vitamin B12, and neuropsychiatric disorders. Nutr. Rev. 1996, 54, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Cherubini, V.; Falsetti, L.; Viticchi, G.; Silvestrini, M.; Toraldo, A. Homocysteine, cognitive functions, and degenerative dementias: State of the art. Biomedicines 2022, 10, 2741. [Google Scholar] [CrossRef] [PubMed]
- Cavalca, V.; Cighetti, G.; Bamonti, F.; Loaldi, A.; Bortone, L.; Novembrino, C.; De Franceschi, M.; Belardinelli, R.; Guazzi, M.D. Oxidative stress and homocysteine in coronary artery disease. Clin. Chem. 2001, 47, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Herrmann, W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006, 580, 2994–3005. [Google Scholar] [CrossRef] [PubMed]
- Kataria, N.; Yadav, P.; Kumar, R.; Kumar, N.; Singh, M.; Kant, R.; Kalyani, V. Effect of vitamin B6, B9, and B12 supplementation on homocysteine level and cardiovascular outcomes in stroke patients: A meta-analysis of randomized controlled trials. Cureus 2021, 13, e14958. [Google Scholar] [CrossRef]
- Gilfix, B.M. Vitamin B12 and homocysteine. CMAJ 2005, 173, 1360. [Google Scholar] [CrossRef]
- Yektaş, Ç.; Alpay, M.; Tufan, A.E. Comparison of serum B12, folate and homocysteine concentrations in children with autism spectrum disorder or attention deficit hyperactivity disorder and healthy controls. Neuropsychiatr. Dis. Treat. 2019, 15, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Fuller, J.A.; Burrell, M.H.; Yee, A.G.; Liyanagama, K.; Lipski, J.; Wickens, J.R.; Hyland, B.I. Role of homeostatic feedback mechanisms in modulating methylphenidate actions on phasic dopamine signaling in the striatum of awake behaving rats. Prog. Neurobiol. 2019, 182, 101681. [Google Scholar] [CrossRef]
- Miranda, A.; Tárraga, R.; Fernández, M.I.; Colomer, C.; Pastor, G. Parenting stress in families of children with autism spectrum disorder and ADHD. Except. Child. 2015, 82, 81–95. [Google Scholar] [CrossRef]
- Bressenot, A.; Pooya, S.; Bossenmeyer-Pourie, C.; Gauchotte, G.; Germain, A.; Chevaux, J.-B.; Coste, F.; Vignaud, J.-M.; Guéant, J.-L.; Peyrin-Biroulet, L. Methyl donor deficiency affects small-intestinal differentiation and barrier function in rats. Br. J. Nutr. 2013, 109, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Miller, M.G.; Scott, T.; Shukitt-Hale, B. Nutritional factors affecting adult neurogenesis and cognitive function. Adv. Nutr. 2017, 8, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-L.; Chen, Y.-J.; Shang, C.-Y.; Tseng, W.-Y.I.; Gau, S.S.-F. Different neural substrates for executive functions in youths with ADHD: A diffusion spectrum imaging tractography study. Psychol. Med. 2016, 46, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Becker, A.; Sundermann, J.; Rothenberger, A.; Herrmann-Lingen, C. Attention deficit-hyperactivity disorder is associated with reduced blood pressure and serum vitamin D levels: Results from the nationwide German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Eur. Child Adolesc. Psychiatry 2017, 26, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Bener, A.; Ehlayel, M.S. Is high prevalence of vitamin D deficiency a correlate for attention deficit hyperactivity disorder? ADHD Atten. Deficit Hyperact. Disord. 2014, 6, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Józefowicz, O.; Rabe-Jabłońska, J.; Woźniacka, A.; Strzelecki, D. Analysis of vitamin D status in major depression. J. Psychiatr. Pract. 2014, 20, 329–337. [Google Scholar] [CrossRef]
- Bradstreet, J.J.; Smith, S.; Baral, M.; Rossignol, D.A. Biomarker-guided interventions of clinically relevant conditions associated with autism spectrum disorders and attention deficit hyperactivity disorder. Altern. Med. Rev. 2010, 15, 15–32. [Google Scholar]
- Fabrazzo, M.; Agnese, S.; Cipolla, S.; Di Vincenzo, M.; Mancuso, E.; Volpicelli, A.; Perris, F.; Sampogna, G.; Catapano, F.; Fiorillo, A.; et al. Vitamin D Deficiency and Risk Factors Related to Acute Psychiatric Relapses in Patients with Severe Mental Disorders: A Preliminary Study. Brain Sci. 2022, 12, 973. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Neyestani, T.R.; Hajifaraji, M.; Omidvar, N.; Eshraghian, M.R.; Shariatzadeh, N.; Kalayi, A.; Gharavi, A.; Khalaji, N.; Haidari, H.; Zowghi, T.; et al. High prevalence of vitamin D deficiency in school-age children in Tehran, 2008: A red alert. Public Health Nutr. 2012, 15, 324–330. [Google Scholar] [CrossRef]
- Mohammadpour, N.; Jazayeri, S.; Tehrani-Doost, M.; Djalali, M.; Hosseini, M.; Effatpanah, M.; Davari-Ashtiani, R.; Karami, E. Effect of vitamin D supplementation as adjunctive therapy to methylphenidate on ADHD symptoms: A randomized, double blind, placebo-controlled trial. Nutr. Neurosci. 2018, 21, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Robberecht, H.; Verlaet, A.A.J.; Breynaert, A.; De Bruyne, T.; Hermans, N. Magnesium, iron, zinc, copper and selenium status in attention-deficit/hyperactivity disorder (ADHD). Molecules 2020, 25, 4440. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Beard, J.; Connor, J.; Felt, B.; Georgieff, M.; Schallert, T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006, 64 (Suppl. S2), S34–S43. [Google Scholar] [CrossRef] [PubMed]
- Santa-Marina, L.; Lertxundi, N.; Andiarena, A.; Irizar, A.; Sunyer, J.; Molinuevo, A.; Llop, S.; Julvez, J.; Beneito, A.; Ibarluzea, J.; et al. Maternal ferritin levels during pregnancy and ADHD symptoms in 4-year-old children: Results from the INMA–infancia y medio ambiente (environment and childhood) prospective birth cohort study. Int. J. Environ. Res. Public Health 2020, 17, 7704. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L. Reevaluation of the metabolic essentiality of the vitamins-review. Asian-Australas. J. Anim. Sci. 2000, 13, 115–125. [Google Scholar] [CrossRef]
- Dereboy, C.; Senol, S.; Sener, S.; Dereboy, F. Validation of the Turkish versions of the short-form Conners’ teacher and parent rating scales. Turk Psikiyatr. Derg. 2007, 18, 48. [Google Scholar]
- Franzen, M.D. The Wechsler Intelligence Scales for Children—The WISC-R, WISC-III, and WPPSI-R. In Reliability and Validity in Neuropsychological Assessment; Springer: Berlin/Heidelberg, Germany, 2000; pp. 71–89. [Google Scholar]
- Bishop, D.V. Test for Reception of Grammar: Test for Reception of Grammar Version TROG-2 Stimulus Book, 1st ed.; Harcourt Assessment: San Antonio, TX, USA, 2003. [Google Scholar]
- Mutlu, G.Y.; Hatun, Ş. Use of vitamin D in children and adults: Frequently asked questions. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 301. [Google Scholar]
- Moretti, R.; Caruso, P. The controversial role of homocysteine in neurology: From labs to clinical practice. Int. J. Mol. Sci. 2019, 20, 231. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Siracusa, R.; Fusco, R.; Cuzzocrea, S.; Di Paola, R.; Impellizzeri, D. Involvements of hyperhomocysteinemia in neurological disorders. Metabolites 2021, 11, 37. [Google Scholar] [CrossRef]
- Kruman, I.I.; Culmsee, C.; Chan, S.L.; Kruman, Y.; Guo, Z.; Penix, L.; Mattson, M.P. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Neurosci. 2000, 20, 6920–6926. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.I.; Ortiz, D.; Rogers, E.; Shea, T.B. Multiple aspects of homocysteine neurotoxicity: Glutamate excitotoxicity, kinase hyperactivation and DNA damage. J. Neurosci. Res. 2002, 70, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Singh, N. Homocysteine excess: Delineating the possible mechanism of neurotoxicity and depression. Fundam. Clin. Pharmacol. 2015, 29, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.K.; Aboud, K.S.; Nguyen, T.Q.; Cutting, L.E. Applying a network framework to the neurobiology of reading and dyslexia. J. Neurodev. Disord. 2018, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.M.; Boy, E.; Miller, J.W.; Green, R.; Sabel, J.C.; Allen, L.H. High prevalence of cobalamin deficiency in Guatemalan schoolchildren: Associations with low plasma holotranscobalamin II and elevated serum methylmalonic acid and plasma homocysteine concentrations. Am. J. Clin. Nutr. 2003, 77, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Refsum, H.; Ueland, P.M.; Nygård, O.; Vollset, S.E. Homocysteine and cardiovascular disease. Annu. Rev. Med. 1998, 49, 31–62. [Google Scholar] [CrossRef] [PubMed]
- Altun, H.; Sahin, N.; Kurutas, E.B.; Gungor, O. Homocysteine, pyridoxine, folate and vitamin B12 levels in children with attention deficit hyperactivity disorder. Psychiatr. Danub. 2018, 30, 310–316. [Google Scholar] [CrossRef]
- Azzini, E.; Ruggeri, S.; Polito, A. Homocysteine: Its possible emerging role in at-risk population groups. Int. J. Mol. Sci. 2020, 21, 1421. [Google Scholar] [CrossRef]
- Saha, T.; Chatterjee, M.; Verma, D.; Ray, A.; Sinha, S.; Rajamma, U.; Mukhopadhyay, K. Genetic variants of the folate metabolic system and mild hyperhomocysteinemia may affect ADHD associated behavioral problems. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 1–10. [Google Scholar] [CrossRef]
- De la Torre-Iturbe, S.; Vázquez-Roque, R.A.; De la Cruz-López, F.; Flores, G.; Garcés-Ramírez, L. Dendritic and behavioral changes in rats neonatally treated with homocysteine; A proposal as an animal model to study the attention deficit hyperactivity disorder. J. Chem. Neuroanat. 2022, 119, 102057. [Google Scholar] [CrossRef]
- Deth, R.; Muratore, C.; Benzecry, J.; Power-Charnitsky, V.-A.; Waly, M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology 2008, 29, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, D. Vitamin B12, Vitamin D and Homocysteine–Trio of Health and Disease; OrionArt: Belgrade, Serbia, 2018; p. 152. [Google Scholar]
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef]
- Lukovac, T.; Pavlović, D. Attention deficit hyperactivity disorder and micronutrities. Engrami 2019, 41, 46–59. [Google Scholar] [CrossRef]
- Oh, R.; Brown, D.L. Vitamin B12 Deficiency. Am. Fam. Physician. 2003, 67, 979–986. [Google Scholar] [PubMed]
- Aparicio-Ugarriza, R.; Palacios, G.; Alder, M.; González-Gross, M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin. Chem. Lab. Med. 2015, 53, 1149–1159. [Google Scholar] [CrossRef]
- Bala, K.A.; Doğan, M.; Kaba, S.; Mutluer, T.; Aslan, O.; Doğan, S.Z. Hormone disorder and vitamin deficiency in attention deficit hyperactivity disorder (ADHD) and autism spectrum disorders (ASDs). J. Pediatr. Endocrinol. Metab. 2016, 29, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xie, Z.; Gu, P.; Li, X.; Zhang, Y.; Wang, X.; Chen, Z.; Deng, S.; Shu, Y.; Li, J.-D. Cry1Δ11 mutation induces ADHD-like symptoms through hyperactive dopamine D1 receptor signaling. JCI Insight 2023, 8, e170434. [Google Scholar] [CrossRef]
- Mathew, A.R.; Di Matteo, G.; La Rosa, P.; Barbati, S.A.; Mannina, L.; Moreno, S.; Tata, A.M.; Cavallucci, V.; Fidaleo, M. Vitamin B12 Deficiency and the Nervous System: Beyond Metabolic Decompensation—Comparing Biological Models and Gaining New Insights into Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2024, 25, 590. [Google Scholar] [CrossRef]
- Monasso, G.S.; Hoang, T.T.; Mancano, G.; Fernández-Barrés, S.; Dou, J.; Jaddoe, V.W.; Page, C.M.; Johnson, L.; Bustamante, M.; Bakulski, K.M.; et al. A meta-analysis of epigenome-wide association studies on pregnancy vitamin B12 concentrations and offspring DNA methylation. Epigenetics 2023, 18, 2202835. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.M.V.; da Luz, M.H.M.; Antunes, H.K.M.; Giampá, S.Q.d.C.; Martins, V.R.; Lee, K.S. Iron-restricted diet affects brain ferritin levels, dopamine metabolism and cellular prion protein in a region-specific manner. Front. Mol. Neurosci. 2017, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Lange, K.M.; Nakamura, Y.; Reissmann, A. Nutrition in the management of ADHD: A review of recent research. Curr. Nutr. Rep. 2023, 12, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Johansson, J.; Falahati, F.; Andersson, M.; Papenberg, G.; Avelar-Pereira, B.; Bäckman, L.; Kalpouzos, G.; Salami, A. The iron-dopamine D1 coupling modulates neural signatures of working memory across adult lifespan. NeuroImage 2023, 279, 120323. [Google Scholar] [CrossRef] [PubMed]
- Konofal, E.; Lecendreux, M.; Arnulf, I.; Mouren, M.-C. Iron deficiency in children with attention-deficit/hyperactivity disorder. Arch. Pediatr. Adolesc. Med. 2004, 158, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.-T.; Cheng, Y.-S.; Yen, C.-F.; Chen, Y.-W.; Stubbs, B.; Whiteley, P.; Carvalho, A.F.; Li, D.-J.; Chen, T.-Y.; Tang, C.-H.; et al. Peripheral iron levels in children with attention-deficit hyperactivity disorder: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 788. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Darakhshan, R.; Bagherian, A. Is there an association between early childhood caries and serum iron and serum ferritin levels? Dent. Res. J. 2012, 9, 294. [Google Scholar]
- Öztürk, Y.; Topal, Z.; Demir, N.; Tufan, A.E. Iron and Ferritin Levels of Children and Adolescents with Attention Deficit Hyperactivity Disorder and Attention Deficit Hyperactivity Disorder-Not Otherwise Specified. J. Pediatr. Res. 2020, 7, 216–222. [Google Scholar] [CrossRef]
- Donfrancesco, R.; Parisi, P.; Vanacore, N.; Martines, F.; Sargentini, V.; Cortese, S. Iron and ADHD: Time to move beyond serum ferritin levels. J. Atten. Disord. 2013, 17, 347–357. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lim, M.H.; Ha, M.; Kim, E.J.; Yoo, S.J.; Kim, J.W.; Paik, K.C. Transferrin in Korean children with attention deficit hyperactivity disorder. Psychiatry Investig. 2011, 8, 366. [Google Scholar] [CrossRef]
- Menegassi, M.; de Mello, E.D.; Guimarães, L.R.; Matte, B.C.; Driemeier, F.; Pedroso, G.L.; Rohde, L.A.; Schmitz, M. Food intake and serum levels of iron in children and adolescents with attention-deficit/hyperactivity disorder. Braz. J. Psychiatry 2010, 32, 132–138. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; El-Mazary, A.-A.M.; Maher, R.M.; Saber, M.M. Zinc, ferritin, magnesium and copper in a group of Egyptian children with attention deficit hyperactivity disorder. Ital. J. Pediatr. 2011, 37, 60. [Google Scholar] [CrossRef] [PubMed]
- Juneja, M.; Jain, R.; Singh, V.; Mallika, V. Iron deficiency in Indian children with attention deficit hyperactivity disorder. Indian Pediatr. 2010, 47, 955–958. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Zhang, L.; Qu, Y.; Mu, D. Iron status in attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0169145. [Google Scholar] [CrossRef] [PubMed]
- Degremont, A.; Jain, R.; Philippou, E.; Latunde-Dada, G.O. Brain iron concentrations in the pathophysiology of children with attention deficit/hyperactivity disorder: A systematic review. Nutr. Rev. 2021, 79, 615–626. [Google Scholar] [CrossRef]
- Szodoray, P.; Nakken, B.; Gaal, J.; Jonsson, R.; Szegedi, A.; Zold, E.; Szegedi, G.; Brun, J.G.; Gesztelyi, R.; Zeher, M.; et al. The complex role of vitamin D in autoimmune diseases. Scand. J. Immunol. 2008, 68, 261–269. [Google Scholar] [CrossRef]
- Saidi, L.; Hammou, H.; Sicard, F.; Landrier, J.-F.; Mounien, L. Maternal vitamin D deficiency and brain functions: A never-ending story. Food Funct. 2023, 14, 6290–6301. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D deficiency: Infertility and neurodevelopmental diseases (attention deficit hyperactivity disorder, autism, and schizophrenia). Am. J. Physiol.-Cell Physiol. 2018, 314, C135–C151. [Google Scholar] [CrossRef] [PubMed]
- Massoodi, A.; Koutanaei, S.J.; Faraz, Z.; Geraili, Z.; Zavarmousavi, S.M. Comparison of serum vitamin D levels between healthy and ADHD children. Casp. J. Intern. Med. 2023, 14, 681. [Google Scholar]
- Pavlović, D.M.; Pavlovic, A.M. Vitamin D across the life span. Spec. Edukac. I Rehabil. 2014, 13, 117–132. [Google Scholar]
- Gouveri, E.; Papanas, N.; Hatzitolios, A.; Maltezos, E. Hypovitaminosis D and peripheral arterial disease: Emerging link beyond cardiovascular risk factors. Eur. J. Intern. Med. 2012, 23, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Wang, Z.; Wang, J.; Xu, H.; Zhou, H. Serum vitamin D levels among children aged 0–12 years in the First Affiliated Hospital of Harbin Medical University, China. J. Public Health 2018, 40, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, P.; Rylander, L.; Lindh, C.H.; Jönsson, B.A.G.; Ode, A.; Olofsson, P.; Ivarsson, S.A.; Rignell-Hydbom, A.; Haglund, N.; Källén, K. Vitamin D status at birth and future risk of attention deficit/hyperactivity disorder (ADHD). PLoS ONE 2015, 10, e0140164. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.R.; Madani, M.; Tabatabaei, F.; Tabatabaee, Z. The relationship between serum vitamin D level and attention deficit hyperactivity disorder. Iran. J. Child Neurol. 2015, 9, 48. [Google Scholar] [PubMed]
- Goksugur, S.B.; Tufan, A.E.; Semiz, M.; Gunes, C.; Bekdas, M.; Tosun, M.; Demircioglu, F. Vitamin D status in children with attention-deficit–hyperactivity disorder. Pediatr. Int. 2014, 56, 515–519. [Google Scholar] [CrossRef]
- Sahin, N.; Altun, H.; Kurutas, E.B.; Balkan, D. Vitamin D and vitamin D receptor levels in children with attention-deficit/hyperactivity disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 581–585. [Google Scholar] [CrossRef]
- Chaplin, G.; Jablonski, N.G. Vitamin D and the Evolution of Human Depigmentation; Wiley-Liss Div John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; pp. 451–461. [Google Scholar]
| Parameter | HC | ADHD |
|---|---|---|
| ADHDT | 63.15 ± 27.26 | 98.81 ± 16.05 |
| WISC-R scores | ||
| – Verbal IQ | 110.38 ± 12.84 | 102.79 ± 12.66 |
| – Non-verbal IQ | 112.65 ± 12.64 | 105.91 ± 12.90 |
| – Total IQ | 111.56 ± 12.05 | 104.04 ± 11.47 |
| Parameter | ADHDT | WISC-R Test | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Verbal IQ | Non-Verbal IQ | Total IQ | |||||||||||
| df | MS | F | p | MS | F | p | MS | F | p | MS | F | p | |
| Group | 1 | 33,835 | 66.491 | 3.153 × 10−13 *** | 1478 | 9.203 | 0.003 *** | 1861 | 12.103 | 0.001 *** | 1815 | 15.039 | 1.692 × 10−4 *** |
| Age | 3 | 337.3 | 0.663 | 0.576 | 296 | 1.845 | 0.142 | 222 | 1.443 | 0.233 | 279 | 2.312 | 0.079 |
| Group × age | 3 | 201 | 0.395 | 0.757 | 30 | 0.19 | 0.903 | 291 | 1.894 | 0.134 | 110 | 0.915 | 0.436 |
| Error | 125 | 508.9 | 161 | 154 | 121 | ||||||||
| Parameter | HC | ADHD |
|---|---|---|
| Iron (μmol/L) | 13.75 ± 3.78 | 16.99 ± 5.69 |
| Ferritin (ng/mL) | 32.72 ± 15.51 | 40.39 ± 18.67 |
| Hcy (μmol/L) | 6.58 ± 1.15 | 8.78 ± 1.75 |
| Vitamin B12 (pg/L) | 469.87 ± 174.37 | 359.66 ± 174.58 |
| Vitamin D (nmol/L) | 64.97 ± 15.52 | 64.16 ± 22.59 |
| Parameter | Age | ADHDT | WISC-R Test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IQ Verbal | IQ Non-Verbal | IQ Total | ||||||||
| r | p | r | p | r | p | r | p | r | p | |
| Iron | 0.050 | 0.689 | −0.102 | 0.410 | 0.086 | 0.487 | 0.039 | 0.753 | 0.105 | 0.400 |
| Ferritin | −0.085 | 0.496 | −0.021 | 0.863 | 0.055 | 0.660 | −0.164 | 0.185 | −0.062 | 0.620 |
| Hcy | 0.211 | 0.086 | −0.233 | 0.058 | −0.048 | 0.703 | 0.061 | 0.626 | −0.006 | 0.960 |
| Vitamin B12 | −0.135 | 0.276 | 0.186 | 0.132 | −0.039 | 0.756 | −0.029 | 0.819 | −0.057 | 0.647 |
| Vitamin D | −0.093 | 0.456 | −0.267 | 0.029 * | −0.098 | 0.430 | −0.207 | 0.092 | −0.216 | 0.079 |
| Parameter | B | SE | Wald χ2 Test | df | p | Exp(B) |
|---|---|---|---|---|---|---|
| Iron | 0.145 | 0.056 | 6.792 | 1 | 0.009 ** | 1.156 |
| Ferritin | 0.028 | 0.015 | 3.393 | 1 | 0.065 | 1.029 |
| Hcy | 1.245 | 0.251 | 24.683 | 1 | 0.000 *** | 3.474 |
| Vitamin B12 | −0.003 | 0.002 | 2.879 | 1 | 0.090 | 0.997 |
| Vitamin D | −0.004 | 0.013 | 0.082 | 1 | 0.775 | 0.996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukovac, T.; Hil, O.A.; Popović, M.; Jovanović, V.; Savić, T.; Pavlović, A.M.; Pavlović, D. Serum Biomarker Analysis in Pediatric ADHD: Implications of Homocysteine, Vitamin B12, Vitamin D, Ferritin, and Iron Levels. Children 2024, 11, 497. https://doi.org/10.3390/children11040497
Lukovac T, Hil OA, Popović M, Jovanović V, Savić T, Pavlović AM, Pavlović D. Serum Biomarker Analysis in Pediatric ADHD: Implications of Homocysteine, Vitamin B12, Vitamin D, Ferritin, and Iron Levels. Children. 2024; 11(4):497. https://doi.org/10.3390/children11040497
Chicago/Turabian StyleLukovac, Tanja, Olivera Aleksić Hil, Milka Popović, Vitomir Jovanović, Tatjana Savić, Aleksandra M. Pavlović, and Dragan Pavlović. 2024. "Serum Biomarker Analysis in Pediatric ADHD: Implications of Homocysteine, Vitamin B12, Vitamin D, Ferritin, and Iron Levels" Children 11, no. 4: 497. https://doi.org/10.3390/children11040497
APA StyleLukovac, T., Hil, O. A., Popović, M., Jovanović, V., Savić, T., Pavlović, A. M., & Pavlović, D. (2024). Serum Biomarker Analysis in Pediatric ADHD: Implications of Homocysteine, Vitamin B12, Vitamin D, Ferritin, and Iron Levels. Children, 11(4), 497. https://doi.org/10.3390/children11040497